Abstract
Objective
To determine the effect of insulinlike growth factor I (IGF-I) in combination with its principal binding protein (IGFBP-3) on the hepatic acute phase response in severely burned children.
Summary Background Data
The hepatic acute phase response is a cascade of events initiated to restore homeostasis after trauma. A prolonged response, however, may contribute to multiple organ failure, hypermetabolism, complications, and death.
Methods
Twenty-two children with a mean total body surface area (TBSA) burn of 57 ± 3% were given a continuous infusion of 1 to 4 mg/kg/day IGF-I/BP-3 for 5 days after wound excision and grafting. Eight children with a TBSA burn of 54 ± 4% were given saline as controls. Before and 5 days after excision and grafting, blood samples were taken for serum hepatic constitutive protein, acute phase protein, and proinflammatory cytokine analysis.
Results
Serum IGF-I levels in burned children given the IGF-I/BP-3 complex increased from 113 ± 15 to 458 ± 40 ng/mL and IGFBP-3 levels increased from 1.8 ± 0.2 to 3.1 ± 0.3 ng/mL. Levels of serum constitutive hepatic proteins (prealbumin, retinol-binding protein, and transferrin) increased with IGF-I/BP-3, whereas levels of type I acute phase proteins (C-reactive protein, α1-acid glycoprotein, and complement C-3) decreased when compared with controls. The complex had no effect on type II acute phase proteins. Tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) levels decreased with IGF-I/BP-3 compared with controls, with no effect on interleukin-6.
Conclusion
Severely burned children receiving IGF-I/BP-3 showed a decrease in IL-1β and TNF-α followed by a decrease in type I acute phase proteins that was associated with a concomitant increase in constitutive hepatic proteins. Attenuating the proinflammatory acute phase with IGF-1/BP-3 response may prevent multiple organ failure and improve clinical outcomes after thermal injury without any detectable adverse side effects.
The hepatic acute phase response represents a cascade of events characterized by the upregulation of type I and type II acute phase proteins and the downregulation of constitutive hepatic proteins. 1–3 Proinflammatory cytokines mediate these events, which are initiated to restore homeostasis after trauma. 1–3 Clinical studies have shown that a sustained or increased acute phase response can be potentially life-threatening, with the uncontrolled and prolonged action of proinflammatory cytokines and acute phase proteins contributing to multiple organ failure, hypermetabolism, complications, and death. 4–6 The downregulation of constitutive hepatic proteins may augment these detrimental effects. 7–10 Multiple clinical trials have been undertaken in an attempt to attenuate the overexpression of proinflammatory cytokines and acute phase proteins. 4,7,8 These clinical trials, however, were unsuccessful in controlling a prolonged acute phase response.
A decrease in serum tumor necrosis factor-α (TNF-α) and an increase in serum albumin levels have been demonstrated in thermally injured pediatric patients given growth hormone (GH). 9,10 GH, however, exerts some of its effects through insulinlike growth factor I (IGF-I). 11,12 The GH/GF-I axis has been proposed as a major pathway in trauma for patient recovery. 11–13 IGF-I is a 7.7 kD single-chain polypeptide of 70 amino acids with sequence homology to proinsulin. 14 In the system, 95% to 99% of IGF-I is bound and transported with one of its six binding proteins. 15 IGF-I has been shown to improve cell recovery, wound healing, peripheral muscle protein synthesis, and gut and immune function after thermal injury. 11,16–19
To investigate the effect of IGF-I on the hepatic acute phase response in severely burned pediatric patients, we administered IGF-I bound to its principal binding protein, IGFBP-3. Adverse side effects of IGF-I given in physiologically effective doses include hypoglycemia, electrolyte imbalance, edema, neuropathies, and cardiac arrest. 12,20 The efficacy of the IGF-I/BP-3 complex was evaluated from its effect on serum constitutive hepatic proteins, acute phase proteins, and proinflammatory cytokines.
PATIENTS AND METHODS
Thirty severely thermally injured children were randomized to receive recombinant human IGF-I in combination with BP-3 or normal saline (control). Inclusion criteria were age younger than 15 years, admission to our hospital within 20 days of injury without evidence of organ failure, and burns covering more than 40% total body surface area (TBSA) that required at least three operations for skin grafting.
The rhIGF-I/BP-3 complex was provided by Celtrix Pharmaceuticals, Inc. (Santa Clara, CA) in a 1:1 molar ratio of rhIGF-I to rhIGFBP-3. This corresponds to the naturally occurring protein complex purified by cation exchange column chromatography. Infusions were prepared from vials containing 10 mg/mL rhIGF-I/BP-3 in sterile 50 mmol/L sodium acetate and 105 mmol/L sodium chloride buffered to pH 5.5.
Study Design
Patients were resuscitated according to the Galveston formula with 5,000 cc/m2 TBSA burned plus 2,000 cc/m2 TBSA lactated Ringer’s solution given in increments over the first 24 hours. Within 48 hours of admission, all patients underwent total burn wound excision, and the wounds were covered with available autograft skin, with allograft used to cover any remaining open areas. After the first surgical procedure, all patients received 0.9% saline for 5 to 10 days until the donor site was healed. To determine baseline and changes in the two study groups, blood samples were taken before surgery and on postoperative day 5. This period was designated period I.
After the first donor site healed, and just before the second surgical procedure (period II), a blood sample was obtained to determine baseline values. All patients were randomized to receive a continuous intravenous infusion of either IGF-I/BP-3 (n = 22) in a dose of 1.0, 2.0, or 4.0 mg/kg/day, or 0.9% NaCl (controls, n = 8) until the donor site healed; again, this was approximately 5 to 10 days after excision and grafting. A blood sample was obtained on the fifth postoperative day. There were seven or eight children in each drug group (total = 22) and eight control patients. No differences in blood concentrations could be shown; thus, the groups were combined.
Serum IGF-I, IGFBP-3, and Growth Hormone
Levels of serum human IGF-I, human IGFBP-3, and GH were determined using a human radioimmunoassay (Endocrine Sciences, Calabasas Hills, CA).
Caloric Intake, Calorimetry, and Albumin Requirements
Intake of proteins, carbohydrates, and fat was measured and recorded each day. The amount of caloric intake was calculated for each study period. All patients received nasoduodenal feedings with Vivonex TEN (Sandoz Nutrition, Minneapolis, MN), containing 82.3% carbohydrate, 3% fat (linoleic acid), and 14.7% protein. Caloric intake was given at a rate calculated to deliver 1,500 kcal/m2 TBSA burned plus 1,500 kcal/m2 TBSA. This feeding regimen was started at admission and continued at a constant rate until the wound was 95% healed. Caloric intake was kept constant throughout the study period. Resting energy expenditure and respiratory quotient were calculated from O2 and CO2 in expired gases. A metabolic cart calorimeter (Sensomedics, Yorba Linda, CA) and standard equations were used.
Serum albumin was measured daily. Children with albumin concentrations less than 2.5 g/dL received albumin substitution based on age to maintain colloid osmotic pressure. Children younger than 2 years of age received 6.25 g/day exogenous albumin, those 2 to 9 years old received 12.5 g/day, and those 10 to 18 years old received 25 g/day. Albumin required during the two study periods was recorded, and the total albumin was compared between patients receiving IGF-I/BP-3 or saline.
Serum Glucose, Electrolytes, and Hepatic Constitutive and Acute Phase Proteins
Serum glucose, electrolytes, constitutive hepatic proteins (prealbumin, retinol-binding protein, and transferrin), type I acute phase proteins (C-reactive protein, C-3 complement, and α1-acid glycoprotein) and type II acute phase proteins (haptoglobin, α2-macroglobulin, and α1-antitrypsin) were measured using a Behring nephelometer (Behring, Dear-field, IL).
Serum Cytokines
Plasma TNF-α levels were determined with a human specific enzyme-linked immunosorbent assay (ELISA, Endogen, Woburn, MA). Standard curves for quantification of human TNF-α were linear from 0 to 400 pg/mL on a logarithmic scale. IL-1β levels were determined using ELISA (Endogen). Standard curves for quantification of human IL-1β were linear from 0 to 400 pg/mL on a logarithmic scale. Serum levels of IL-6 were determined by human ELISA (Biosource, Camarillo, CA). Standard curves for quantification of human IL-6 were linear from 0 to 500 pg/mL on a logarithmic scale.
Ethics and Statistics
Informed consent approved by the Institutional Review Board of the University of Texas Medical Branch was obtained from all patients, parents, or guardians. Statistical comparisons were made by analysis of variance and the Student t test with post-hoc Bonferroni’s correction where appropriate. Data are expressed as means ± SEM. Significance was accepted at P < .05.
RESULTS
No differences between children receiving 1, 2, or 4 mg/kg/day of IGF-I/BP-3 could be shown for the hepatic acute phase response; therefore, patients were combined and treated as one group. No difference in IGF-1/BP-3 blood levels was found between the 1, 2, and 4 mg/kg/day groups; thus, no valid dose response was established.
Patient Demographics
There were no significant differences in age, sex, size and depth of burns, or death rate between children treated with rhIGF-I/BP-3 or saline. No differences could be shown between groups for time after burn to hospital admission or to the start and end of study periods I and II. Total body wound healing showed no significant change during the study period, and this was not considered to be a major source contributing to the decrease in proinflammatory factors.
Serum IGF-I, IGFBP-3, and GH
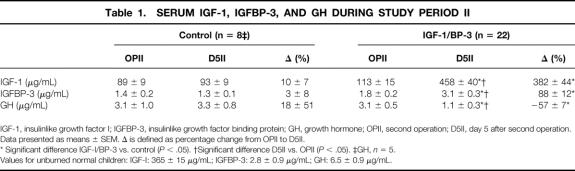
Serum IGF-I levels significantly increased from 113 ± 15 to 458 ± 40 μg/mL with IGF-I/BP-3 administration (P < .05); serum IGFBP-3 levels increased from 1.8 ± 0.2 to 3.1 ± 0.3 μg/mL (P < .05). Serum GH levels decreased from 3.1 ± 0.5 to 1.1 ± 0.3 μg/mL (P < .05). No changes in serum IGF-I, IGFBP-3, or GH were found in the control group (Table 1).
Table 1. SERUM IGF-1, IGFBP-3, AND GH DURING STUDY PERIOD II
IGF-1, insulinlike growth factor I; IGFBP-3, insulinlike growth factor binding protein; GH, growth hormone; OPII, second operation; D5II, day 5 after second operation.
Data presented as means ± SEM. Δ is defined as percentage change from OPII to D5II.
* Significant difference IGF-I/BP-3 vs. control (P < .05). †Significant difference D5II vs. OPII (P < .05). ‡GH, n = 5.
Values for unburned normal children: IGF-I: 365 ± 15 μg/mL; IGFBP-3: 2.8 ± 0.9 μg/mL; GH: 6.5 ± 0.9 μg/mL.
Caloric Intake and Indirect Calorimetry
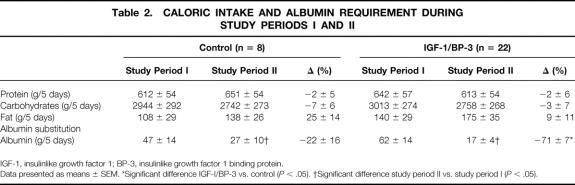
There were no differences in the caloric intake of protein, carbohydrate, and fat between the treatment and the control groups. No differences could be demonstrated between the groups for oxygen consumption (treatment group: 1625 ± 295 kcal/day vs. control group: 1637 ± 288 kcal/day) or respiratory quotient (treatment group: 1.0 ± 0.03 vs. control: 0.97 ± 0.03), indicating there were no significant changes in substrate utilization. Children receiving the IGF-I/BP-3 complex required significantly less albumin substitution than controls (P < .05, Table 2).
Table 2. CALORIC INTAKE AND ALBUMIN REQUIREMENT DURING STUDY PERIODS I AND II
IGF-1, insulinlike growth factor 1; BP-3, insulinlike growth factor 1 binding protein.
Data presented as means ± SEM. *Significant difference IGF-I/BP-3 vs. control (P < .05). †Significant difference study period II vs. study period I (P < .05).
Serum Electrolytes and Hepatic Constitutive and Acute Phase Proteins
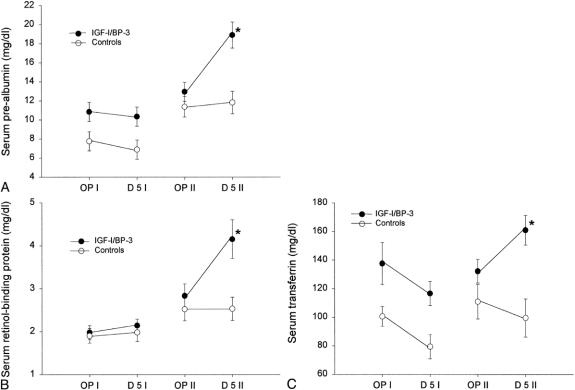
No hypoglycemia or electrolyte imbalance could be found in the treatment group. There were no differences between the groups for constitutive hepatic proteins during study period I. During study period II, children in the treatment group had increased levels of serum prealbumin, retinol-binding protein, and transferrin compared with the control group (P < .05;Fig. 1).
Figure 1. Changes in serum constitutive hepatic proteins between the two study periods. (A) Serum prealbumin changes. (B) Serum retinol-binding protein changes. (C) Serum transferrin changes. For all proteins, there were no significant changes from the first operation to postoperative day 5 between the groups, but there were significant changes (*P < .05) between the groups from the second operation to postoperative day 5. Data are presented as means ± SEM. Normal serum prealbumin level is 25 to 45 mg/dL. Normal serum retinol-binding protein level is 3 to 6 m/dL. Normal serum transferrin level is 203 to 430 mg/dL.
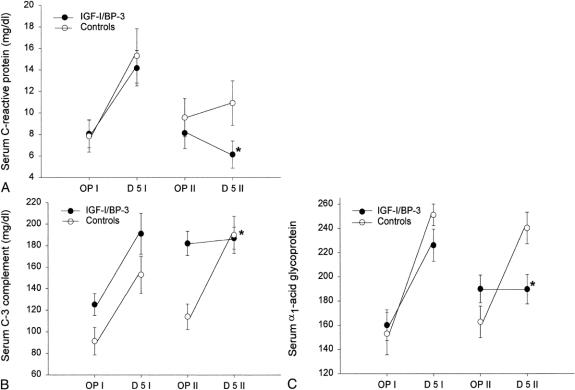
No significant difference between the two groups for type I and II acute phase proteins could be shown during study period I. RhIGF-I/BP-3 treatment decreased the level of type I acute phase proteins compared with saline treatment (P < .05;Fig. 2). IGF-I/BP-3 treatment had no effect on the levels of type II acute phase proteins, haptoglobin, α2-macroglobulin, and α1-antitrypsin.
Figure 2. Changes in serum acute phase proteins between the two study periods. (A) Serum C-reactive protein changes. (B) Serum C-3 complement changes. (C) Serum α1-acid glycoprotein changes. For all proteins, there were no significant changes from the first operation to postoperative day 5 between the groups, but there were significant changes (*P < .05) between the groups from the second operation to postoperative day 5. Data are presented as means ± SEM. Normal serum C-reactive protein level is less than 5 mg/dL. Serum reference range for C-3 complement is 50 to 90 mg/dL. Serum reference range for α1-acid glycoprotein is 55 to 140 mg/dL.
Serum Cytokines
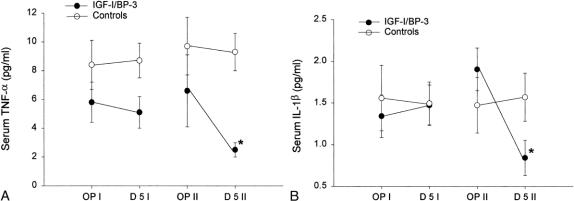
There were no differences in serum levels of TNF-α or IL-1β between groups during study period I. IGF-I/BP-3 administration decreased the level of serum TNF-α and serum IL-1β 5 days after the initiation of the drug when compared with the control group (P < .05;Fig. 3). No difference between groups could be shown for serum IL-6 levels.
Figure 3. Changes in serum proinflammatory cytokines between the two study periods. (A) Changes in serum tumor necrosis factor-alpha (TNF-α). (B) Changes in serum interleukin-1β *IL-1β. For both cytokines, there were no significant changes from the first operation to postoperative day 5 between the groups, but there were significant changes (*P < .05) between the groups from the second operation to postoperative day 5. Data are presented as means ± SEM. Serum reference range for TNF-α is 0 to 4.9 pg/mL. Serum reference range for IL-1β is 0.1 to 0.8 pg/mL.
White Blood Cells
The white blood cell count was 12,710 ± 1,592 for the control group and 10,590 ± 917 for the treatment group. The distribution of monocytes, lymphocytes, and eosinophils was not significantly different. Immature to mature polymorphonuclear cell ratios were 1:4 for the control group and 1:13 for the treatment group. The polymorphonuclear cells were 45 ± 17% for the control group and 59 ± 11% for the treatment group (P = .02); the percentages of immature polymorphonuclear cells (band cells) were 13 ± 4% for the control group and 5 ± 5% for the treatment group.
DISCUSSION
A prolonged increased hepatic acute phase response has been shown to contribute to multiple organ failure and death. 4–6 The overexpression of proinflammatory cytokines, such as IL-1β and TNF-α, has been shown to inhibit the GH/IGF-I axis, and these studies suggested that this may be one reason for an increased hypermetabolic response, with resulting increases in complications and death. 13,21,22 Therefore, several clinical trials attempted to downregulate exaggerated levels of proinflammatory cytokines in an effort to attenuate this response and improve clinical outcome. 4–6 However, the antiinflammatory agents used failed to control the exaggerated synthesis of proinflammatory cytokines and acute phase proteins because they focused on only one pathway or mediator in the inflammatory cascade, leading to a compensation through other pathways. 4,7,8 In the present study, we demonstrated that IGF-I in combination with its principal binding protein decreases the proinflammatory cytokines IL-1β and TNF-α, with subsequent decreases in the type I acute phase proteins C-reactive protein, complement C-3, and α1-acid glycoprotein. The IGF-I/BP-3 and control groups had significantly different starting points for study periods I and II. We have no explanation for this phenomenon; however, the change during study period II was significantly different between the treatment and the control groups (P < .02). Because we did not observe an increase in IL-6 or type II acute phase proteins, we suggest that IGF-I effectively decreased IL-1β and TNF-α without a compensatory elevation of IL-6 and type II acute phase proteins.
The pathway by which IGF-I modulates the hepatic acute phase response is not entirely defined. However, IGF-I may exert some of its effects on IL-1β and TNF-α and subsequently on type I acute phase proteins through a downregulation of hepatic nuclear factor (NF)-κB activation (unpublished observations). NF-κB controls the transcriptional regulation of many proinflammatory cytokines, including IL-1β and TNF-α and type I acute phase proteins. 23–25 Many type I acute phase proteins contain NF-κB response elements in their promoter region. A downregulation of NF-κB activation may therefore result in decreased levels of IL-1-like cytokines and type I acute phase proteins. The relative specificity of NF-κB for IL-1-like cytokines and type I acute phase proteins may explain why IGF-I had no effect on IL-6 and type II acute phase proteins.
Another possible pathway by which IGF-I decreases acute phase proteins and proinflammatory cytokines may be through the C-enhancer binding protein family (C/EBP). 26–29 The C/EBPβ subtype increases after trauma and regulates acute phase proteins and proinflammatory cytokine synthesis. 26,27 IGF-I decreases C/EBPβ and therefore may subsequently decrease acute phase protein and cytokine synthesis.
A decreased concentration of acute phase protein and proinflammatory cytokines was associated with increased synthesis of constitutive hepatic proteins, such as prealbumin, retinol-binding protein, and transferrin. After thermal injury, constitutive hepatic proteins have been shown to decrease by 50% to 70% below normal levels because of the reprioritization of liver protein synthesis. 2 Constitutive hepatic proteins, however, have important physiologic functions, and their downregulation after trauma has been described as potentially harmful. Synthesis of these proteins has been used to predict death and to serve as clinical markers for nutritional status, severity of stress, and improved recovery. 3,30,31
The exact mechanisms by which IGF-I exerts the beneficial effect of stimulating constitutive hepatic proteins are not defined. We suggest that one possible mechanism is that decreased acute phase proteins redirect the liver synthesis toward the physiologic status, with subsequently increased constitutive hepatic protein synthesis. Another possible mechanism may be that IGF-I affects the signal transduction pathway. Recent studies demonstrated that IGF-I can modulate the C/EBP family. 26–29,32 C/EBPα is a transcription factor for constitutive hepatic proteins, such as prealbumin. 26,27 Unlike C/EBPβ, C/EBPα levels decrease after trauma and thus it can be considered a negative regulator. 26,27 IGF-I stimulates C/EBPα and thus may upregulate constitutive hepatic protein synthesis. 32
Increased expression of proinflammatory cytokines has been associated with increased weight loss and hypermetabolism. We found that children treated with the IGF-I/BP-3 complex who demonstrated decreased proinflammatory cytokine levels showed an increase in peripheral muscle fractional synthetic rates compared with controls. These findings suggest that an attenuation of proinflammatory cytokines and acute phase proteins is associated with increased systemic protein synthesis or decreased protein catabolism.
It has been recently shown that GH attenuates the hepatic acute phase response and stimulates albumin synthesis in pediatric burn patients. 10 However, a side effect of GH that has been delineated is an increase in the hepatic triglyceride concentration and development of a fatty liver. 33,34 In burned rats, GH given for 7 days increased the hepatic triglyceride concentration by nearly 50% (unpublished observations). The mechanisms have been discussed in clinical studies; it has been proposed that GH increases peripheral lipolysis, and because of a lack of transporter proteins (low- and high-density lipoprotein), triglycerides accumulate in the liver. GH, given after thermal injury, acutely increased free fatty acid concentrations compared with placebo, indicating that GH stimulates peripheral lipolysis and subsequently the free fatty acid concentration. 33,34 In the present study, IGF-I/BP-3 did not cause an increase in free fatty acids and triglycerides; rather, it decreased free fatty acids 5 days after the initiation of the treatment.
From our data, we conclude that attenuating the hepatic acute phase response with IGF-I/BP-3 modulates the hypermetabolic response. This may prevent multiple organ failure and improve clinical outcome after a thermal injury without any detectable adverse side effects. The benefit of the amelioration of the hepatic acute phase response may also help reduce the incidence of multiple organ failure and mortality often observed in other forms of severe trauma.
Footnotes
Correspondence: Robert E. Barrow, PhD, Dept. of Surgery, University of Texas Medical Branch, Shriners Burns Hospital, Galveston, TX 77550.
Supported by Shriners Hospital for Children Grants 8660 and 8490, NIH Grants 1 RO1-GM56687-01 and 5 T32 GM 0825607, and the Celtrix Pharmaceutical Company.
Accepted for publication August 24, 1999.
References
- 1.Moshage H. Cytokines and the hepatic acute phase response. J Pathol 1997; 181:257–266. [DOI] [PubMed] [Google Scholar]
- 2.Fey G, Gauldie J. The acute phase response of the liver in inflammation. In: Popper H, Schaffner F, eds. Progress in Liver Disease. Philadelphia: WB Saunders; 1990: 89–116. [PubMed]
- 3.Brown RO, Bradley JE, Bekemeyer WB, Luther RW. Effect of albumin supplementation during parenteral nutrition on hospital mortality. Crit Care Med 1988; 16:1177–1183. [DOI] [PubMed] [Google Scholar]
- 4.Livingston DH, Mosenthal AC, Deitch EA. Sepsis and multiple organ dysfunction syndrome: a clinical-mechanistic overview. New Horizons 1995; 3:276–287. [PubMed] [Google Scholar]
- 5.Selzman CH, Shames BD, Miller SA, et al. Therapeutic implications of interleukin-10 in surgical disease. Shock 1998; 10:309–318. [DOI] [PubMed] [Google Scholar]
- 6.De Maio A, de Mooney ML, Matesic LE, Paidas CN, Reeves RH. Genetic component in the inflammatory response induced by bacterial lipopolysaccharide. Shock 1998; 10:319–323. [DOI] [PubMed] [Google Scholar]
- 7.Pruitt JH, Copeland EM, Moldawer LL. Interleukin-1 and interleukin-1 antagonism in sepsis systemic inflammatory response syndrome and septic shock. Shock 1995; 3:235–251. [DOI] [PubMed] [Google Scholar]
- 8.Williams G, Giroir B. Regulation of cytokine gene expression: tumor-necrosis factor, interleukin-1, and the emerging biology of cytokine receptors. New Horizons 1995;276–287. [PubMed] [Google Scholar]
- 9.Chrysopoulo MT, Jeschke MG, Ramirez R, Barrow RE, Herndon DN. Recombinant human growth hormone decreases tumor necrosis factor-alpha in burned children. Arch Surg 1999; 134:283–287. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez RJ, Wolf SE, Barrow RE, Herndon DN. Growth hormone treatment in pediatric burns. Ann Surg 1998; 228:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemmons DR. Insulin-like growth factor-1 as an anabolic agent in catabolic states. Ann Intern Med 1994; 120:596–597. [Google Scholar]
- 12.Bondy CA, Underwood LE, Clemmons DR, et al. Clinical uses of insulin-like growth factor-I. Ann Intern Med 1994; 120:593–601. [DOI] [PubMed] [Google Scholar]
- 13.Thissen JP, Verniers J. Inhibition by interleukin-1β and tumor necrosis factor-α of the insulin-like growth factor I messenger ribonucleic acid response to growth hormone in rat hepatocyte primary culture. Endocrinology 1997; 138:1078–1084. [DOI] [PubMed] [Google Scholar]
- 14.Humbel RE. Insulin-like growth factor I and factor II. Eur J Biochem 1990; 190:445–462. [DOI] [PubMed] [Google Scholar]
- 15.Baxter RC. Circulating levels and molecular distribution of the acid-labile (alpha) subunit of the high molecular weight insulin-like growth factor-binding protein complex. J Clin Endocrinol Metab 1990; 70:1347–1353. [DOI] [PubMed] [Google Scholar]
- 16.Huang KF, Chung DH, Herndon DN. Insulin-like growth factor-1 (IGF-I) reduces gut atrophy and bacterial translocation after severe burn injury. Arch Surg 1993; 128:47–54. [DOI] [PubMed] [Google Scholar]
- 17.Strock LL, Singh H, Abdullah A. The effect of insulin-like growth factor-1 on postburn hypermetabolism. Surgery 1990; 108:161–164. [PubMed] [Google Scholar]
- 18.Steenfos HH. Growth factors and wound healing. Scand J Plast Reconstr Hand Surg 1994; 28:95–105. [DOI] [PubMed] [Google Scholar]
- 19.Michelopoulos GK, DeFrances M. Liver regeneration. Science 1997; 276:60–66. [DOI] [PubMed] [Google Scholar]
- 20.Jabri N, Schalch DS, Schwartz SL, et al. Adverse effects of recombinant human insulin-like growth factor-I in obese insulin-resistant type II diabetic patients. Diabetes 1994; 43:369–374. [DOI] [PubMed] [Google Scholar]
- 21.Lang CH, Fan J, Cooney R, Vary TC. IL-1 receptor antagonist attenuates sepsis-induced alterations in the IGF system and protein synthesis. Am J Physiol 1996; 270:E430–E437. [DOI] [PubMed] [Google Scholar]
- 22.Delhanty PJ. Interleukin-1 beta suppresses growth hormone-induced acid-labile subunit mRNA levels and secretion in primary hepatocytes. Biochem Biophys Res Com 1998; 243:269–272. [DOI] [PubMed] [Google Scholar]
- 23.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I (kappa) B kinase-beta. Nature 1998; 6706:77–80. [DOI] [PubMed] [Google Scholar]
- 24.Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhances are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med 1990; 171:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Ann Rev Cell Biol 1994; 10:405–455. [DOI] [PubMed] [Google Scholar]
- 26.Gilpin DA, Hsieh CC, Kuninger DT, Herndon DN, Papaconstantinou J. Effect of thermal injury on the expression of transcription factors that regulate acute phase response genes: the response of C/EBPβ, C/EBPβ, and C/EBPβ to thermal injury. Surgery 1996; 119:674–683. [DOI] [PubMed] [Google Scholar]
- 27.Alam T, An MR, Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem 1992; 267:5021–5024. [PubMed] [Google Scholar]
- 28.Umayahara Y, Ji C, Centrella M, Rotwein P, McCarthy TL. CCAAT/enhancer-binding protein delta activates insulin-like growth factor-I gene transcription in osteoblasts. Identification of a novel cyclic AMP signaling pathway in bone. J Biol Chem 1997; 272:31793–31800. [DOI] [PubMed] [Google Scholar]
- 29.Nolten LA, Steenbergh PH, Sussenbach JS. Hepatocyte nuclear factor 1 alpha activates promoter 1 of the human insulin-like growth factor I gene via two distinct binding sites. Mol Endocrinol 1995; 9:1488–1499. [DOI] [PubMed] [Google Scholar]
- 30.Barnum-Huckins KM, Martinez AO, Rivera EV. A comparison of the suppression of human transferrin synthesis by lead and lipopolysaccharide. Toxicology 1997; 118:11–22. [DOI] [PubMed] [Google Scholar]
- 31.Smith DJ, Roberts D. Effects of high volume and/or intense exercise on selected blood chemistry parameters. Clin Biochem 1994; 27:435–440. [DOI] [PubMed] [Google Scholar]
- 32.Nolten LA, van Schaik FM, Steenbergh PH, Sussenbach JS. Expression of the insulin-like growth I gene is stimulated by the liver-enriched transcription factors C/EBP alpha and LAP. Mol Endocrinol 1994; 8:1636–1645. [DOI] [PubMed] [Google Scholar]
- 33.Aarsland A, Chinkes D, Wolfe RR, et al. Beta-blockade lowers peripheral lipolysis in burn patients receiving growth hormone. Rate of hepatic very low density lipoprotein triglyceride secretions remains unchanged. Ann Surg 1996; 223:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aarsland A, Chinkes D, Wolfe RR. Contributions of de novo synthesis of fatty acids to total VLDL-triglyceride secretion during prolonged hyperglycemia/hyperinsulinemia in normal man. J Clin Invest 1996; 98:2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]