Abstract
Objective
To examine the role of total parenteral nutrition (TPN) in predisposing infants to infection caused by coagulase-negative staphylococci.
Summary Background Data
Total parenteral nutrition is an important means of providing essential nutrients to newborn infants. However, its use has been associated with complications, particularly infection caused by coagulase-negative staphylococci. Recent data suggest that TPN may modulate immune function; however, reports directly indicating impaired immunity against coagulase-negative staphylococci during TPN are limited.
Methods
Study 1 involved 31 infants younger than 4 months who had undergone surgery and were not receiving antibiotics; 20 were receiving TPN and 11 were receiving a normal enteral diet. An in vitro whole blood model was used to measure the host bactericidal activity against coagulase-negative staphylococci. Bacterial killing and phagocytosis were measured after a 45-minute challenge with viable coagulase-negative staphylococci. In study 2, whole blood killing and intracellular killing of coagulase-negative staphylococci were measured in five newborn infants (younger than 2 months) who were receiving long-term TPN (>10 days), five control infants receiving a normal enteral diet, and five healthy adults.
Results
In study 1, infants receiving a normal enteral diet showed a high capacity to ingest and kill coagulase-negative staphylococci. In contrast, the blood of infants receiving long-term TPN showed a reduction in coagulase-negative staphylococci phagocytosis and killing. There were significant negative linear correlations between the duration of TPN and killing of coagulase-negative staphylococci and phagocytosis of coagulase-negative staphylococci. In study 2, infants receiving long-term TPN had lower whole blood killing and intracellular killing than infants receiving a normal enteral diet and healthy adult volunteers. These data seem to indicate a neutrophil dysfunction mediated by TPN in infancy.
Conclusions
Host defense mechanisms, including phagocytosis and killing of coagulase-negative staphylococci, are impaired during long-term TPN. The impaired bactericidal activity seems to be related to defective intracellular killing in neutrophils. These findings may explain the high rate of septicemia caused by coagulase-negative staphylococci in infants receiving TPN.
Total parenteral nutrition (TPN) has contributed to the recent improvement in the care of newborn infants. However, this form of nutritional support is associated with complications, particularly infection. Five percent to 37% of infants receiving TPN become bacteremic, 1–3 most frequently with coagulase-negative staphylococci. 4 Infection may result in impairment of liver function or systemic inflammatory response syndrome, necessitating the removal of central venous catheters. It therefore represents a major clinical problem of TPN in infancy.
It is generally believed that infection with coagulase-negative staphylococci results from contamination of indwelling catheters by bacteria of the skin flora during handling of intravenous catheters. It has also been suggested that TPN itself may impair host defense mechanisms and contribute to the occurrence of infection. In preliminary studies, predominantly in adults, the lipid component of TPN has been shown to affect several immunologic parameters, including cytokine production, 5,6 bactericidal activity, 7 and neutrophil function. 8 To date, data on human infants are limited.
In this study, we demonstrate that long-term TPN in infants has a direct effect on the capacity to eradicate coagulase-negative staphylococci in vitro. We also postulate that this impairment in host defense contributes to the high incidence of TPN-associated bacteremia in infants with coagulase-negative staphylococci.
PATIENTS AND METHODS
Patients
Study 1
Whole blood bactericidal activity and the phagocytic activity of neutrophils were assessed in 20 infants younger than 4 months who were receiving TPN after surgery for gastroschisis (n = 7), necrotizing enterocolitis (n = 6), intestinal atresia (n = 3), congenital diaphragmatic hernia (n = 2), and esophageal atresia (n = 2). None of these patients was receiving enteral feeding at the time of the study. Eleven control infants not affected by gastrointestinal problems and receiving a normal enteral diet were studied before operations such as inguinal hernia repair or lensectomy. Patients receiving antibiotics after surgery were studied at least 36 hours after cessation of antimicrobial therapy. Nine patients were studied on more than one occasion, resulting in a total of 33 studies in this group. Immunocompromised patients and infants with a neutrophil count less than the normal range (1.8 × 109/L) were excluded from the control group. 9
Study 2
Whole blood bactericidal activity and neutrophil intracellular killing of coagulase-negative staphylococci were studied in five infants undergoing surgery and receiving TPN for more than 10 days (mean age 18 days, range 16–37; mean body weight 2.4 kg, range 1.8–3.1), five infants receiving a normal enteral diet (mean age 23 days, range 7–48; mean body weight 3.4 kg, range 2.0–3.7), and five healthy adult volunteers (mean age 30 years, range 29–32; mean body weight 65 kg, range 55–80). Infants receiving TPN were clinically stable with no symptoms of sepsis and were not receiving enteral feeding at the time of the study. The clinical indications for TPN in this group of infants were gastroschisis (n = 4) and necrotizing enterocolitis (n = 1). Patients were studied at least 10 days after surgery or after the onset of necrotizing enterocolitis and at least 36 hours after cessation of the antimicrobial therapy. Control infants were studied before minor operations performed under general anesthesia, such as inguinal hernia repair or lensectomy. They were receiving a normal enteral diet, had no gastrointestinal anomalies, and were not receiving antibiotics.
Both studies were approved by the Ethical Committee of Great Ormond Street Hospital for Children NHS Trust, and informed written consent was obtained from parents before each study.
Total Parenteral Nutrition
A standardized protocol for intravenous nutrition was used in all patients. Children received TPN through a central venous catheter. Daily intake was 100 to 180 mL/kg and consisted of carbohydrate, amino acids, fat, electrolytes, vitamins, and trace elements 10 prepared in the hospital pharmacy under aseptic conditions. Daily intravenous carbohydrate intake was 15 to 18 g/kg. Intravenous amino acids were contained in the Vamin 9 solution (Pharmacia & Upjohn, Milton Keynes, Buckinghamshire, UK); daily intake was 2 to 3 g/kg. The source of intravenous fat was Intralipid 20%, an emulsion based on long-chain fatty acids (Pharmacia & Upjohn), given at a rate of 4 g/kg per day.
Bacterial Suspension
A single strain of coagulase-negative staphylococci isolated from the blood of a patient receiving TPN was used for the bacterial challenge. The strain was inoculated into brain-heart infusion broth (Oxoid; Unipath Ltd., Basingstoke, UK) for 18 hours at 37°C and was prepared as suspensions containing 108 organisms per milliliter. The bacterial suspension was mixed with sterile glycerol in a ratio of 1:10. After this, 300 μL was placed in Eppendorf tubes and held at −40°C for use in the study.
Whole Blood Model of Bacteremia
An in vitro whole blood model 11,12 of bacteremia was used to measure host–pathogen interactions in coagulase-negative staphylococci infection. Blood samples were taken from patients who were not clinically septic and were not receiving antibiotic therapy. Blood samples were withdrawn from the central venous catheter or a peripheral vein without stasis and were anticoagulated with heparin (1 u/mL). Five microliters of the bacterial suspension described above was added to 500 μL whole blood and was incubated at 37°C on a rocking table at nine revolutions per minute.
Bacterial Killing
To assess in vitro killing of coagulase-negative staphylococci, 100 μL patient blood was examined after 45 minutes of incubation. The number of viable organisms was determined using a modification of the Miles and Misra technique. 12,13 Tenfold dilutions of the samples were made in sterile water, and five 20-μL samples of each dilution were spotted onto blood agar. After 18 hours of incubation at 36°C in 6% carbon dioxide, the number of colonies was counted and expressed as colony-forming units per milliliter. The viable count of the original bacterial suspension was also measured to calculate the percentage of coagulase-negative staphylococci killed.
Phagocytic Activity of Neutrophils
Fifty microliters patient blood was used to assess the phagocytic activity of neutrophils after 40 minutes of incubation. Phagocytosis was measured by inoculating the blood with bacteria labeled with fluorescence isothiocyanate (FITC; Sigma, St. Louis, MO). 13 Red cells were lysed with 2 mL FACS lysing solution (Becton-Dickinson, Mountain View, CA) for 10 minutes. Samples were then spun at 1200 rpm for 5 minutes, and the pellets were washed with phosphate-buffered saline and fixed in 500 μL 0.5% formaldehyde. The percentage of neutrophils containing labeled bacteria (phagocytosis of coagulase-negative staphylococci) was measured by flow cytometry using FACScan (Becton-Dickinson, Mountain View, CA) and analyzed using Lysis II and CELLQuest software (Becton-Dickinson, Cowley, UK).
Neutrophil Intracellular Killing
For the analysis of neutrophil intracellular killing, 50 μL bacteria/blood suspension was taken at 0, 15, 30, 45, and 60 minutes from the bacterial challenge. Red blood cells were lysed with 2 mL of a red cell lysis solution. Samples were then spun at 1200 rpm for 5 minutes, and pellets were washed twice with phosphate-buffered saline. After red cell lysis, 200 μL 1% 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propane sulfonate (CHAPS; BDH, Lutterworth, UK) solution was added to destroy the neutrophil membranes. After 5 minutes of incubation, 800 μL distilled water was added and incubated for a further 5 minutes. Then the samples were fixed in 500 μL 0.5% formaldehyde. 14 The survival rate of bacteria in the neutrophils was measured by flow cytometry using a FACS-Calibur and analyzed using CELLQuest software. The changes in median fluorescence intensity after 15, 30, 45, and 60 minutes of bacterial challenge were analyzed in comparison with time 0 (100% survival) and expressed as the percentage of organisms killed by neutrophils.
Data Analysis
Results are expressed as median and range. Differences between study groups were analyzed using the Mann-Whitney test. SAS Proc Mixed 15 was used to quantify the relation with duration of TPN while accounting for the nonindependence of repeat measurements within patients. Least-squares linear regression was used to investigate relations among the control infants.
RESULTS
Study 1
There was no difference between patients receiving TPN and control patients in terms of gestational age and postnatal age (Table 1). Body weight was significantly lower (P < .01) in patients receiving TPN, reflecting their impaired nutritional status. Twelve patients received TPN for less than 2 weeks; the remaining eight patients received long-term TPN.
Table 1. CLINICAL DATA OF PATIENTS IN STUDY 1
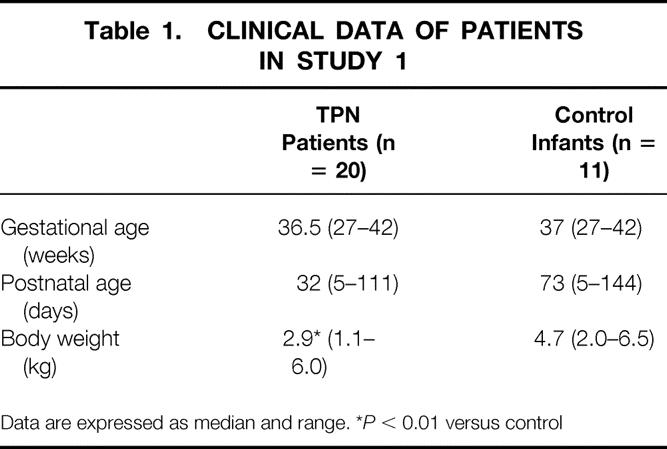
Data are expressed as median and range. *P < 0.01 versus control
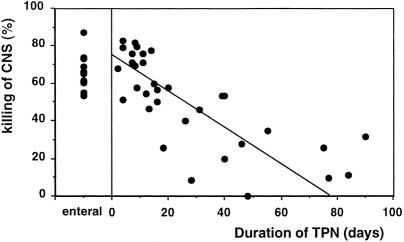
When coagulase-negative staphylococci were added to whole blood from control patients receiving enteral feeding, the median killing of coagulase-negative staphylococci was 65% (range 54–88). In contrast, blood from patients receiving long-term TPN failed to kill this organism as effectively. Indeed, there was a significant negative linear correlation between the duration of TPN in days and killing of coagulase-negative staphylococci (P = .002) (Fig. 1). For every additional week of TPN, there was a 7% reduction in killing of coagulase-negative staphylococci. The percentage of neutrophils that phagocytosed coagulase-negative staphylococci in vitro was 43% (range 26–67) in the control group. In contrast, patients receiving long-term TPN had reduced phagocytosis. There was a significant negative linear correlation between the duration of TPN in days and phagocytosis of coagulase-negative staphylococci (P = .008) (Fig. 2). Coagulase-negative staphylococci septicemia developed in three infants (38%) receiving long-term TPN (coagulase-negative staphylococci killing 20%, 34%, 57%). None of the infants receiving short-term TPN (coagulase-negative staphylococci killing 52–82%) developed septicemia.
Figure 1. (Left) Killing of coagulase-negative staphylococci (CNS; %) in a control infant receiving enteral feeding. (Right) Correlation between the duration of TPN (days) and killing of coagulase-negative staphylococci (%) (y = −0.96x + 74.0;P = .002).
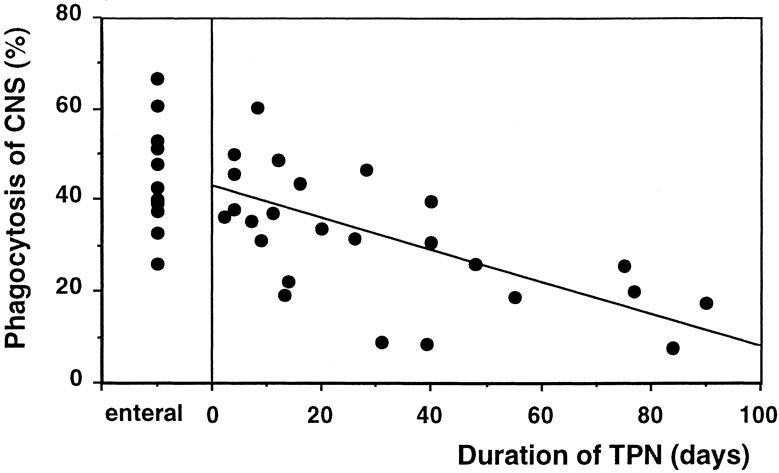
Figure 2. (Left) Phagocytosis of coagulase-negative staphylococci (percentage of neutrophils that phagocytosed coagulase-negative staphylococci) in a control infant receiving enteral feeding. (Right) Correlation between the duration of TPN (days) and phagocytosis of coagulase-negative staphylococci. (y = −0.32x + 41.2;P = .008).
There was no correlation between gestational age, postnatal age, and body weight with killing and phagocytosis of coagulase-negative staphylococci.
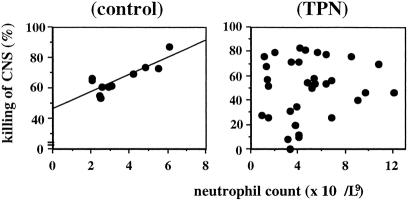
There was no significant difference in neutrophil count between patients receiving TPN (mean 4.1, range 1–12) and control enterally fed infants (mean 2.9, range 2–6). There was a significant positive correlation between neutrophil count and killing of coagulase-negative staphylococci (r = .8, P = .001) in control infants receiving enteral feeding; this correlation was not observed in patients receiving TPN, implying a lack of normal neutrophil function in these patients (Fig. 3).
Figure 3. (Left) Correlation between neutrophil count and killing of coagulase-negative staphylococci in a control infant (y = 5.6x + 46.6;r = .8, P = .001). (Right) There was no correlation between neutrophil count and killing of coagulase-negative staphylococci in TPN patients.
Study 2
To explore the effects of TPN on neutrophil function in infancy, we studied the intracellular killing of coagulase-negative staphylococci and correlated this with the whole blood killing. Figure 4 shows the whole blood killing and the intracellular killing in the three study groups. The highest levels of whole blood killing and intracellular killing were observed in control infants receiving enteral feeding and in healthy adult volunteers. Infants receiving long-term TPN had the lowest levels of both whole blood killing and intracellular killing, indicating that the reduced killing of coagulase-negative staphylococci in whole blood is at least in part due to a defect in neutrophil function.
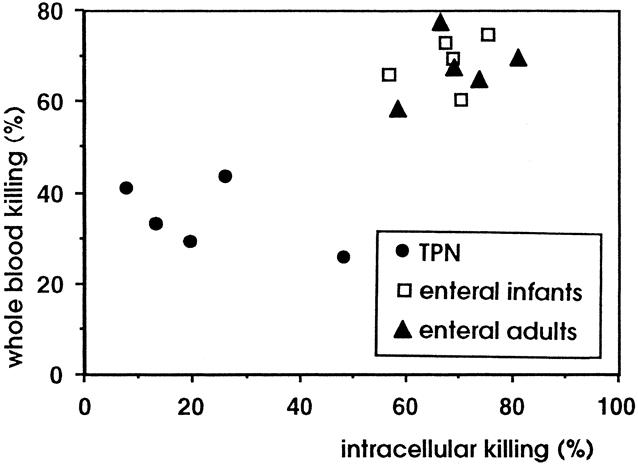
Figure 4. Correlation between whole blood killing and intracellular killing in the three study groups. The lowest levels of both whole blood killing and intracellular killing were observed in infants receiving long-term TPN.
DISCUSSION
The association of TPN and impairment of immune function has been known for some time. Animal studies have demonstrated that TPN can modulate the phagocytosis by neutrophils and monocytes, macrophage function, bactericidal activity, and cytokine production. 16–18 Studies in human adults are less clear, but effects on several immune parameters have been observed. 5,6
In this study, we have shown for the first time that infants receiving long-term TPN have impaired bactericidal activity against coagulase-negative staphylococci, the major cause of TPN-associated bacteremia in children. 19 Killing of coagulase-negative staphylococci closely correlated with neutrophil counts in healthy controls but not in patients receiving TPN (Fig. 3). In addition, the phagocytosis of coagulase-negative staphylococci after 40 minutes of incubation was significantly decreased in patients receiving long-term TPN compared with either controls or patients receiving short-term TPN (Fig. 2). Both of these findings support previous data that implicate an important role for neutrophils in the killing of coagulase-negative staphylococci 20 and suggest that the decrease in killing of coagulase-negative staphylococci is due to impaired neutrophil function. This was investigated further in the second part of this study, in which we examined the capacity of neutrophils to kill ingested coagulase-negative staphylococci. We showed that killing of coagulase-negative staphylococci by neutrophils from infants fed exclusively with long-term TPN was impaired compared with enterally fed infants and a group of healthy adults. Taken together, our findings show that TPN impairs the capacity of whole blood to kill coagulase-negative staphylococci and that this is at least in part due to an effect of TPN on neutrophil function.
The possible causes of the reduction in bactericidal activity in vitro include malnutrition, specific nutrient deficiency, and deleterious effects of TPN components on cells of the immune system or other organs, particularly the liver.
Malnutrition has been related to impairment of host defenses against invading pathogens, resulting in an increased incidence and severity of infection. Protein energy malnutrition and deficiencies of micronutrients such as zinc, iron, vitamin A, and vitamin B6 have all been implicated. However, modern intravenous regimens include all these nutrients and are capable of both correcting nutritional deficiencies and promoting normal growth. In this study, no correlation between body weight and bactericidal activity was found. It is possible, however, that the lack of specific nutrients such as glutamine, not currently included in TPN regimens, may influence immune function. 21,22
Impairment of the host defenses directly by the TPN solution has been suggested as an important factor in the high rate of TPN-associated infections. Previous studies in human adults have shown that the administration of lipid emulsions containing long-chain fatty acids results in a depletion in the host defense mechanisms, particularly in neutrophil bactericidal activity and migration. 5–7 Conversely, TPN regimens containing short-chain fatty acids and medium-chain triglycerides seem to be beneficial in reducing the TPN-associated immunosuppression in rats undergoing major surgery. 6,23,24 The exact mechanisms by which lipid solutions may influence immune function are yet to be determined. However, in a recent study, we showed that the production of the proinflammatory cytokine tumor necrosis factor-alpha was reduced in response to a challenge with coagulase-negative staphylococci in blood from TPN-fed infants. 25 This may indicate that cytokine hyporesponsiveness is a factor in TPN-mediated immunosuppression. 26
The central venous catheter has been assumed to be the major portal of entry for microorganisms causing septicemia in patients receiving TPN. 2,27 However, recent data indicate that bacteria, including coagulase-negative staphylococci, may gain access to the circulation from other sites, including the gastrointestinal tract. 28–31 Whatever the source of the organism, the results of this study indicate that host factors are also important in determining susceptibility to infection with this organism. Current strategies to prevent bacteremia during TPN are directed toward avoiding contamination of infusion lines and TPN solutions. In light of our findings, therapy directed toward modulating bactericidal activity may be a useful adjunct to management of coagulase-negative staphylococci infections in infants receiving TPN.
Acknowledgment
The authors thank Angela Wade, Epidemiology and Biostatistics Unit, Institute of Child Health, for the statistical analysis.
Footnotes
Correspondence: Agostino Pierro, MD, Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust, University College London, 30 Guilford St., London WC1N 1EH, United Kingdom.
Partly supported by Grant #22 from Tommy’s Campaign.
Accepted for publication July 26, 1999.
References
- 1.Bos AP, Tibboel D, Hazebroeck FWJ, et al. Total parenteral nutrition associated cholestasis: a predisposing factor for sepsis in surgical neonates? Eur J Pediatr 1990; 149:351–353. [DOI] [PubMed] [Google Scholar]
- 2.Wesley JR, Coran AG. Intravenous nutrition for the pediatric patient. Sem Pediatr Surg 1992; 1:212–230. [PubMed] [Google Scholar]
- 3.Seashore JH. Central venous access devices in children: trends over 543 patient years. Clin Nutr 1994; 13:27–A079. [Google Scholar]
- 4.Pierro A, van Saene HKF, Donnell SC, et al. Microbial translocation in neonates on long-term parenteral nutrition. Arch Surg 1996; 131:176–179. [DOI] [PubMed] [Google Scholar]
- 5.Monson JRT, Ramsden CW, MacFie J, et al. Immunorestorative effects of lipid emulsion during total parenteral nutrition. Br J Surg 1986; 73:843–846. [DOI] [PubMed] [Google Scholar]
- 6.Sedman PC, Somers SS, Ramsden CW, et al. Effects of different lipid emulsions on lymphocyte function during total parenteral nutrition. Br J Surg 1991; 78:1396–1399. [DOI] [PubMed] [Google Scholar]
- 7.Palmblad J, Broström O, Lahnborg G, et al. Neutrophil functions during total parenteral nutrition and intralipid infusion. Am J Clin Nutr 1982; 35:1430–1436. [DOI] [PubMed] [Google Scholar]
- 8.Fisher GW, Hunter KW, Wilson SR, Mease AD. Diminished bacterial defences with intralipid. Lancet 1980; 2:819–820. [DOI] [PubMed] [Google Scholar]
- 9.Gregory J, Hey E. Blood neutrophil response to bacterial infection in the first month of life. Arch Dis Child 1972; 47:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones MO, Pierro A, Hammond P, et al. Glucose utilisation in the surgical newborn infant receiving total parenteral nutrition. J Pediatr Surg 1993; 28:1121–1125. [DOI] [PubMed] [Google Scholar]
- 11.Klein NJ, Kalabalikis P, Curtis N, et al. Ex-vivo assessment of candidate anti-inflammatory agents in the treatment of Gram-negative sepsis. Imm Infect Dis 1994; 4:33–35. [Google Scholar]
- 12.Ison CA, Heyderman R, Klein NJ, et al. Whole blood model of meningococcal bacteraemia—a method for exploring host-bacterial interactions. Microbial Pathogenesis 1995; 18:97–107. [DOI] [PubMed] [Google Scholar]
- 13.Klein NJ, Ison CA, Peakman M, et al. The influence of capsulation and lipooligosaccharides structure on neutrophil adhesion molecule expression and endothelial injury by Neisseria meningitidis. J Infect Dis 1996; 173:172–179. [DOI] [PubMed] [Google Scholar]
- 14.Martin E, Bhakdi S. Flow cytometric assay for quantifying opsonophagocytosis and killing of Staphylococcus aureus by peripheral blood leukocytes. J Clin Microbiol 1992; 30:2246–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Littell RC, Millikan JA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute Inc.; 1996.
- 16.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg 1996; 223:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shou J, Lappin J, Minnard EA, Daly JM. Total parenteral nutrition, bacterial translocation, and host immune function. Am J Surg 1994; 167:145–150. [DOI] [PubMed] [Google Scholar]
- 18.Shou J, Lappin J, Daly JM. Impairment of pulmonary macrophage function with total parenteral nutrition. Ann Surg 1994; 219:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogata ES, Schulman S, Raffensperger J, et al. Caval catheterization in the intensive care nursery: a useful means for providing parenteral nutrition to the extremely low birth-weight infant. J Pediatr Surg 1984; 19:258–262. [DOI] [PubMed] [Google Scholar]
- 20.Shultz GE, Hall MA, Baker CJ, Edwards MS. Role of neutrophil receptors in opsonophagocytosis of coagulase-negative staphylococci. Infect Immun 1991; 59:2573–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Riordain MG, Fearon KC, Ross JA, et al. Glutamine-supplemented total parenteral nutrition enhances T-lymphocyte response in surgical patients undergoing colorectal resection. Ann Surg 1994; 220:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alverdy JC, Aoys E, Weiss-Carrington P, Burke DA. The effect of glutamine-enriched TPN on gut immune cellularity. J Surg Res 1992; 52:34–38. [DOI] [PubMed] [Google Scholar]
- 23.Gogos CA, Kalfarentzos FE, Zoumbos NC. Effect of different type of total parenteral nutrition on T-lymphocyte subpopulations and NK cells. Am J Clin Nutr 1990; 51:119–122. [DOI] [PubMed] [Google Scholar]
- 24.Platt VC, Tappenden KA, McBurney MI, Field CJ. Short-chain fatty acid-supplemented total parenteral nutrition improves nonspecific immunity after intestinal resection in rats. JPEN 1996; 29:264–271. [DOI] [PubMed] [Google Scholar]
- 25.Okada Y, Klein N, van Saene HKF, Pierro A. Small volumes of enteral feedings normalise immune function in infants receiving parenteral nutrition. J Pediatr Surg 1998; 33:16–19. [DOI] [PubMed] [Google Scholar]
- 26.Peters M, Dixon G, Petros A, Klein N. Acquired immunoparalysis in intensive care. Br Med J 1999; 4:609–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiro H, Muller E, Takeda S, et al. Potentiation of Staphylococcus epidermidis catheter-related bacteremia by lipid infusions. J Infect Dis 1995; 171:220–224. [DOI] [PubMed] [Google Scholar]
- 28.Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 1988; 104:185–190. [PubMed] [Google Scholar]
- 29.Pierro A. Cholestatic jaundice in infants receiving parenteral nutrition. Semin Neonatal 1996; 1:1–4. [Google Scholar]
- 30.Eastick K, Leeming JP, Bennett D, Millar MR. Reservoirs of coagulase-negative staphylococci in preterm infants. Arch Dis Child 1996; 74:F99–F104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnell SC, Taylor N, van Saene HKF, et al. Nutritional implications of gut overgrowth and selective decontamination of the digestive tract. Proc Nutrit Soc 1998; 57:381–387. Pharmacia & Upjohn, Milton Keynes, Buckinghamshire, UK [DOI] [PubMed]