Abstract
Objective
To evaluate the value of 18fluorodeoxyglucose (FDG) positron emission tomography (PET) in primary head and neck cancer.
Background Data
Head and neck carcinomas tend to metastasize to regional lymph nodes rather than to spread hematogenously. With nodal metastases, cure rates decrease by approximately 50%. Moreover, in approximately 3% of the patients, a second primary tumor is found at initial presentation.
Methods
Fifty-four consecutive patients (31 men and 23 women; mean age 60 years, range 34–81 years) with previously untreated squamous cell carcinomas of the oral cavity or oropharynx were studied. Before surgery and within a period of 3 weeks, clinical examination, chest x-ray, computed tomography (CT), ultrasonography with fine-needle aspiration cytology (US/FNAC), and FDG-PET were performed. All study results were scored per neck side and were also classified as 0 (no metastases), 1 (single metastasis), or 2 (multiple metastases).
Results
The sensitivity for the detection of lymph node metastases per neck side was 96%, 85%, and 64% for FDG-PET, CT, and US/FNAC, respectively. The specificity was 90%, 86%, and 100% for FDG-PET, CT, and US/FNAC, respectively. In terms of the classification, FDG-PET showed the best correlation with the histologic data. Finally, in nine patients (17%), a second primary tumor was detected by FDG-PET and confirmed by histologic evaluation.
Conclusion
Because of the high prevalence of second primary tumors detected by FDG-PET and the decreased error rate in the assessment of lymph node involvement compared with CT and US, FDG-PET should be routinely performed in patients with primary head and neck cancer.
Head and neck squamous cell carcinomas originating from the mucous membranes of the upper aerodigestive tract account for approximately 5% of all malignant neoplasms. 1 These tumors tend to metastasize to regional lymph nodes rather than to spread hematogenously. Distant metastases are uncommon in patients who have never had nodal metastases in the neck. The incidence of lymph node metastases depends mainly on the site and size of the primary tumor, ranging from 1% for T1 glottic cancers to 80% for nasopharyngeal cancer. 2 The status of the cervical lymph nodes is an important prognostic factor. When nodal metastases exist at initial presentation or develop subsequently, cure rates decrease by approximately 50%. 3,4 The management of the involved neck is usually surgical in most institutions. When extranodal spread or multiple positive nodes are present in the neck dissection specimen, there is a high risk of recurrence in the neck. In these circumstances, postoperative radiotherapy can reduce the recurrence rate in the neck considerably. 5 However, patients with N0 lymph node status are the subpopulation who would benefit most from a better pretreatment evaluation of the regional lymph nodes, because an elective neck dissection may be avoided in these patients.
Despite the use of conventional imaging modalities, such as ultrasonography (US), magnetic resonance imaging (MRI), and computed tomography (CT), the overall error rate of assessing the presence or absence of cervical lymph node metastases is still 7.528% for both CT and MRI. 6 Therefore, it would be helpful to have a diagnostic tool that gives a better identification of the subpopulation.
One of the causes of poor outcome in patients with early-stage head and neck squamous cell carcinoma is the occurrence of second primary tumors. Most of these second tumors appear in the same organ or organ systems. Slaughter et al 7 explained this phenomenon with their “field cancerization” concept. The entire epithelial lining, covering the aerodigestive and upper digestive tract, undergoes extensive cytologic changes as a result of being exposed to repeated insults by the same carcinogens and as such is prone to multifocal cancers. Epidemiologic studies have established that second primary tumors appear at a continuing annual rate of approximately 3%, 8 depending on geographic and racial circumstances. The ultimate incidence of second primary tumors varies from 10% to 40% during 5 years. 9 A few of these tumors are synchronous tumors (those detected within 6 months after the initial tumor), whereas only 2% to 3% are detected simultaneously (within 1 month after the initial tumor) by using panendoscopy. 10–13
Recent reports have demonstrated the value of using 18fluorodeoxyglucose (FDG) positron emission tomography (PET) in the assessment of lymph node involvement in patients with primary head and neck cancer. 14–16 Because of the limited availability of dedicated PET cameras, alternative techniques have been introduced for the detection of FDG, but data on its use in head and neck cancer are limited.
The aims of the present study were to assess the value of imaging FDG using a dual-head PET camera in the evaluation of patients with primary head and neck cancer and to perform a prospective comparison with US, CT, and histology in the assessment of lymph node involvement.
PATIENTS AND METHODS
Patients
We prospectively studied 54 consecutive patients (31 men and 23 women; mean age 60 years, range 34–81 years) referred to the Department of Oral and Maxillofacial Surgery, University Hospital Utrecht, The Netherlands. All had previously untreated squamous cell carcinomas of the oral cavity or oropharynx. Patients with a history of malignancy were excluded from the study. Before surgery and within a period of 3 weeks all studies were performed, including clinical examination, chest x-ray, CT scanning, US with fine-needle aspiration cytology (FNAC) of the head and neck, and FDG-PET. Because of the low yield of panendoscopy (2–3%) for the detection of simultaneous tumors, this time-consuming procedure is not performed in the clinical setting in patients with oral or oropharyngeal cancer in our hospital. Therefore, based on the disappointing results described in the literature and the fact that the T stage of these tumors can be assessed by clinical examination, panendoscopy was not performed in the present study.
FDG-PET
All patients were studied after a 6-hour fast. Before the PET studies, plasma glucose levels were measured with a standard clinical test. At 60 minutes after the intravenous administration of 185 MBq (5 mCi) F18 FDG, imaging of the head and neck and chest was performed using a dual-head PET camera (Vertex MCD, ADAC, Milpitas, CA). Two acquisitions were made in each patient; both involved a rotation of each detector by 180° with 32 stops at 45 seconds per stop. During the interval between the administration of FDG and acquisition, patients were not allowed to speak, move, or chew to avoid artifacts. PET images were generated using iterative reconstruction (OS-ML, 2 iterations, 8 OS).
CT Scanning
CT scans of the cervical region were performed with a conventional CT scanner (Phillips SR 8000, Eindhoven, The Netherlands). Contiguous 3-mm slices were obtained; contrast enhancement was achieved using 100 mL nonionic contrast material (Ultravist 300, Schering, Berlin, Germany) with a power injector rate of 1.5 mL/sec.
Ultrasonography
High-resolution US studies (5–10 MHz, linear array; HDI-3000, ATL, Woerden, The Netherlands) of the regional lymph nodes were performed.
Analysis of Data
All studies were visually analyzed by experienced observers. The results were classified as 0 (no metastases), 1 (one metastasis), or 2 (multiple metastatic lymph nodes). Because the presence of metastatic disease is an indication for a neck dissection (class 0 vs. class 1 and 2), correct identification of the presence or absence of metastatic neck disease was chosen for statistical analysis. In addition, because the presence of two or more metastatic lymph nodes is one of the indications for radiotherapy (class 0 and 1 vs. class 2), the classifications of the imaging studies were assessed in relation to the pathologic data. With respect to the identification of the primary tumors, FDG-PET was compared with CT. Finally, in patients demonstrating additional sites of increased uptake, panendoscopy with biopsy or CT scanning of the chest was performed to assess the presence of second primary tumors.
Sensitivity was calculated by dividing the number of true-positive cases by the sum of the number of true-positive cases and the number of false-negative cases (×100%). The specificity was calculated by dividing the number of true-negative cases by the sum of the number of true-negative cases and the number of false-positive cases (×100%). The positive predictive value was calculated by dividing the number of true-positive cases by the sum of the true-positive cases and false-positive cases (×100%). The negative predictive value was calculated by dividing the number of true-negative cases by the sum of the true-negative cases and false-negative cases (×100%). The accuracy was calculated by dividing the sum of the true-positive cases and true-negative cases by the total number of cases (×100%). We estimated the prevalence with 95% confidence intervals of the detected second primary tumors.
RESULTS
The mean glucose level was 5.2 mmol/L (range 4.2–6.8 mmol/L). Of the 54 patients, 15 had T1, 21 had T2, 5 had T3, and 13 had T4 tumor stage. Of these tumors, 26 were in the floor of the mouth, 9 in the tongue, 8 in the gingiva, 4 in the oropharynx, 3 in the trigonum retromolare, 2 in the tonsil, and 2 in the lip. The mean diameter and the infiltration depth of these tumors were 2.67 cm (range 0.6–7.0 cm) and 1.0 cm (range 0.1–3.3 cm), respectively. FDG-PET identified all primary lesions (100%) versus 78% detected by CT. All primary tumors missed by CT has a depth of infiltration of 4 mm or less.
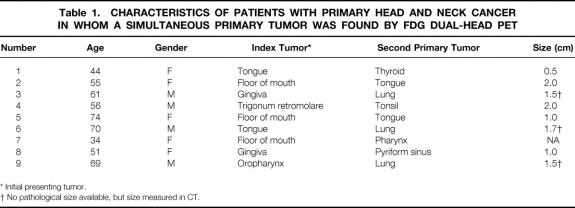
In nine patients, an unknown second primary tumor was detected by FDG-PET and confirmed by histologic evaluation (Table 1, Fig. 1). The mean size of these second primary tumors was 1.4 cm (range 0.5–2.0 cm). In eight of the nine patients, these tumors were localized in the aerodigestive and upper digestive tract, for a yield of 15% (95% confidence interval 6–24%) for FDG dual-head PET in screening the “field of cancerization” for unknown second primaries. In one patient, the second primary tumor was found in the thyroid; this was assumed to be a coincident tumor.
Table 1. CHARACTERISTICS OF PATIENTS WITH PRIMARY HEAD AND NECK CANCER IN WHOM A SIMULTANEOUS PRIMARY TUMOR WAS FOUND BY FDG DUAL-HEAD PET
* Initial presenting tumor.
† No pathological size available, but size measured in CT.
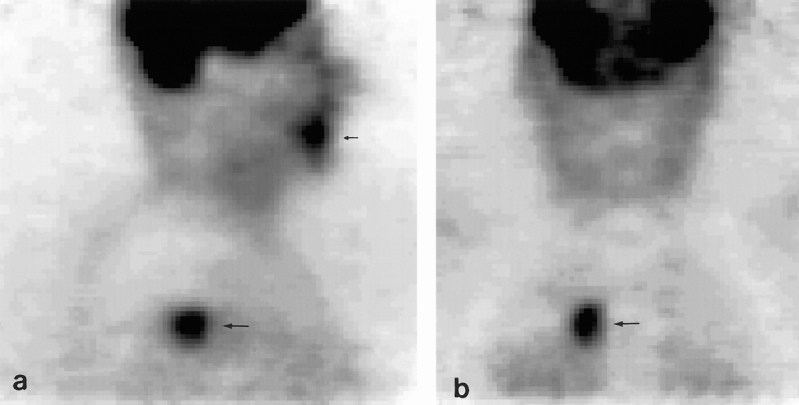
Figure 1. Sagittal (A) and coronal (B) slices of an FDG dual-head positron emission tomography study in a patient with a squamous cell carcinoma of the gingiva, demonstrating increased uptake at that site (small arrow). Unexpectedly, increased FDG accumulation was also seen in the upper lobe of the right lung (large arrow). Histologic examination of the biopsy specimen revealed an adenocarcinoma; therefore, this tumor was classified as an unknown second primary tumor.
In the 54 patients, 81 neck sides were available for evaluation. In 24 of these patients, metastases were found; 18 patients had unilateral metastases and 6 had bilateral metastases. With respect to the neck sides, the sensitivity, specificity, accuracy, and predictive values of US with and without FNAC, CT, and FDG-PET are shown in Table 2. The specificity of FDG-PET was influenced by a biopsy in two patients, causing false-positive uptake at that site. With knowledge of the preceding biopsies, the specificity would have been 94%.
Table 2. COMPARISON OF FDG DUAL-HEAD PET AND CONVENTIONAL IMAGING MODALITIES WITH HISTOPATHOLOGIC FINDINGS
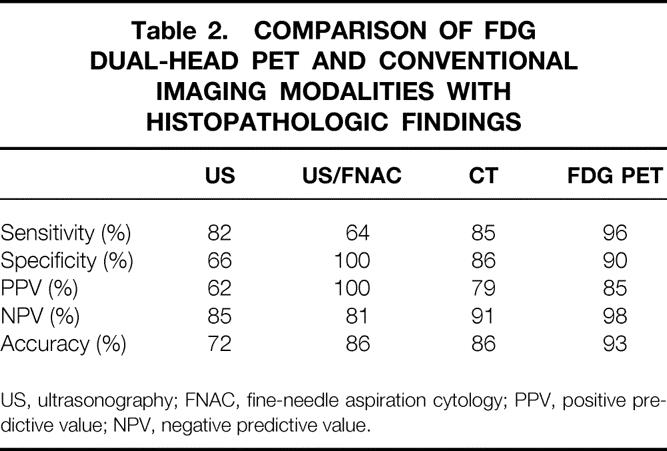
US, ultrasonography; FNAC, fine-needle aspiration cytology; PPV, positive predictive value; NPV, negative predictive value.
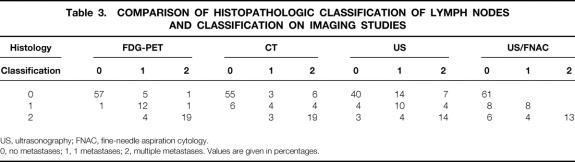
With respect to the classification of metastasis (0, 1, or 2), FDG-PET demonstrated the best overall correlation with the histologic data (88%), followed by US/FNAC (82%), CT (78%), and US without FNAC (64%) (Table 3).
Table 3. COMPARISON OF HISTOPATHOLOGIC CLASSIFICATION OF LYMPH NODES AND CLASSIFICATION ON IMAGING STUDIES
US, ultrasonography; FNAC, fine-needle aspiration cytology.
0, no metastases; 1, 1 metastases; 2, multiple metastases. Values are given in percentages.
In 14 patients, discordant results were found between FDG-PET and CT. In three patients, FDG PET demonstrated false-positive uptake (resulting from a preceding biopsy in two patients), whereas CT showed a correct correlation with pathologic findings. In four patients, CT revealed a false-positive result, whereas in three patients the number of metastases were overestimated on CT. In these seven patients, FDG-PET revealed the correct classification. Finally, in four patients, FDG-PET demonstrated metastatic disease that was not demonstrated by CT. Histologic examination of the neck dissection specimen showed that one patient was found to have four metastases, whereas three patients had one metastasis. Measurement of these metastases revealed a mean diameter of 9 mm. Five of seven metastases had a diameter less than 10 mm, whereas two had a diameter of 15 mm.
DISCUSSION
The presence of metastatic disease in patients with head and neck cancer indicates a high risk of tumor recurrence in the neck. In addition, the occurrence of second primary tumors is an important cause of poor survival rates in patients with early-stage disease. Therefore, an adequate preoperative evaluation of patients with a tumor in this area is a necessity. In the present study, using FDG-PET, we were able to identify an unknown second primary tumor in the field of cancerization in 15% (confidence interval 6–24%) of the patients with a tumor of the oral cavity or oropharynx. FDG-PET demonstrated the highest accuracy in identifying metastatic disease when compared with CT, US, and US/FNAC. To our knowledge, this is the largest case series performed evaluating FDG detection with a dual-head PET camera in head and neck cancer.
FDG-PET depicted all primary tumors; CT detected only 78% of the tumors. All tumors with a depth of infiltration of 4 mm or less were missed on CT images. These results are in agreement with the results found in the literature. 17,18 Primary tumors that do not distort tissue planes or invade contiguous structures may not be detected by CT scanning. However, in patients with a tumor in the oral cavity, as described in the present study, there is little or no benefit of FDG-PET’s higher detection rate. Clinical assessment by palpation of the primary tumor alone may be sufficient to stage the local extent of tumor correctly, despite the fact that in many patients only the mucosal surface is visible. Because correct T staging is necessary to define the appropriate surgical approach, the need for more accurate and reliable methods is well recognized in patients with a tumor located outside the oral cavity. In this respect, Jabour et al 17 and Laubenbacher et al 19 demonstrated that despite the high detection rate, tumor size is often overestimated on FDG- PET, which makes this technique less valuable in the assessment of T stage. However, in these studies, the blurring of tumor borders was also described in contrast-enhanced MRI, because Gd-DTPA tends to migrate in peritumoral interstitial tissue. With respect to the identification of primary tumors, FDG-PET may have a role in patients with cervical metastases from an unknown primary tumor. Recent reports have shown promising results with both FDG- PET and FDG/single photon emission computed tomography. 20–22 Depending on the criteria for patient selection, unknown primary tumors were found in approximately 30% to 80% of the patients.
Patients with primary head and neck cancer have a high risk for developing a second primary tumor in the “field of cancerization.” The histologic criteria for the diagnosis of multicentric neoplasm were originally described by Warren and Gates 23 : the neoplasms must be clearly malignant, each neoplasm must be geographically separated and not connected by either submucosal or intraepithelial neoplastic changes, and the second neoplasm must not represent a metastasis. Because of second primary tumors, survival rates are poor. Five-year survival rates are approximately 48%, 24,25 and the 5-year survival rate after diagnosis of the second neoplasm is 8% to 23%. 9,26,27 The reasons for this poor survival include the high prevalence of second cancers in the lung and esophagus, which have extremely poor survival rates, and the often late diagnosis of these second lesions, with resultant advanced disease. In a metaanalysis of second malignant tumors in head and neck cancer derived from 25 studies, a mean overall prevalence of 11.4% (range 5–26%) was found. 28 Most of these tumors are found metachronously (detected >6 months after the primary tumor). Approximately 4% of these second primaries are detected within 6 months after the initial tumor as synchronous tumors. The use of panendoscopy in identifying these second primaries is still a matter of debate. Because of the low yield described in the literature of approximately 2% to 3% in detecting simultaneous tumors (tumors occurring within 1 month after the initial primary tumor), this technique is not routinely performed in our hospital in patients with tumors in the oral cavity. In the present study, we were able to identify a second primary tumor in 15% of the patients. Regarding the lower limit of 6% of the confidence interval, we were probably able to identify all simultaneous and synchronous tumors as well as some of the metachronous tumors. To confirm the value of FDG-PET in the early detection of second primary tumors and to assess its value with respect to prognosis and survival, however, follow-up studies are required.
In the present study, we chose to use a clinical approach to the assessment of lymph node involvement. The classification used was described by Braams et al 29 and is based on the therapeutic options for no, one, or multiple metastases. 2,5 A second rationale for this classification is that without image fusion, it is difficult to assess the agreement between pathologic and nonpathologic lymph nodes on FDG-PET and CT images. For example, if CT shows an enlarged and a normal lymph node near each other and pathologic examination shows tumor in the smaller one, it is difficult to assess whether the focally increased uptake on the PET images corresponds to the small node (true-positive) or the enlarged lymph node. In the latter situation, we are dealing not only with a false-positive but also a false-negative result. Nevertheless, compared with the results obtained with CT (78%), US (64%), and US/FNAC (82%), FDG-PET showed the best correlation (88%) with the pathologic examinations by using this classification. Moreover, in the cases in which FDG-PET and CT yielded discordant results, PET was found to show the correct result in 11 of 14 patients. In 4 of these 11 patients, FDG PET correctly depicted metastatic disease missed by CT, whereas in 7 patients metastatic disease shown by CT was excluded. These findings demonstrate the value of metabolic imaging compared with anatomic imaging.
Based on the neck sides, the high sensitivity of 96% in the present study is comparable with the results found in the literature with dedicated PET cameras. 19,29 Because of the underestimation of the number of metastases, as can be seen in Table 2, the true sensitivity for the identification of individual involved lymph nodes is lower. However, with respect to the decision to perform surgery or not, only 1% of the patients would have been undertreated. In contrast, because of our high negative predictive value of 98%, we recommend that neck dissection should not be performed in patients if FDG-PET does not show lymph node involvement (ie, N0 stage). Our negative predictive values of 85% and 91% for US and CT, respectively, demonstrate the necessity for elective neck dissections in patients at risk for lymph node metastases. Consequently, the patients with N0 clinical status based on examination and CT who will be considered for elective neck dissection are the subpopulation who would benefit most from a better pretreatment assessment of regional nodes—and this can be achieved by introducing FDG-PET into the diagnostic workup.
A major problem of FDG-PET remains the 90% specificity, which means that patients are being overstaged. In two patients, false-positive uptake was caused by a biopsy that had been performed shortly before the FDG-PET study. By postponing the biopsy, the specificity would have been 94%, which is comparable to the recent results of Adams et al. 16 Despite the lower overall error rate of FDG-PET compared with CT and US, 6% of the patients would still have been overtreated. In other words, using FDG-PET as an indicator for surgery is still controversial.
The availability and cost of dedicated PET scanners may limit the use of FDG-PET. We used a dual-head gamma camera for coincidence detection. The lower cost of this camera ($500,000) compared with that of dedicated PET scanners ($1–2 million) and the results achieved in the present study may encourage more widespread use of FDG detection in clinical oncology.
In summary, we assessed the value of FDG detection with a dual-head PET camera in the preoperative evaluation of patients with primary head and neck cancer. Because of the high prevalence of second primary tumors obtained with FDG-PET as well as the decreased error rate in the assessment of lymph node involvement compared with CT and US, FDG-PET should be routinely performed in these patients at initial presentation. Moreover, based on the results, we conclude that FDG detection with a dual-head PET camera is useful for this indication.
Footnotes
Correspondence: Marcel P.M. Stokkel, MD, Dept. of Nuclear Medicine, LUMC, Albinusdreef 2, 2300 RC Leiden, The Netherlands.
Accepted for publication May 19, 1999.
References
- 1.Parker SL, Tong T, Bolden S, et al. Cancer statistics 1996. CA Cancer J Clin 1996; 46:5–27. [DOI] [PubMed] [Google Scholar]
- 2.Snow GB, Patel P, Leemans CR, Tiwari R. Management of cervical lymph nodes in patients with head and neck cancer. Eur Arch Otorhinolaryngol 1992; 249:187–194. [DOI] [PubMed] [Google Scholar]
- 3.Whitehurst JO, Droulias CA. Surgical treatment of squamous cell carcinoma of the oral tongue. Arch Otolaryngol 1987; 103:212–215. [DOI] [PubMed] [Google Scholar]
- 4.Sham JS, Choy D. Prognostic factors of nasopharyngeal carcinoma: a review of 759 patients. Br J Radiol 1990; 63:51–58. [DOI] [PubMed] [Google Scholar]
- 5.Leemans TR, Tiwari R, van der Waal I, Karim ABMF, Nauta JJP, Snow GB. The efficacy of comprehensive postoperative radiotherapy in nodal metastases of squamous cell carcinoma of the upper respiratory and digestive tract. Laryngoscope 1990; 100:1194–1198. [DOI] [PubMed] [Google Scholar]
- 6.Van den Breekel MWM, Stel HV, Castelijns JA, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990; 177:379–384. [DOI] [PubMed] [Google Scholar]
- 7.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium. Clinical implications of multicentric origin. Cancer 1953; 6:963–968. [DOI] [PubMed] [Google Scholar]
- 8.Jovanovic A, van der Tol IGH, Kostense PJ, et al. Second respiratory and upper digestive tract cancer following oral squamous cell carcinoma. Eur J Cancer B Oral Oncol 1994; 30B:225–229. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz LH, Oszahin M, Zhang GN, et al. Synchronous and metachronous head and neck carcinomas. Cancer 1994; 74:1933–1938. [DOI] [PubMed] [Google Scholar]
- 10.Parker JT, Hill JH. Panendoscopy in screening for synchronous primary malignancies. Laryngoscope 1988; 98:147–149. [DOI] [PubMed] [Google Scholar]
- 11.Hordijk GJ, Bruggink T, Ravasz LA. Panendoscopy: a valuable procedure. Laryngol Head Neck Surg 1989; 101:426–428. [DOI] [PubMed] [Google Scholar]
- 12.Dhooge IJ, de Vos J, Albers FWJ. Panendoscopy as a screening procedure for simultaneous primary tumors in head and neck cancer. Eur Arch Otorhinolaryngol 1996; 253:319–324. [DOI] [PubMed] [Google Scholar]
- 13.McGarry GW, Mackenzie K, Periasamy P, McGurk F, Gatehouse S. Multiple primary malignant tumours in patients with head and neck cancer: the implications for follow-up. Clin Otolaryngol 1992; 17:558–562. [DOI] [PubMed] [Google Scholar]
- 14.Myers LL, Wax MK, Nabi H, Simpson GT, Lamonica D. Positron emission tomography in the evaluation of the N0 neck. Laryngoscope 1998; 108:232–236. [DOI] [PubMed] [Google Scholar]
- 15.Benchaou M, Lehman W, Slosman DO, et al. The role of FDG PET in the preoperative assessment of N staging in head and neck cancer. Acta Otolaryngol 1996; 116:332–335. [DOI] [PubMed] [Google Scholar]
- 16.Adams S, Baum RP, Stuckensen T, Bitter K, Hor G. Prospective comparison of F18-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med 1998; 25:1255–1260. [DOI] [PubMed] [Google Scholar]
- 17.Jabour BA, Choi Y, Hoh CK, et al. Extracranial head and neck PET imaging with 2-(F18)-fluoro-2-deoxy-D-glucose and MR imaging correlation. Radiology 1993; 186:27–35. [DOI] [PubMed] [Google Scholar]
- 18.Steinkamp HJ, Maurer J, Heim T, Knobber D, Felix R. Magnetresonanz-tomographie und computertomographie im tumorstaging des mundhohlen oropharynxkarzinoms. HNO 1993; 41:519–525. [PubMed] [Google Scholar]
- 19.Laubenbacher C, Saumweger D, Wagner-Manslau C, et al. Comparison of fluorine-18-fluorodeoxyglucose PET, MRI and endoscopy for staging head and neck squamous cell carcinomas. J Nucl Med 1995; 36:1714–1757. [PubMed] [Google Scholar]
- 20.Mukherji SK, Drane WE, Mancuso AA, Parsons JT, Mendenhall WM, Stringer S. Occult primary tumors of the head and neck: detection with 2-(F18)-fluoro-2-deoxy-D-glucose SPECT. Radiology 1996; 199:761–766. [DOI] [PubMed] [Google Scholar]
- 21.Kole AC, Nieweg OE, Pruim J, et al. Detection of unknown occult primary tumors using positron emission tomography. Cancer 1998; 82:1160–1166. [DOI] [PubMed] [Google Scholar]
- 22.Lastoria S, Mainolfi C, Panico R, et al. Potential role of whole-body PET with F18 FDG in patients with unknown origin tumors. J Nucl Med 1995; 36(suppl):194P. [Google Scholar]
- 23.Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer 1932; 51:1358–1403. [Google Scholar]
- 24.Dhooge IJ, de Vos M, van Cauwenberge PB. Multiple primary malignant tumors in patients with head and neck cancer: results of a prospective study and future perspectives. Laryngoscope 1988; 108:250–256. [DOI] [PubMed] [Google Scholar]
- 25.Jones AS, Morar P, Phillips DE, Field JK, Husband D, Helliwell TR. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer 1995; 75:1343–1353. [DOI] [PubMed] [Google Scholar]
- 26.Gluckman JL, Crissman JD. Survival rates in 548 patients with multiple neoplasms of the upper aerodigestive tract. Laryngoscope 1983; 93:71–74. [DOI] [PubMed] [Google Scholar]
- 27.Larson JT, Adams GL, Fattah HA. Survival statistics for multiple primaries in head and neck cancer. Otolaryngol Head Neck Surg 1990; 103:14–24. [DOI] [PubMed] [Google Scholar]
- 28.Haughey BH, Gates GA, Arfken CL, Harvey J. Metaanalysis of second malignant tumors in head and neck cancer: the case for an endoscopic screening protocol. Ann Otol Rhinol Laryngol 1992; 101:105–112. [DOI] [PubMed] [Google Scholar]
- 29.Braams JW, Pruim J, Freling NJM, et al. Detection of lymph node metastases of squamous cell cancer of the head and neck with FDG-PET and MRI. J Nucl Med 1995; 36:211–216. Vertex MCD, ADAC, Milpitas, CA [PubMed]