Abstract
Objective
The authors reviewed their institution’s experience treating mammographically detected ductal carcinoma in situ (DCIS) of the breast with breast-conserving therapy (BCT) to determine 10-year rates of local control and survival, patterns of failure, and factors associated with outcome.
Summary Background Data
From January 1980 to December 1993, 177 breasts in 172 patients were treated with BCT for mammographically detected DCIS of the breast at William Beaumont Hospital, Royal Oak, Michigan.
Methods
All patients underwent an excisional biopsy, and 65% were reexcised. Thirty-one breasts (18%) were treated with excision alone, whereas 146 breasts (82%) received postoperative radiation therapy (RT). All patients undergoing RT received whole-breast irradiation to a median dose of 50.0 Gy. One hundred thirty-six (93%) received a boost to the tumor bed for a median total dose of 60.4 Gy. Median follow-up was 5.9 years for the lumpectomy alone group and 7.2 years for the lumpectomy + RT group.
Results
In the entire population, 15 patients had an ipsilateral breast recurrence. The 5- and 10-year actuarial rates of ipsilateral breast recurrence were 7.8% and 7.8% for lumpectomy alone and 8.0% and 9.2% for lumpectomy + RT, respectively. Eleven of the 15 recurrences developed within or immediately adjacent to the lumpectomy cavity and were designated as true recurrences or marginal misses (TR/MM). Four recurred elsewhere in the breast. Eleven of the 15 recurrences were invasive, whereas 4 were pure DCIS. Only one patient died of disease, yielding 5- and 10-year actuarial cause-specific survival rates of 100% and 99.2%, respectively. Eleven patients were diagnosed with subsequent contralateral breast cancer, yielding 5- and 10-year actuarial rates of 5.1% and 8.3%, respectively. Clinical, pathologic, and treatment-related factors were analyzed for an association with ipsilateral breast failure or TR/MM. No factors were significantly associated with ipsilateral breast failure. In the entire population, the omission of RT and younger age at diagnosis were significantly associated with TR/MM. Patients younger than 45 years at diagnosis had a significantly higher rate of TR/MM in both the lumpectomy + RT and lumpectomy alone groups. None of the 37 patients who received a postexcisional mammogram had an ipsilateral breast failure versus 15 in the patients who did not receive a postexcisional mammogram.
Conclusions
Patients diagnosed with mammographically detected DCIS of the breast appear to have excellent 10-year rates of local control and overall survival when treated with BCT. These results suggest that the use of RT reduces the risk of local recurrence and that patients diagnosed at a younger age have a higher rate of local recurrence with or without the use of postoperative RT.
The widespread use of screening mammography to detect breast cancer has led to a significant increase in the diagnosis of clinically occult ductal carcinoma in situ (DCIS). 1,2 In an effort to establish appropriate treatment recommendations for patients undergoing breast-conserving therapy (BCT), multiple groups have attempted to identify subsets of patients who are at greater risk for developing a local recurrence. Various authors have reported that certain histopathologic factors (margin status, nuclear grade, tumor size) may be associated with a higher local recurrence rate. 3–15 Several of these studies have analyzed treatment results and prognostic factors for DCIS in general. However, studies reporting prognostic variables for clinically detected DCIS may not be directly applicable to mammographically detected lesions. Only three prior studies have confined their analyses to mammographically detected DCIS. 13,14,16 Each of these studies included only patients who were treated with excision followed by radiation therapy (RT), and follow-up for most studies has been relatively short. This report describes the 10-year outcome of a large group of patients with mammographically detected DCIS treated with BCT, including those treated with either excision alone or excision followed by RT. Patterns of failure are analyzed to help clarify the factors associated with outcome.
PATIENTS AND METHODS
From January 1980 through December 1993, 210 breasts in 205 patients with DCIS were treated with BCT at William Beaumont Hospital in Royal Oak, Michigan. One hundred eighty-seven of these cases (89%) were detected by screening mammography. Of the cases that were mammographically detected, 170 (91%) underwent complete pathologic review. Ten of the 187 cases were excluded based on findings at histologic review (3 for invasive cancer, 6 for atypical ductal hyperplasia, and 1 for lobular carcinoma in situ). The study population included 167 patients with unilateral DCIS and 5 patients with bilateral DCIS for a total of 177 breasts with DCIS in 172 patients.
All women had American Joint Committee on Cancer (AJCC) clinical stage 0 (Tis N0 M0) DCIS of the breast at presentation. 17 Patients with the following findings were excluded in this analysis: invasive carcinoma of the breast, microinvasive carcinoma of the breast, or initial detection by any method other than mammography. Nine patients (5%) with previous or simultaneous contralateral breast cancer (five with DCIS and four with invasive cancer) were included in the study population. However, the four patients with previous or simultaneous contralateral invasive breast cancer were excluded from all survival analyses.
All women were treated with either excision alone or excision followed by breast irradiation. The surgical treatment in all cases consisted of excision of tissue around the tip of the needle localization wire. The initial excision was guided by mammographic needle localization in 174 cases (98%). One hundred fifteen cases (65%) underwent a reexcision of the primary tumor site due to close (≤2 mm), positive, or uncertain margins at the discretion of the surgeon or radiation oncologist. In some cases, postexcisional (preirradiation) mammograms were obtained to exclude residual microcalcifications in the breast.
Pathologic lymph node staging was performed in 81 cases (46%). All lymph nodes excised were free of metastasis. The median number of lymph nodes removed was 14 (range 1–39 nodes). Since 1990, surgical staging of the axilla has not been routinely performed.
One hundred forty-six breasts (82%) received postoperative radiation therapy, whereas 31 breasts (18%) were treated with excision alone. Reasons for omitting RT were often difficult to determine, but included short life expectancy, favorable tumor characteristics, and patient preference.
Our radiation technique has been previously reported. 14,15 Briefly, RT was initiated at a median interval of 5.1 weeks after the last surgical procedure (range 0–32 weeks). The entire breast was irradiated with 4–6 MV photons to a median dose of 50.0 Gy (range 43.1–56.0 Gy). Whole-breast irradiation was followed by a supplemental boost to the tumor bed in 136 cases (93%) for a median total dose of 60.4 Gy (range 45.0–71.8 Gy) to the tumor bed. The tumor bed was boosted with electrons in 107 cases, an interstitial implant in 28 cases, or photons in 1 case. The 10 cases not boosted received a median of 50.4 Gy to the entire breast (range 45.0–56.0 Gy). Regional lymphatics were not treated in any patient, and no adjuvant chemotherapy was administered. Six patients (3%) received adjuvant tamoxifen.
All specimen slides were reviewed for this study by one of the authors (NSG) without knowledge of the clinical outcome. For both the initial biopsy and reexcision specimens, the following were recorded:
• Maximum specimen dimensions
• Pattern of DCIS involvement, categorized as tumorous, tumorous with dispersion, or predominantly dispersed. DCIS formed a tumorous lesion when the DCIS ducts were closely grouped such that a tumor size could be measured. DCIS had a dispersed pattern when one or more DCIS ducts were separated by normal breast parenchyma in a random or discontinuous pattern (sometimes on several slides) such that a tumor size could not be measured.
• Maximum DCIS tumor dimensions, measured from the portion of DCIS that had a tumorous pattern. If a tumorous lesion was contiguous across several slides, this tumor dimension was calculated using an estimated maximal tumor block thickness of 0.4 cm and multiplying by the number of slides containing the tumor. The dispersed pattern associated with tumorous DCIS was not measured.
• Predominant histologic subtype, categorized as comedo, cribriform, papillary, micropapillary, solid, clinging, or cystic 18
• Predominant nuclear grade
• Highest nuclear grade
• Margin status, classified as positive (any DCIS duct transected at the margin), close (DCIS located within 0.2 cm of the inked margin edge, but not transected), negative (no DCIS within 0.2 cm of the inked margin), or uncertain (specimen was not inked or was fragmented such that the specimen margin could not be determined).
Based on the findings of the complete pathologic review, those 92 patients with a measurable tumor size were classified according to the Van Nuys Prognostic Index (VNPI). 11 Patients with an uncertain margin status after pathologic review were excluded from Van Nuys scoring. For the purposes of this analysis, the margin status was considered close (Van Nuys margin score 3) if DCIS was within 0.2 cm of the margin.
Mammographic findings were recorded from a retrospective review of reports on patient charts. Results were categorized as mass alone (with no calcifications), calcifications alone, or mass and calcifications. The vast majority of patients underwent standard two-view film screening mammography (craniocaudal and mediolateral oblique) with magnification views of suspicious calcifications or masses.
After completion of treatment, patients were evaluated every 3 months for the initial 2 years of follow-up, and at 6-month intervals thereafter. Patients frequently alternated follow-up visits between their surgeon and radiation oncologist. Mammograms were performed 6 months after completion of treatment and annually thereafter, unless a mammographic finding warranted earlier follow-up.
An ipsilateral breast failure was defined as the reappearance of cancer in the treated breast before or at the time of metastases. Ipsilateral breast failures were classified by clinical location in relation to the initial boost volume according to the criteria described by Recht et al. 19 A true recurrence/marginal miss (TR/MM) was defined as a recurrence within or immediately adjacent to the boost volume (or primary tumor site in patients not receiving RT). An “elsewhere” failure was defined as an ipsilateral breast recurrence several centimeters from the primary site and was generally thought to be a new primary cancer or multicentric cancer. Contralateral breast failure was defined as the subsequent development of breast cancer in the opposite, untreated breast.
Overall survival reflects all deaths, cancer-related or otherwise. Cause-specific survival was based on deaths attributed to breast cancer. The four patients with previous or simultaneous contralateral invasive breast cancer were excluded from all contralateral breast failure and survival analyses. However, these patients were included in all ipsilateral breast failure, TR/MM, and elsewhere failure analyses. The five patients with previous or simultaneous contralateral DCIS were also excluded from contralateral breast failure analyses, but they were included in survival analyses.
Actuarial results for ipsilateral breast failure, contralateral breast failure, disease-free survival, overall survival, and cause-specific survival were calculated by the Kaplan-Meier method. 20 The association of clinical, pathologic, and treatment-related variables with any given event was analyzed using Fisher’s exact test (two-tailed) for categorical variables and logistic regression for continuous variables. The statistical significance of differences between actuarial curves was calculated with the log-rank test. 21 Multivariate analysis was performed using multiple logistic regression. P ≤ .05 was considered statistically significant. For calculations of survival, the number of patients was used instead of the number of cases. All time intervals were calculated from the date of diagnosis. Statistical analysis was performed using SAS version 6.12 (SAS Institute, Inc., Cary, NC).
The median follow-up for all patients was 7.0 years (range 1.3–14.2 years). One hundred thirty-nine patients (79%) were followed for at least 5 years, whereas 34 patients (19%) were followed for more than 10 years. Median follow-up was 5.9 years for the lumpectomy alone group and 7.2 years for the lumpectomy + RT group.
RESULTS
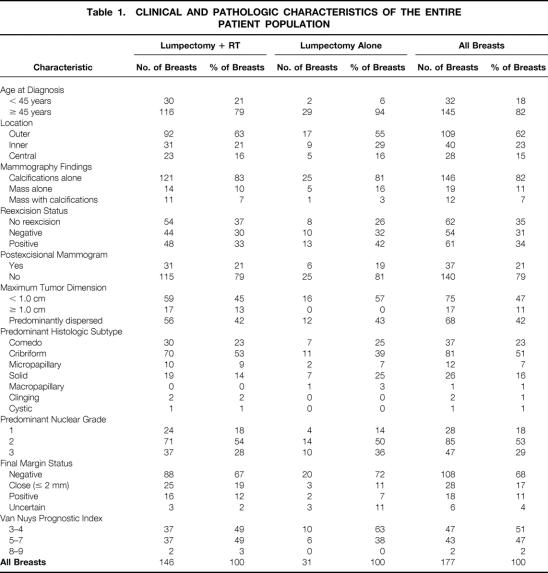
Table 1 lists the various clinical, pathologic, and treatment-related characteristics of the entire study population. Thirty-seven cases (21%) underwent postexcisional mammography to rule out residual microcalcifications. In seven of these cases (19%), suspicious findings were noted, prompting or confirming the need for reexcision. Residual DCIS was present in the reexcision specimen in six of the seven patients (86%) who underwent reexcision for suspicious postexcisional mammograms. Of the 160 cases with complete pathologic review, a negative final margin status was confirmed in 68%. Only 4% of specimens were not inked or were fragmented such that the final margin status could not be determined.
Table 1. CLINICAL AND PATHOLOGIC CHARACTERISTICS OF THE ENTIRE PATIENT POPULATION
Outcome for All Patients
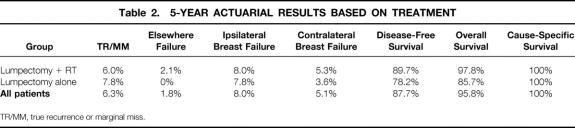
Tables 2 and 3 list 5- and 10-year actuarial outcome data for ipsilateral breast failure, TR/MM, elsewhere failure, contralateral breast failure, disease-free survival, overall survival, and cause-specific survival for the entire patient population. A total of 15 patients had recurrence in the ipsilateral breast, yielding 5- and 10-year rates of 8.0% and 9.1%, respectively. One hundred twenty-seven patients were at risk for an ipsilateral breast recurrence at 5 years and 31 at 10 years. In 11 cases (73%), the recurrences were classified as a TR/MM, and in 4 cases (27%), the recurrences were noted elsewhere in the breast (eg, a new primary lesion). The 5- and 10-year actuarial rates of a TR/MM were 6.3% and 7.4%, respectively. The mean interval from diagnosis to TR/MM was 3.8 years (range 1.1–6.8 years, median 3.9 years). For recurrences that occurred elsewhere in the breast, the 5- and 10-year actuarial rates were 1.8%. The mean interval from diagnosis to elsewhere failure was 4.8 years (range 2.5–10.3 years, median 3.2 years). The mean interval for all ipsilateral breast failures was 3.8 years (median 3.3 years). Thirteen ipsilateral breast failures (87%) occurred at 5 years or less. One TR/MM and one elsewhere failure occurred at 6.8 and 10.3 years, respectively.
Table 2. 5-YEAR ACTUARIAL RESULTS BASED ON TREATMENT
TR/MM, true recurrence or marginal miss.
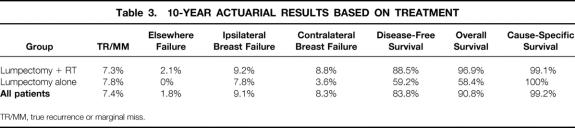
Table 3. 10-YEAR ACTUARIAL RESULTS BASED ON TREATMENT
TR/MM, true recurrence or marginal miss.
Outcome by Treatment Technique
In the group treated with lumpectomy + RT, 13 patients had recurrence, yielding 5- and 10-year actuarial rates of 8.0% and 9.2%, respectively. In those treated with lumpectomy alone, two patients experienced an ipsilateral breast failure, yielding a 5- and 10-year rate of 7.8%. For the recurrences classified as TR/MM, the 10-year actuarial rate for the lumpectomy + RT group was 7.3%, versus 7.8% for those treated with lumpectomy alone. The 10-year actuarial rate of elsewhere failure was 2.1% in the lumpectomy + RT group. No patients in the lumpectomy alone group experienced an elsewhere failure.
Because of patient selection and death from intercurrent illness, the 5- and 10-year disease-free and overall survival rates varied significantly between the two treatment groups. The 10-year disease-free survival rate was 88.5% in the lumpectomy + RT group versus 59.2% for the lumpectomy alone group. The 10-year overall survival rate was 96.9% in the lumpectomy + RT group versus 58.4% for the lumpectomy alone group. The 5-year cause-specific survival rate was 100% in both treatment groups. One patient in the lumpectomy + RT group had an invasive ipsilateral breast failure 2.3 years after diagnosis and then developed distant metastasis 3.0 years after ipsilateral breast failure. This patient died of disease shortly after distant metastasis. The 10-year actuarial cause-specific survival rate was 99.1% for those receiving lumpectomy + RT. No patient treated with lumpectomy alone died of breast cancer.
Failure Characteristics
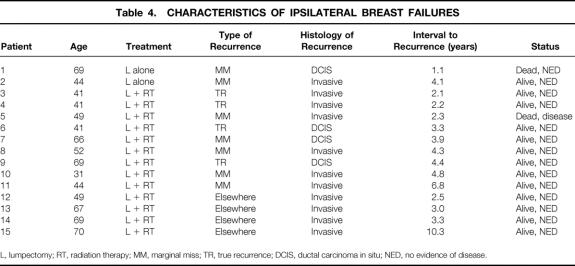
The characteristics of the 15 patients with ipsilateral breast failure are presented in Table 4. Of the 15 ipsilateral breast recurrences, the histology at the time of recurrence was invasive carcinoma (with or without associated DCIS) in 11 cases (73%) and pure DCIS in 4 cases (27%). Of the 11 recurrences classified as TR/MM, 7 were invasive and 4 were pure DCIS. All four elsewhere failures were invasive. In the patients treated with lumpectomy + RT, 10 (77%) of the recurrences were invasive and 3 were pure DCIS. In the lumpectomy alone group, one recurrence was invasive and one was pure DCIS. For all 11 invasive recurrences, the mean interval to recurrence was 4.2 years (range 2.1–10.3 years, median 3.3 years). The four noninvasive recurrences appeared 1.1, 3.3, 3.9, and 4.4 years after diagnosis. Thirteen recurrences (87%) were detected by mammography. One was detected by self-examination and one by physician examination.
Table 4. CHARACTERISTICS OF IPSILATERAL BREAST FAILURES
L, lumpectomy; RT, radiation therapy; MM, marginal miss; TR, true recurrence; DCIS, ductal carcinoma in situ; NED, no evidence of disease.
Salvage treatment at the time of ipsilateral breast failure consisted of mastectomy in 14 cases (93%). One patient initially treated with lumpectomy alone received salvage treatment consisting of lumpectomy followed by RT. This patient is alive without evidence of disease 2.0 years after salvage treatment. Seven of the patients treated with mastectomy had lymph nodes excised. In all seven patients, all nodes were free of metastases. No patient with invasive recurrence was treated with chemotherapy, and no patient developed a chest wall recurrence. Fourteen patients are without evidence of disease at a median of 3.2 years (range 0.2–7.1 years) after salvage treatment. The 5-year actuarial disease-free survival rate after an ipsilateral breast recurrence was 89%. One patient had distant failure 3.0 years after salvage mastectomy and eventually died of disease.
For the patients with ipsilateral breast failure, the survival status at last follow-up was alive with no evidence of disease in 13 patients (87%), dead with no evidence of disease in 1 patient (7%), and dead of disease in 1 patient (7%).
Contralateral Breast Failure
Eleven patients were diagnosed with subsequent contralateral breast cancer after conservative treatment for DCIS. Three of these were diagnosed with subsequent contralateral DCIS (one of whom underwent mastectomy), and eight were diagnosed with contralateral invasive cancer. The 5- and 10-year actuarial rates for the development of subsequent contralateral breast cancer after diagnosis of DCIS were 5.1% and 8.3%, respectively. The median interval between ipsilateral DCIS and subsequent contralateral breast cancer was 3.3 years (range 0.8–10.8 years). None of these 11 patients have had recurrence in either breast.
Statistical Analysis
An analysis was performed for several potential clinical, pathologic, and treatment-related factors relative to ipsilateral breast failure. The factors analyzed included age at diagnosis, mammographic findings (calcifications, mass, or both), location of the primary tumor (central, outer quadrant, or inner quadrant), reexcision status (no reexcision, negative reexcision, or positive reexcision), and use of postexcisional mammography to verify complete excision. Multiple pathologic factors were analyzed after a complete pathologic review of 160 cases, as discussed above. Additional treatment-related factors analyzed in the lumpectomy + RT group included whole breast dose, tumor bed dose, elapsed days during RT, boost type (no boost, interstitial implant, electrons, or photons), boost electron energy, boost volume, and interval from surgery to start of RT.
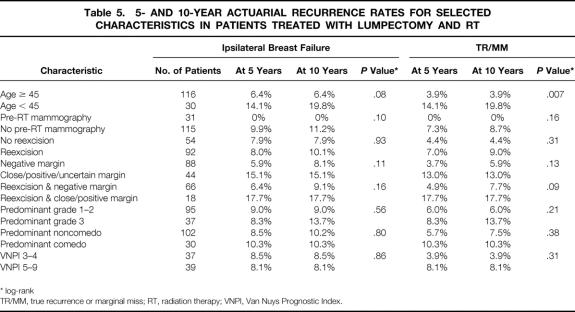
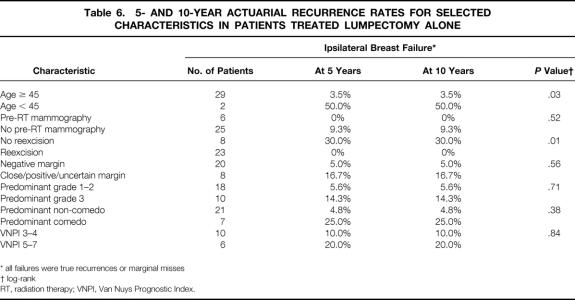
Univariate analysis of selected characteristics was performed for the entire population, the lumpectomy + RT group, and the lumpectomy alone group. Results of univariate analyses are summarized in Tables 5 (lumpectomy + RT) and 6 (lumpectomy alone). For the entire population, patients who were diagnosed at a younger age had a significantly higher risk of TR/MM when analyzed as a continuous variable (P = .02, logistic regression) or with a cutoff of 45 years of age (P = .002, log-rank). Patients younger than 45 years at diagnosis had a significantly higher recurrence rate in both the lumpectomy + RT (P = .007) and lumpectomy alone (P = .03) groups. In the lumpectomy alone group, two of the eight patients who did not undergo reexcision developed an ipsilateral recurrence, versus none of the 23 patients who underwent reexcision (P = .01, log-rank). The reexcision status was not significantly associated with recurrence for the lumpectomy + RT group or for all 177 cases. None of the 37 patients who received a postexcisional mammogram had an ipsilateral breast failure, versus 15 (10.7%) of the patients who did not undergo postexcisional mammography. For the entire patient population, this difference was statistically significant (P = .04, Fisher’s exact test). Regardless of the method used to analyze pathologic factors (ie, when combining important factors using the VNPI or considering them individually), these factors were not significantly associated with ipsilateral breast failure or TR/MM in the entire population or in either treatment group.
Table 5. 5- AND 10-YEAR ACTUARIAL RECURRENCE RATES FOR SELECTED CHARACTERISTICS IN PATIENTS TREATED WITH LUMPECTOMY AND RT
* log-rank
TR/MM, true recurrence or marginal miss; RT, radiation therapy; VNPI, Van Nuys Prognostic Index.
Table 6. 5- AND 10-YEAR ACTUARIAL RECURRENCE RATES FOR SELECTED CHARACTERISTICS IN PATIENTS TREATED LUMPECTOMY ALONE
* all failures were true recurrences or marginal misses
† log-rank
RT, radiation therapy; VNPI, Van Nuys Prognostic Index.
Multivariate analyses were performed for the entire population (Table 7) and for each treatment group (Tables 8 and 9) using the variables that were significant on univariate analyses as well as selected pathologic variables that were associated with recurrence in other series. No factor was significantly associated with ipsilateral breast failure in the whole population or in either treatment group. In the entire population, omitting RT (P = .03) and younger age at diagnosis (P = .03) were significantly associated with TR/MM. In the lumpectomy + RT group, younger age at diagnosis was significantly associated with TR/MM. No factor was significantly associated with TR/MM in the lumpectomy alone group on multivariate analysis.
Table 7. MULTIVARIATE ANALYSIS (P VALUES) OF FACTORS ASSOCIATED WITH RECURRENCE FOR THE ENTIRE POPULATION
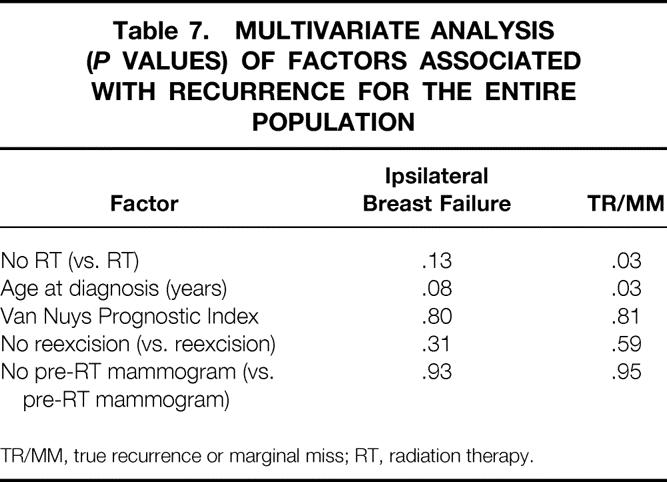
TR/MM, true recurrence or marginal miss; RT, radiation therapy.
Table 8. MULTIVARIATE ANALYSES (P VALUES) FOR PATIENTS TREATED WITH LUMPECTOMY FOLLOWED BY RT
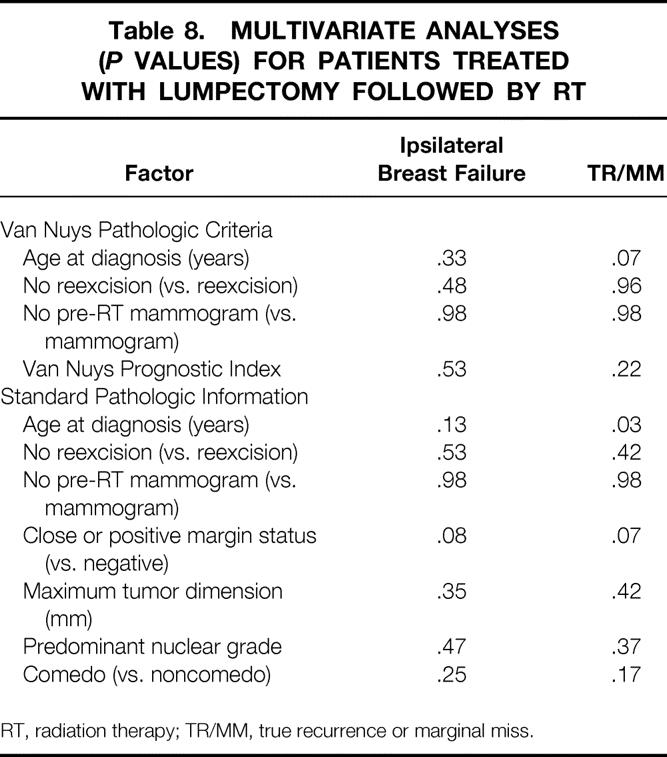
RT, radiation therapy; TR/MM, true recurrence or marginal miss.
Table 9. MULTIVARIATE ANALYSES (P VALUES) FOR PATIENTS TREATED WITH LUMPECTOMY ALONE
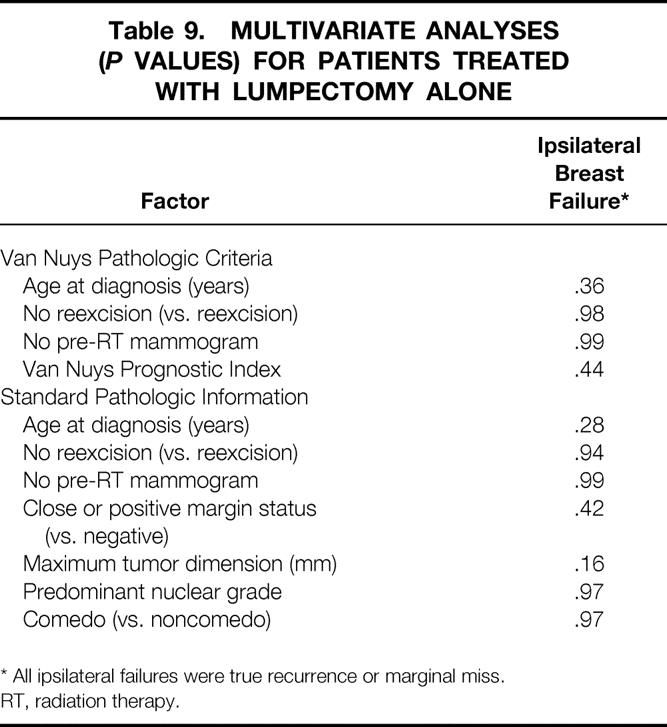
* All ipsilateral failures were true recurrence or marginal miss.
RT, radiation therapy.
DISCUSSION
In this study, we retrospectively reviewed our institution’s experience treating a large group of patients with mammographically detected DCIS of the breast with BCT. With a median follow-up of 7.2 years, the 5- and 10-year actuarial rates of ipsilateral breast failure were 8.0% and 9.1%, respectively. The corresponding 5- and 10-year cause-specific survival rates were 100% and 99.2%, respectively. When analyzing risk factors for TR/MM in the entire patient population, the omission of RT and younger age were statistically significant on multivariate analysis. Patients diagnosed at a younger age had a higher risk of TR/MM in both the lumpectomy + RT and lumpectomy alone treatment groups. The use of postoperative mammography was also significantly associated with outcome on univariate analysis. These findings demonstrate the excellent long-term rates of local control and survival with BCT for mammographically detected DCIS and emphasize both the importance of RT in reducing local recurrence and the impact of patient age on outcome.
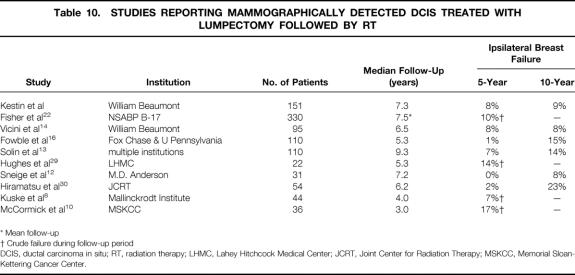
Several other groups have recently reported their experience with BCT for mammographically detected DCIS. Table 10 lists the largest studies reported to date. The recent NSABP B-17 update reported the results of 814 patients treated with BCT. 22 Six hundred fifty-four (80%) of these cases were detected by mammography only. Three hundred thirty of the mammographically detected cases were randomized to treatment with lumpectomy + RT, and 324 received lumpectomy alone. With a mean follow-up of 7.5 years, 33 patients (10%) in the lumpectomy + RT arm and 86 patients (26%) in the lumpectomy only arm developed an ipsilateral breast recurrence. Only 14 deaths (1.7%) in the entire study population were attributable to breast cancer.
Table 10. STUDIES REPORTING MAMMOGRAPHICALLY DETECTED DCIS TREATED WITH LUMPECTOMY FOLLOWED BY RT
* Mean follow-up
† Crude failure during follow-up period
DCIS, ductal carcinoma in situ; RT, radiation therapy; LHMC, Lahey Hitchcock Medical Center; JCRT, Joint Center for Radiation Therapy; MSKCC, Memorial Sloan-Kettering Cancer Center.
Additional studies on mammographically detected DCIS treated with BCT have been reported by Fowble et al 16 and Solin et al. 13 Each of these studies analyzed 110 patients treated with lumpectomy + RT. Fowble et al reported only three ipsilateral breast failures after a median follow-up of 5.3 years, yielding 5- and 10-year actuarial rates of 1% and 15%, respectively. Two of these recurrences were in a separate quadrant of the breast from the original primary, whereas one recurred in the same quadrant. The 10-year cause-specific survival rate was 100%. No factors were significantly associated with local recurrence. In the multiinstitutional series published by Solin et al, 15 patients had recurrence in the ipsilateral breast, yielding 5- and 10-year actuarial rates of 7% and 14%, respectively. Eleven of these were classified as TR/MM, and three were elsewhere failures. One recurrence was classified as diffuse. The 10-year cause-specific survival rate was 96%. Again, no factors were significantly associated with local recurrence.
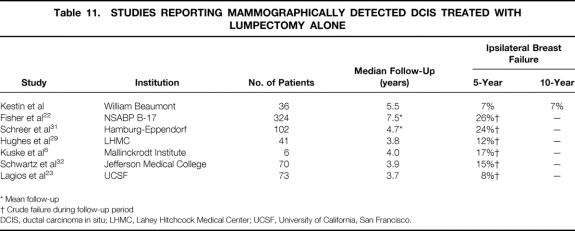
There are only limited studies reporting treatment outcome with excision alone for mammographically detected DCIS. Table 11 lists the only series we are aware of that have been published on this issue. Lagios et al 23 analyzed 79 patients treated with lumpectomy alone for DCIS. Seventy-three of these were detected mammographically. At a median follow-up of 3.7 years, six of these patients (8%) had recurrence in the ipsilateral breast. A higher rate of ipsilateral breast recurrence was reported in the lumpectomy alone arm of NSABP B-17. Of the 324 patients with DCIS detected by mammography only, 86 (26%) developed an ipsilateral breast recurrence. An update of the series by Lagios et al was recently published with a median follow-up of 10.3 years. 24 Of the entire cohort of 79 patients, 15 had recurrence in the ipsilateral breast, yielding a 15-year actuarial rate of 19%.
Table 11. STUDIES REPORTING MAMMOGRAPHICALLY DETECTED DCIS TREATED WITH LUMPECTOMY ALONE
* Mean follow-up
† Crude failure during follow-up period
DCIS, ductal carcinoma in situ; LHMC, Lahey Hitchcock Medical Center; UCSF, University of California, San Francisco.
Multiple studies have attempted to define a subset of DCIS patients at a lower risk of recurrence who may not require RT after lumpectomy. However, no clear subset has been identified. In the NSABP B-17 study, all subsets analyzed benefited from the addition of RT. 22 In the study by Lagios et al, 23 the selection criteria for treatment with lumpectomy alone evolved to include only patients with mammographically detected DCIS with histologically and mammographically confirmed complete excision measuring less than 2.5 cm. In the current study, patients treated with lumpectomy alone had a significantly shortened overall survival compared with the lumpectomy + RT group because of older age and intercurrent illness. These selection factors may partially explain the similar rate of ipsilateral breast failure with or without the use of RT in our study versus the higher failure rate in the lumpectomy alone arm of NSABP B-17. However, when all prognostic factors were considered with multivariate analysis, omitting RT was significantly associated with local recurrence. Patients diagnosed at a younger age also had a higher recurrence rate. Of note, six of seven patients (86%) with suspicious mammography findings after the initial excision had residual DCIS in their reexcision specimens, and none of the 37 patients with postexcisional mammograms had a recurrence. However, larger patient numbers and other studies will be required to confirm the utility of postexcisional mammography.
The VNPI was developed as a tool to assist in treatment selection by combining multiple pathologic factors to predict the probability of local recurrence after BCT. 11 In our study, the VNPI was not significantly associated with recurrence in either treatment group or the entire population on univariate or multivariate analysis. This lack of association may be partially related to the inherent difficulty of measuring DCIS tumor size unless the specimen has been totally and sequentially embedded, as other authors have suggested. 25–28 In the current analysis, tumor size could be accurately measured in only 92 cases (52%). Even in these 92 cases with precise tumor measurement, the VNPI was not significantly associated with recurrence. Because this analysis included only mammographically detected cases, only two cases received a VNPI of 8 or 9 due to smaller tumor size, possibly reducing the power to detect a statistical association. However, these smaller, mammographically detected lesions represent the vast majority of cases in which treatment selection may be controversial and which would benefit most from the use of a tool to assist in treatment decision making. Despite its limitations, the VNPI is an important step toward identifying and quantifying the effect of multiple risk factors on the probability of recurrence.
Multiple studies have analyzed various factors in an attempt to predict ipsilateral failure after lumpectomy + RT. However, only three studies have confined their analysis to patients with mammographically detected DCIS. 13,14,16 Similar to the current analysis, no consistent clinical, pathologic, or treatment-related risk factor for ipsilateral breast recurrence was identified in these studies. This may be related in part to small patient numbers, inadequate follow-up, and inconsistencies in defining pathologic criteria. In addition, failure rates with BCT are small, thereby weakening the statistical power to demonstrate an association with outcome.
When reporting treatment results with BCT, it may also be important to determine the type and location of the recurrence to differentiate a new primary breast cancer from a failure to control the initial lesion. This becomes particularly important when analyses are then performed to demonstrate an association of a particular clinical, pathologic, or treatment-related factor with the development of local recurrence. As we have previously shown, erroneous conclusions may be reached regarding the association of a particular variable with outcome if analyses are not performed in this manner. 14 Unfortunately, most of the studies listed in Tables 5 and 6 do not report outcome in this manner, making data comparisons problematic. Because 27% of the recurrences in our study were considered elsewhere failures, the dilutional effect of adding these events to TR/MM makes statistical analyses imprecise. For example, when analyzing all ipsilateral failures, no factor was found to be associated with outcome, whereas when analyzing for TR/MM, younger age was found to be associated with outcome in all subsets of patients. We hope that future studies will analyze treatment results in this fashion so that valid conclusions can be reached regarding factors associated with optimal results.
As noted above, young age appears to be associated with a higher rate of local recurrence in patients treated with lumpectomy, with or without postoperative RT. The factors responsible for this higher risk remain undefined. Additional studies will be needed to determine whether this risk can be reduced or eliminated with optimal treatment techniques or whether younger patients have an inherently worse outcome, independent of currently defined risk factors.
In conclusion, patients diagnosed with mammographically detected DCIS of the breast appear to have excellent long-term rates of local control and overall survival when treated with BCT. Our results suggest that the use of RT reduces the risk of local recurrence and that patients diagnosed at a younger age have a higher rate of local recurrence with or without the use of postoperative RT. The use of postexcisional mammography to rule out residual microcalcifications appears to be critical in optimizing the treatment outcome for all patients treated with BCT.
Footnotes
Correspondence: Frank A. Vicini, MD, Dept. of Radiation Oncology, William Beaumont Hospital, 3601 West Thirteen Mile Rd., Royal Oak, MI 48073.
Accepted for publication April 5, 1999.
References
- 1.Choi WS, Parker BA, Pierce JP, Greenberg ER. Regional differences in the incidence and treatment of carcinoma in situ of the breast. Cancer Epidemiol Biomark Prev 1996; 5:317–320. [PubMed] [Google Scholar]
- 2.Ernster VL, Barclay J, Kerlikowske K, et al. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA 1996; 275:913–918. [PubMed] [Google Scholar]
- 3.Bornstein BA, Recht A, Connolly JL, et al. Results of treating ductal carcinoma in situ of the breast with conservative surgery and radiation therapy. Cancer 1991; 67:7–13. [DOI] [PubMed] [Google Scholar]
- 4.Fisher E, Constantino J, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) protocol B-17: intraductal carcinoma (ductal carcinoma in situ). Cancer 1995; 75:1310–1319. [DOI] [PubMed] [Google Scholar]
- 5.Fourquet A, Zafrani B, Compana F, et al. Breast-conserving treatment of ductal carcinoma in situ. Semin Radiat Oncol 1992; 2:116–125. [DOI] [PubMed] [Google Scholar]
- 6.Haffty BG, Peschel RE, Papadopoulis D, Pattare P. Radiation therapy for ductal carcinoma in situ of the breast. Conn Med 1990; 54:482–484. [PubMed] [Google Scholar]
- 7.Holland P, Gandhi A, Knox WF, et al. The importance of complete excision in the prevention of local recurrence of ductal carcinoma in situ. Br J Cancer 1998; 77:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuske RR, Bean JM, Garcia DM, et al. Breast conservation therapy for intraductal carcinoma of the breast. Int J Radiat Oncol Biol Phys 1993; 26:391–396. [DOI] [PubMed] [Google Scholar]
- 9.Lagios MD. Ductal carcinoma in situ. Pathology and treatment. Surg Clin North Am 1990; 70:853–871. [DOI] [PubMed] [Google Scholar]
- 10.McCormick B, Rosen PO, Kinne D, et al. Duct carcinoma in situ of the breast: an analysis of local control and conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys 1991; 21:289–292. [DOI] [PubMed] [Google Scholar]
- 11.Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer 1996; 77:2267–2274. [DOI] [PubMed] [Google Scholar]
- 12.Sneige N, McNeese M, Atkinson E, et al. Ductal carcinoma in situ treated with lumpectomy and irradiation: histopathological analysis of 49 specimens with emphasis on risk factors and long-term results. Hum Pathol 1995; 26:642–649. [DOI] [PubMed] [Google Scholar]
- 13.Solin LJ, McCormick B, Recht A, et al. Mammography detected clinically occult ductal carcinoma in situ (intraductal carcinoma) treated with breast-conserving surgery and definitive breast irradiation. Cancer J Sci Am 1996; 2:158–165. [PubMed] [Google Scholar]
- 14.Vicini FA, Lacerna MD, Goldstein NS, et al. Ductal carcinoma in situ detected in the mammographic era: an analysis of clinical, pathologic, and treatment-related factors affecting outcome with breast-conserving therapy. Int J Radiat Oncol Biol Phys 1997; 39:627–635. [DOI] [PubMed] [Google Scholar]
- 15.White J, Levine A, Gustafson G, et al. Outcome and prognostic factors for local recurrence in mammographically detected ductal carcinoma in situ of the breast treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 1995; 31:791–797. [DOI] [PubMed] [Google Scholar]
- 16.Fowble B, Hanlon AL, Fein DA, et al. Results of conservative surgery and radiation for mammographically detected ductal carcinoma in situ (DCIS). Int J Radiat Oncol Biol Phys 1997; 38:949–957. [DOI] [PubMed] [Google Scholar]
- 17.American Joint Committee on Cancer. Breast. In: Fleming ID, Cooper JS, Henson DE, et al, eds. AJCC Cancer Staging Manual, 5th ed. Philadelphia: Lippincott-Raven; 1998:159–170.
- 18.Page DL, Anderson TJ. Diagnostic Histopathology of the Breast. Edinburgh: Churchill Livingstone; 1987.
- 19.Recht A, Silver B, Schnitt S, et al. Breast relapse following primary radiation therapy for early stage breast cancer. I. Classification, frequency and salvage. Int J Radiat Oncol Biol Phys 1985; 11:1271–1276. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–459. [Google Scholar]
- 21.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50:163–170. [PubMed] [Google Scholar]
- 22.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 1998; 16:441–452. [DOI] [PubMed] [Google Scholar]
- 23.Lagios MD, Margolin FR, Westdahl PR, Rose MR. Mammographically detected duct carcinoma in situ. Frequency of local recurrence following tylectomy and prognostic effect of nuclear grade on local recurrence. Cancer 1989; 63:618–624. [DOI] [PubMed] [Google Scholar]
- 24.Lagios MD. Lagios experience. In: Silverstein MJ, ed. Ductal Carcinoma in Situ of the Breast. Baltimore: Williams & Wilkins; 1997: 361–365.
- 25.Fisher ER. Pathobiological considerations relating to the treatment of intraductal carcinoma (ductal carcinoma in situ) of the breast. CA Cancer J Clin 1996; 47:52–64. [DOI] [PubMed] [Google Scholar]
- 26.Schnitt SJ, Connolly J. Processing and evaluation of breast excision specimens. A clinically oriented approach. Am J Clin Pathol 1992; 98:125–137. [DOI] [PubMed] [Google Scholar]
- 27.Schnitt SJ, Harris JR, Smith BL. Developing a prognostic index for ductal carcinoma in situ of the breast: are we there yet? Cancer 1996; 77:2189–2192. [DOI] [PubMed] [Google Scholar]
- 28.Hetelekidis S, Collins L, Silver B, et al. Predictors of local recurrence following excision alone for ductal carcinoma in situ. Cancer 1999; 85:427–431. [PubMed] [Google Scholar]
- 29.Hughes KS, Lee AKC, McLellan R, et al. Breast-conserving therapy for patients with ductal carcinoma in situ. Breast Dis 1996; 9:255–268. [Google Scholar]
- 30.Hiramatsu H, Bornstein BA, Recht A, et al. Local recurrence after conservative surgery and radiation therapy for ductal carcinoma in situ. Possible importance of family history. Cancer J Sci Am 1995; 1:55–61. [PubMed] [Google Scholar]
- 31.Schreer I. Conservation therapy of DCIS without irradiation. Breast Dis 1996; 9:27–36. [Google Scholar]
- 32.Schwartz GF, Finkel GC, Garcia JC, Patchefsky AS. Subclinical ductal carcinoma in situ of the breast. Treatment by local excision and surveillance alone. Cancer 1992; 70:2468–2474. [DOI] [PubMed] [Google Scholar]