Abstract
Objective
To compare the short- and long-term results of pancreaticoduodenectomy with pylorus preservation (PPPD) or with antrectomy (Whipple procedure) in the treatment of selected patients with chronic pancreatitis.
Background
PPPD may be preferred over Whipple because of its purported nutritional advantages and the reduced likelihood of postgastrectomy syndromes.
Methods
A retrospective review was performed of 72 consecutive patients undergoing pancreaticoduodenectomy for chronic pancreatitis between 1991 and 1997.
Results
PPPD was performed in 39 patients and Whipple in 33. The two patient populations had similar characteristics. Short-term complications included (PPPD vs. Whipple): pancreatic or biliary fistulas (5.1% vs. 15%), delayed gastric emptying (33% vs. 12%), cholangitis (2.6% vs. 6.1%), and death (0 vs. 3%). Delayed gastric emptying was not associated with other complications and resulted in longer hospital stays for PPPD than for Whipple patients (15 vs. 12 days). The duration of follow-up averaged 41 ± 24 months. Long-term weight status was similar, with body-mass indices of 22.1 and 22.9 after PPPD and Whipple, respectively. Postoperative enzyme supplementation (63% vs. 77%) and new-onset diabetes (10% vs. 12%) did not differ significantly between the PPPD and Whipple groups. Dumping, bile gastritis, or peptic ulcer disease occurred in three patients after PPPD and in three after Whipple. Complete or partial pain relief was attained in 60% and 70% of patients after PPPD and Whipple, respectively. Multivariate analysis of preoperative variables revealed that site-specific pathology in the head of the pancreas was the only independent factor associated with successful pain relief after pancreatic resection.
Conclusion
PPPD results in higher frequencies of postoperative delayed gastric emptying compared with the Whipple procedure. Both operations achieve comparable long-term nutritional results, cause new insulin dependence in surprisingly few patients, and provide equivalent pain relief to 65% of selected patients. Patients with disproportionate pathology in the head of the pancreas have a higher likelihood of successful pain relief.
Surgical therapy for chronic pancreatitis is reserved for patients with complications of the disease, intractable abdominal pain, or suspected underlying carcinoma. 1 The choice of operation for chronic pancreatitis is predicated on the two anatomical variants of the disease, which are distinguished by the size of the main pancreatic duct. 2 Large duct disease (>6 mm in diameter) accounts for approximately 40% of cases and is thought to develop from increased pressure in the pancreatic ductal system. 3 In this circumstance, the longitudinal pancreaticojejunostomy (or modified Puestow procedure) has been the treatment of choice, benefiting close to 80% of patients. 4,5 Patients with small duct disease (<6 mm in diameter) generally are thought not to be candidates for that operation and require pancreatic resection. Because of the historical ineffectiveness of distal resections 6,7 and the complications associated with total pancreatectomy, 1 resections targeting the head of the pancreas are the primary operations for patients with chronic pancreatitis of the small duct form that is refractory to medical treatment. Three main operations are available to these patients today: the Whipple-type pancreaticoduodenectomy (with antrectomy), 8 the pylorus-preserving pancreaticoduodenectomy (PPPD), 9 and the Beger duodenum-preserving pancreatic head resection. 10
Regarding pancreaticoduodenectomy for chronic pancreatitis, controversy exists with respect to the choice of operation and the expected rate of successful outcomes. The PPPD was first described by Watson in 1944 11 and reintroduced by Traverso and Longmire in 1978 9 to improve on the nutritional deficiencies associated with the classic Whipple. Large published series, however, report successful weight maintenance or gain in more than 80% of patients after either operation. 12,13 Relief of pain after pancreaticoduodenectomy is another focus of debate. Information from the literature is commonly based on anecdotal experience of a single approach, often with small cohorts or inadequate follow-up information. 12–17 There are few studies comparing alternative surgical treatments. 1 Evaluation of published reports is further complicated by a lack of standard measurements to assess success. 1,18 Successful pain relief after pancreaticoduodenectomy by either technique is reported over the wide range of 60% to 100% of patients.
In this study, we reviewed our experience with pancreaticoduodenectomy in the treatment of chronic pancreatitis for the past 7 years. During this time, we performed an almost equal number of Whipple and PPPD operations. Our goals were twofold. First, we wished to ascertain the advantages or disadvantages of one operation over the other, both in the short term and the long term. Secondly, and perhaps more important, we performed an outcome analysis of these patients in the hopes of providing prospective selection criteria that will improve the likelihood of successful surgical results.
PATIENTS AND METHODS
Seventy-two consecutive pancreaticoduodenectomies with a final pathologic diagnosis of chronic pancreatitis were performed by the senior authors (CFC, DWR, ALW) between January 1991 and November 1997. The hospital and office records of these patients were reviewed retrospectively. Preoperative information collected included patient age, sex, etiology and duration of pancreatitis, frequency of pain episodes, frequency of hospital admissions, history of diabetes, smoking history, medications on presentation, previous surgery, findings on preoperative radiologic workup, and presence or absence of jaundice and weight loss. Perioperative parameters tabulated included preoperative height and weight, preoperative diagnosis, surgical procedure, size of pancreatic duct, blood loss and transfusion requirement, postoperative complications, and length of hospital stay (LOS). Complications were subdivided into nine categories: death, pancreatic or biliary fistula, delayed gastric emptying (DGE), intraabdominal abscess, cholangitis, myocardial infarction or arrhythmia, pneumonia, infections (wound, urine, venous catheter), and reoperation.
Follow-up was obtained through telephone interviews by one investigator (REJ) during September and October 1998, complemented by physicians’ and surgeons’ office notes. Data for 13% of the patients not contacted directly was derived from office notes only or from the relevant primary care physicians. Patients requiring subsequent completion pancreatectomy had their follow-up terminated at the time of this surgery, and pancreaticoduodenectomy was judged to be a complete failure in these patients. Information for patients who died before October 1998 was obtained from family members.
Outcome was measured as good, fair, or poor according to postoperative pain relief. Patients with a good outcome had no postoperative pain. Those with fair outcomes had residual pain that was judged by the patient to have been significantly improved by the operation. Patients with poor results had pain unchanged from or worse than preoperative levels. All patients were assessed for narcotic use, and those with residual pain were asked to rate it on a scale from 1 (no pain) to 10 (severe). Other follow-up parameters collected included pancreatic enzyme supplement requirement, occurrence of bile gastritis or dumping, new onset of diabetes or peptic ulcer disease, subsequent relevant operations, postoperative weight trends, frequency of hospital readmissions for symptom management, and employment status.
Values are expressed as mean ± standard deviation. Medians are also provided when appropriate. Student t tests and Fisher’s exact tests were used for comparisons between groups, and a P value of .05 or less was considered significant. Multivariate analyses with logistic regression were performed where indicated.
RESULTS
Seventy-two consecutive pancreaticoduodenectomies for chronic pancreatitis were performed during the 82-month study period. Thirty-nine of these resections were of the pylorus-preserving type (end-to-side duodenojejunostomy 19;Fig. 1) , and 33 were standard Whipple procedures (antrectomy and Billroth II reconstruction). 20 All three surgeons involved in the study performed these operations in identical fashion. PPPD was the preferred operation for this cohort of patients with chronic pancreatitis. A Whipple operation was performed when there was suspicion for cancer, when there was a history of peptic ulcer disease, or when previous gastric or pancreatic surgery precluded the feasibility of a PPPD. Reconstruction after pancreatic resection involved a two-layer end-to-side pancreaticojejunostomy (stented), end-to-side choledochojejunostomy (not stented), and gastrojejunostomy (Whipple) or retrocolic duodenojejunostomy (Fig. 1). Aside from sacrifice or preservation of the pylorus, the only other difference in the conduct of the operation involved postoperative gastric drainage. Nasogastric intubation was used in patients after the Whipple procedure, but all patients undergoing PPPD had gastrostomy tubes inserted at the time of surgery. Vagotomy was not performed in either group.
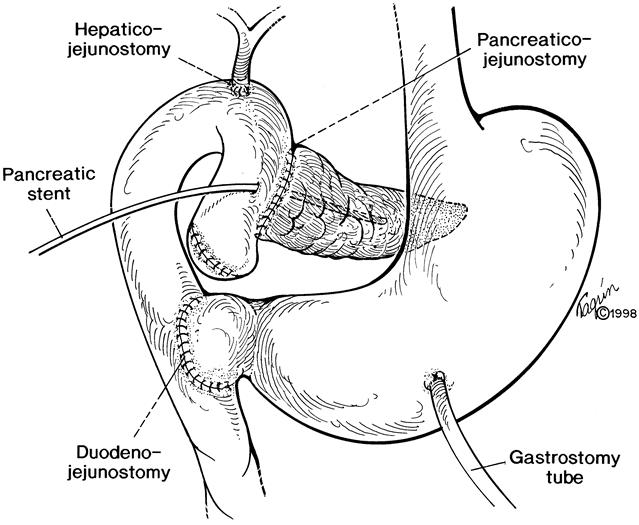
Figure 1. Pylorus-preserving pancreaticoduodenectomy.
Whipple Versus Pylorus-Preserving Pancreaticoduodenectomy
The clinical characteristics of our patients can be seen in Table 1 grouped according to the type of operation performed (PPPD vs. Whipple). The average age was 47.6 years, with ampullary stenosis, pancreas divisum, and alcohol abuse being the most frequent etiologies for chronic pancreatitis. Our series seems to be unusual in its high percentage of patients with late stages of obstructive changes secondary to pancreas divisum or ampullary stenosis, reflecting the clinical interests of our gastroenterologists and surgeons. More than half of the patients had severe daily pain requiring narcotic treatment. Preoperative diabetes was present in 11 (15%) patients, 5 of whom were insulin-dependent. Pancreatic enzyme supplements were taken by 28 (39%) patients. In 11 (15%) patients, previous pancreatic procedures had failed, including six longitudinal pancreaticojejunostomies. Radiologic tests used for preoperative evaluation included endoscopic retrograde cholangiopancreatography (93% of patients), computed tomography (67%), endoscopic ultrasound (24%), and visceral angiography (14%). A stricture or mass in the head of the pancreas was noted in 60% of patients, six of whom had jaundice. The PPPD and Whipple groups were similar in every respect, except that more alcohol abusers were treated by PPPD.
Table 1. PATIENT POPULATION
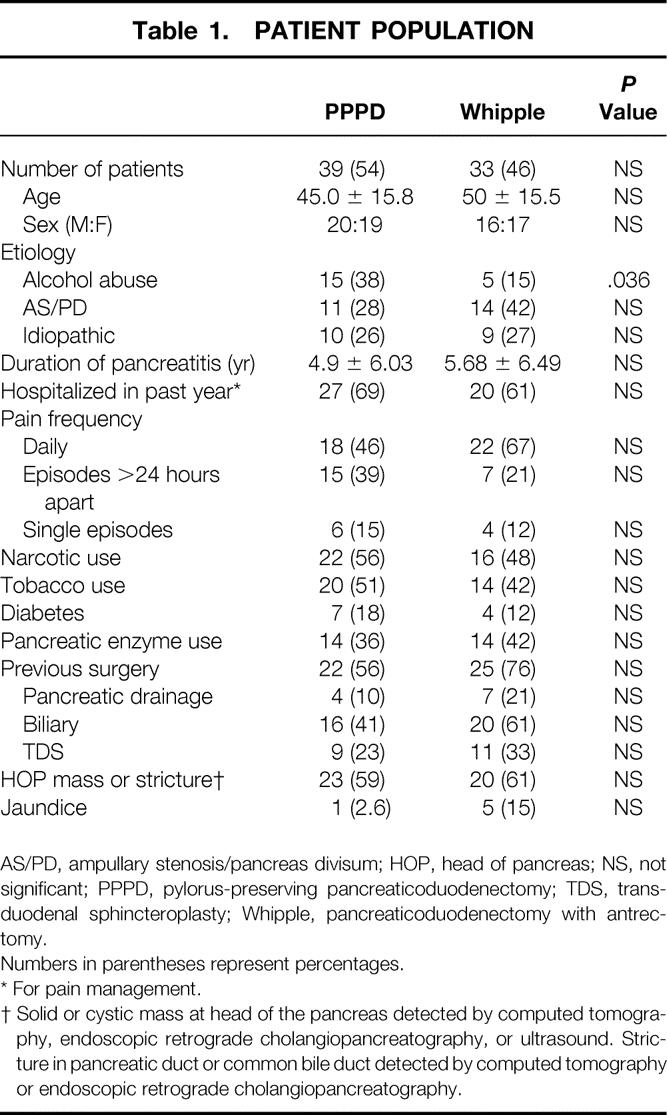
AS/PD, ampullary stenosis/pancreas divisum; HOP, head of pancreas; NS, not significant; PPPD, pylorus-preserving pancreaticoduodenectomy; TDS, transduodenal sphincteroplasty; Whipple, pancreaticoduodenectomy with antrectomy.
Numbers in parentheses represent percentages.
* For pain management.
† Solid or cystic mass at head of the pancreas detected by computed tomography, endoscopic retrograde cholangiopancreatography, or ultrasound. Stricture in pancreatic duct or common bile duct detected by computed tomography or endoscopic retrograde cholangiopancreatography.
Table 2 summarizes the postoperative complications, perioperative death rate, and LOS. Forty-five percent of patients had complications after pancreaticoduodenectomy. Operation-specific complications limited to fistulas, DGE, intraabdominal abscess, hemorrhage, or cholangitis occurred in 38% of patients. None of the complications, particularly regarding DGE and fistulas, were related to leakage, disruption, or obstruction at the gastrojejunostomies or duodenojejunostomies.
Table 2. HOSPITAL COURSE
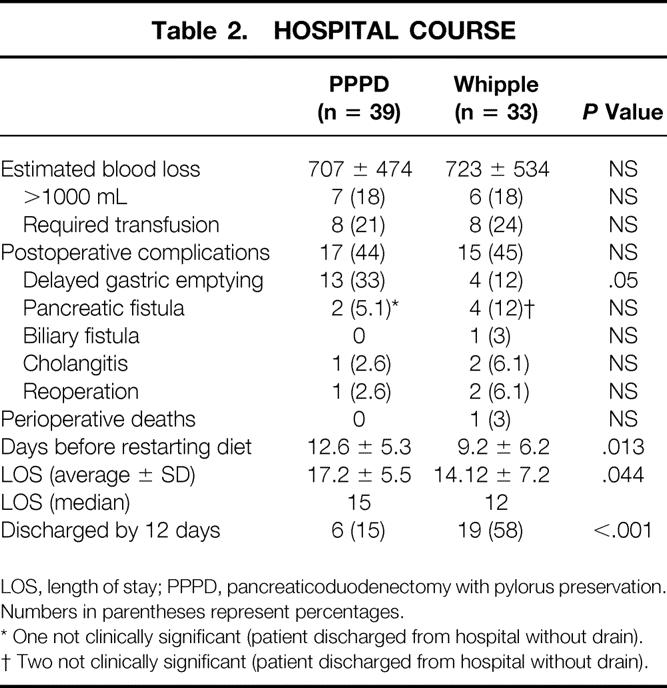
LOS, length of stay; PPPD, pancreaticoduodenectomy with pylorus preservation.
Numbers in parentheses represent percentages.
* One not clinically significant (patient discharged from hospital without drain).
† Two not clinically significant (patient discharged from hospital without drain).
There was only one death in this series, for a perioperative death rate of 1.4%. This patient was a 66-year-old man with a complicated past medical history including end-stage renal disease who could not tolerate postoperative hemodialysis. Subsequent institution of peritoneal dialysis resulted in peritonitis, anastomotic breakdown at the pancreaticojejunostomy (requiring reoperation), sepsis, and multiorgan system failure resulting in death.
For this study, DGE was defined as lack of significant postoperative oral intake for more than 14 days, requiring institution of total parenteral nutrition. DGE was the most common complication encountered. DGE developed in 33% of patients undergoing PPPD. This was significantly different from the 12% frequency of DGE observed in patients after the Whipple operation. Even PPPD patients who did not meet the criteria for DGE started oral feedings later than their Whipple counterparts (10 vs. 7 days). DGE was always a consequence of gastrointestinal paresis or ileus and never resulted from anatomical obstruction. Associated intraabdominal complications (an abscess) occurred in only 1 (6%) of the 17 patients with DGE; all other cases were isolated events. None of the other 16 patients had any evidence of right upper quadrant inflammation. A single patient required reoperation after Whipple resection for management of DGE, and this was for gastrostomy and jejunostomy tube placement.
There was no significant difference in the rate of pancreatic or biliary cutaneous fistula between the two groups. A fistula of this type was defined as surgical drain output greater than 25 mL beyond postoperative day 7, and amylase determination in drain fluid was used to differentiate between pancreatic and biliary sources. Pancreatic fistula had an overall frequency of 8.3%. Biliary fistula occurred in one patient after Whipple resection and closed spontaneously. Only three of the six pancreatic fistulas persisted long enough to necessitate discharge from the hospital with a surgical drain in place. No patient required reoperation for fistula management; all closed under conservative treatment. Conservative fistula management consisted of drain advancement and, occasionally, octreotide therapy.
The median LOS was 15 days for patients after PPPD and 12 days after Whipple (Fig. 2). Fifty-eight percent of patients were discharged on or before the 12th postoperative day after Whipple resection, whereas only 15% achieved this goal after PPPD. The difference in LOS is largely a consequence of the difference in postoperative DGE. Even among patients free of postoperative complications, there was a 3-day difference in median LOS (10 days for PPPD, 7 days for Whipple). The interval until oral feeding was tolerated in PPPD patients accounted for the need for a prolonged hospital stay and was unrelated to concomitant leaks, fistulas, or infections.
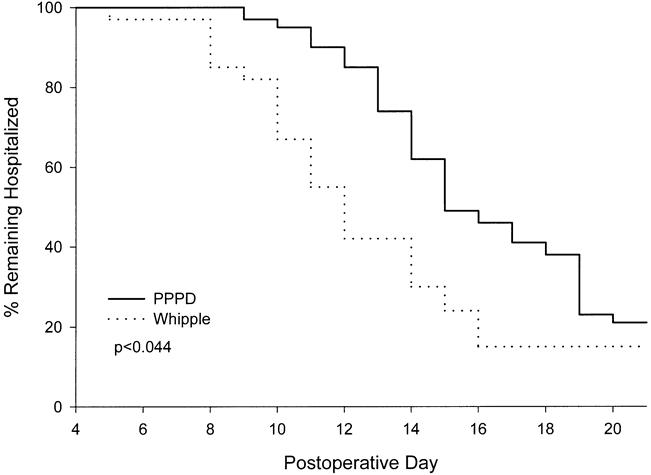
Figure 2. Length of stay after pancreatic resection. P value was calculated by the Wilcoxon rank test.
Current information was available for 90% of our patients (35 post-PPPD, 30 post-Whipple), as shown in Table 3. The average duration of follow-up exceeded 40 months, and no living patient had a duration of follow-up of less than 11 months. During this period, there were five deaths. Three of these deaths (1/35 after PPPD and 2/30 after Whipple resection) resulted from malnutrition and failure to thrive as a consequence of progressive chronic pancreatitis (at 19, 60, and 77 months). Two other deaths resulted from pulmonary embolism (at 2 months) and amyotrophic lateral sclerosis (at 17 months).
Table 3. FOLLOW-UP DATA
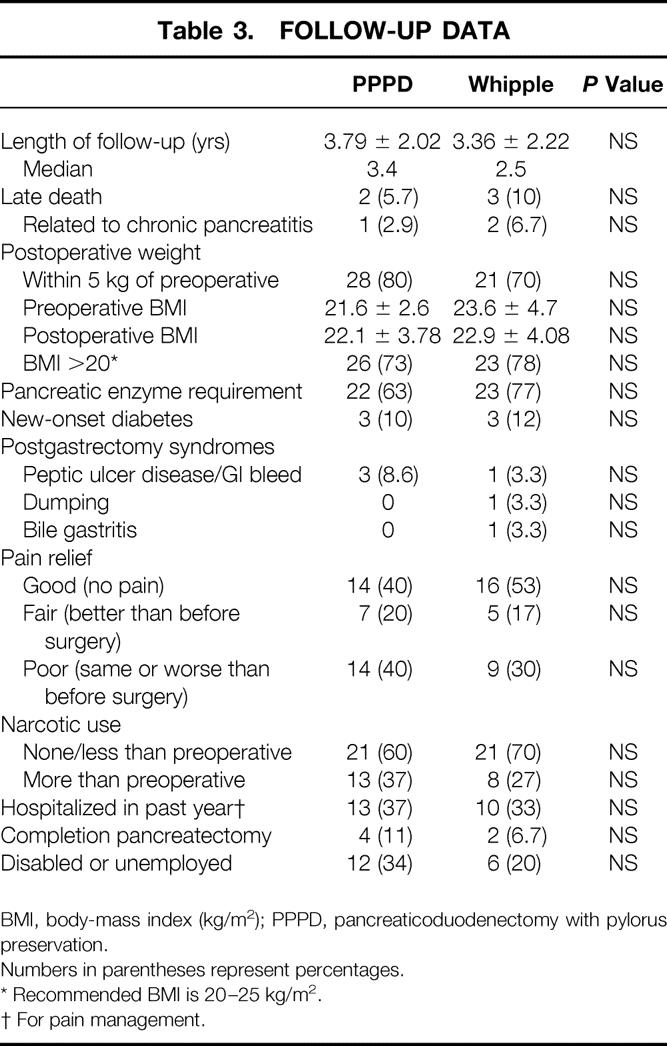
BMI, body-mass index (kg/m2); PPPD, pancreaticoduodenectomy with pylorus preservation.
Numbers in parentheses represent percentages.
* Recommended BMI is 20–25 kg/m2.
† For pain management.
Long-term gastrointestinal and pancreatic function and nutritional status were indirectly measured by quantification of body weight, pancreatic enzyme supplement use, new-onset diabetes, and occurrence of bile gastritis, dumping, or peptic ulcer disease. Seventy percent and 80% of patients have maintained postoperative weights greater than 90% of their preoperative levels after Whipple and PPPD, respectively. Patients’ heights and weights were used to calculate postoperative body-mass indices (BMI). No significant difference in BMI after Whipple or PPPD was noted, with approximately 75% of patients (73% after PPPD and 78% after Whipple) maintaining indices within or greater than the recommended range of 20 to 25 during long-term follow-up. 21
Sixty-nine percent of patients required pancreatic enzyme supplements for control of steatorrhea after surgery, with no significant difference between the Whipple and PPPD groups. Postoperative diabetes developed in 6 patients other than the 11 who already had it before surgery. Four of these six patients required insulin for glucose control. Among patients with no preoperative diabetes, therefore, 11% had new-onset diabetes after pancreaticoduodenectomy. Also, three patients with preoperative diabetes controlled by oral medications were insulin-dependent after resection. No difference in the incidence of diabetes, whether insulin-dependent or not, was detected between the groups. Postoperative enzyme requirement but not diabetes was associated with long-term weight loss (Table 4).
Table 4. FACTORS INFLUENCING LONG-TERM WEIGHT MAINTENANCE
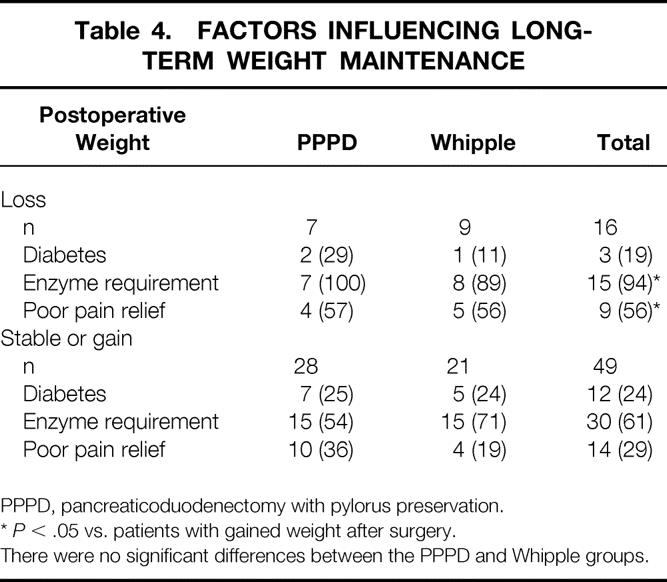
PPPD, pancreaticoduodenectomy with pylorus preservation.
*P < .05 vs. patients with gained weight after surgery.
There were no significant differences between the PPPD and Whipple groups.
Bile gastritis occurred in one patient after Whipple resection; it required reconstruction to a Roux-en-Y gastrojejunostomy. Dumping was diagnosed in only one patient after Whipple resection. Bile gastritis or dumping did not occur in any patient after PPPD. Four patients had endoscopically documented peptic ulceration during evaluation of gastrointestinal bleeding or new-onset abdominal pain. Although peptic ulceration occurred more frequently in the PPPD than in the Whipple group (three vs. one patients), this difference did not attain statistical significance. No patient required reoperation for management of ulcer disease.
The success rate of pancreaticoduodenectomy in either eliminating or greatly reducing the pain and narcotic use associated with chronic pancreatitis was 65% (Table 3). There was no significant difference in outcome with respect to the type of operation performed. Complete pain relief occurred in 46% of patients, 14 after PPPD and 16 after Whipple. Twelve more patients (19%) had residual episodic pain after surgery, which was judged by the patient to be markedly reduced from preoperative levels (pain scale scores 1–3). Eight of these 12 patients had pain-free periods longer than 1 month between exacerbations, and only 4 required occasional narcotics for pain control. Poor results occurred in 23 patients: 20 had daily pain, 21 required more narcotics than before surgery, and pain scale scores ranged from 7 to 10. Among the patients with failed treatment, six (four PPPD and two Whipple) underwent completion pancreatectomy for continuing pain approximately 1 year after pancreaticoduodenectomy. Poor pain relief after pancreaticoduodenectomy was associated with postoperative weight loss (Table 4).
Outcome Analysis
The preoperative and hospital variables (Tables 1 and 2) were subjected to univariate and multivariate analysis with respect to postoperative pain relief (good or fair vs. poor). Univariate analysis revealed six factors associated with good or fair surgical results (Table 5) : age older than 50 years, duration of disease less than 2 years, presentation after an isolated episode of pain, history of diabetes, no previous longitudinal pancreaticojejunostomy, and positive localized pathology in the head of the pancreas. Findings in the head of the pancreas included pancreatic duct or common bile duct strictures, or discrete solid or cystic masses detected by computed tomography, endoscopic retrograde cholangiopancreatography, or endoscopic ultrasound (Fig. 3). Of these six variables, only preoperative pathology in the head of the pancreas was found to be an independent predictor of a good or fair outcome in the multiple logistic regression model, indicating that all six factors identified by the univariate analyses were highly correlated and represent a single group of patients for whom surgery is likely to have successful results.
Table 5. OUTCOME ANALYSIS: PREOPERATIVE FACTORS
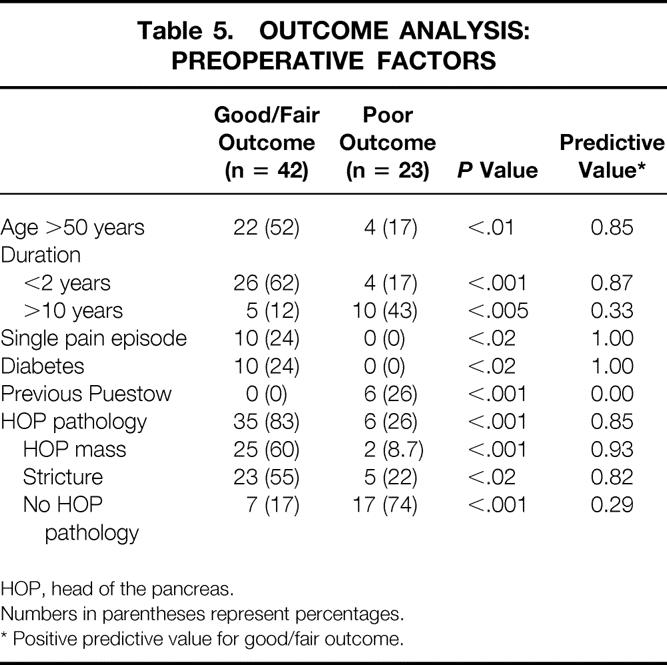
HOP, head of the pancreas.
Numbers in parentheses represent percentages.
* Positive predictive value for good/fair outcome.
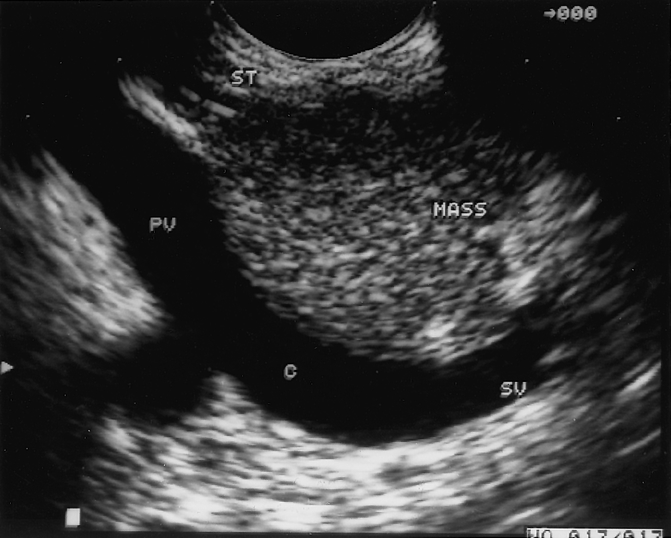
Figure 3. Mass in the head of the pancreas secondary to chronic pancreatitis. This image was obtained by endoscopic ultrasound and demonstrates a mass in the head of the pancreas adjacent to the portal vein. The patient underwent a Whipple resection, resulting in complete resolution of pain. PV, portal vein; SV, splenic vein; C, confluence of splenic and superior mesenteric veins.
Outcome analysis with respect to pain relief showed no difference between the Whipple and PPPD procedures. None of the immediate postoperative complications was predictive of adverse outcome. In particular, development of postoperative DGE did not influence a patient’s long-term surgical results.
Factors significantly associated with poor pain relief by univariate analysis included duration of symptoms more than 10 years and previous longitudinal pancreaticojejunostomy (modified Puestow procedure).
DISCUSSION
In the past two decades, advances in surgical technique and critical care and the emergence of medical centers specializing in the treatment of pancreatic disease have led to dramatic changes in the death rates associated with pancreaticoduodenectomy. Multiple series have documented death rates well below 5%, 22,23 confirmed by the rate of 1.4% in this series. The newfound safety of the operation has led to a greater willingness to consider it for less than life-threatening illness, including chronic pancreatitis. 14 PPPD has been advocated over Whipple resection with antrectomy primarily because of its purported nutritional advantages and the reduced likelihood of postgastrectomy syndromes, including dumping and bile reflux gastritis. 24
Our study represents the largest published series systematically comparing the standard Whipple resection and the PPPD in the treatment of chronic pancreatitis. In the short term (in-hospital), DGE was found to be twice as common after PPPD than after Whipple resection. We noted this complication initially in 1985, 25 and other series confirm that DGE occurs in 25% to 30% of patients after PPPD. 19,26–30 Based on these results, we have recommended placement of gastrostomy tubes in all patients at the time of PPPD to minimize the discomfort of prolonged nasogastric intubation and its attendant complications. Higher frequencies of DGE have translated into significantly longer hospital stays for PPPD versus Whipple patients. The difference in LOS for the group of patients as a whole averaged 3 days, but discharge on or before the 12th postoperative day was remarkably different between the groups (15% PPPD, 58% Whipple). DGE not only adds to the hospital cost of the PPPD compared with the Whipple but can also increase complications because it adds the complications associated with central venous access and institution of total parenteral nutrition.
Some investigators have noted a correlation between the incidence of DGE and other complications such as abscesses, fistulas, cholangitis, and right upper quadrant inflammation. 27,31,32 They suggest that DGE is a direct consequence of these other processes and is not a primary motility disorder. Our results do not confirm such a correlation: only 1 of the 17 patients with DGE had an associated intraabdominal complication. We surmise that DGE is most likely to be related to temporary gastroparesis of unknown pathogenesis. DGE did not influence long-term gastrojejunal function or nutritional status.
Historically, the short-term drawbacks of PPPD related to DGE have been tolerated in exchange for its putative nutritional advantages. Multiple studies have shown that PPPD preserves well-coordinated gastric and pyloric function in the long term. 24,33 As a result, emptying of liquids takes a significantly shorter time after PPPD than after Whipple resection. 34,35 Likewise, physiologic measurements of serum gastrin have been shown to be nearly normal after PPPD but markedly depressed after standard Whipple (in which antrectomy removes the source of gastrin). 21,36 Other, more ambitious studies have failed to demonstrate nutritional advantages in terms of glucose homeostasis and iron absorption. 34 In general, most studies have used postoperative patient weights as parameters of nutritional status and have reported weight gain in 67% to 95% of patients after PPPD. 13,16,37,38
Our data, however, demonstrate no differences in nutritional parameters between the two procedures. The frequency of postoperative diabetes or pancreatic enzyme requirement was similar. Seventy percent and 80% of our patients maintained weights greater than 90% of their preoperative levels after Whipple or PPPD, respectively, and this difference was not statistically significant. Because postoperative nutritional assessment by isolated weight measurements can be misleading, primarily because of variable preoperative nutritional status, standardization of weight measurements by determination of postoperative BMI has been advocated for nutritional assessment in patients after pancreaticoduodenectomy. 21 Our results showed no significant differences in BMI after Whipple or PPPD, with 73% and 78% of patients maintaining indices above the recommended range, respectively. Long-term weight loss was more likely to occur in association with pancreatic insufficiency or poor pain relief, but this was irrespective of the operation performed. Therefore, our data do not support the nutritional superiority of one operation over the other.
Development of postgastrectomy syndromes after pancreaticoduodenectomy may also affect well-being. Several studies have reported that the frequency of peptic ulcer disease is higher after PPPD than after Whipple, perhaps because of preserved antral gastrin secretion. 27,29,38 Our series found a prevalence of peptic ulcer disease of 9% and 3% after PPPD and Whipple, respectively, which is consistent with published data but not conclusive. Neither bile gastritis nor dumping occurred in any patient in whom the pylorus was preserved; in contrast, these complications were observed in two patients after Whipple resection. The patient with bile gastritis had complete resolution after revision gastrojejunostomy, but the one with dumping syndrome remains under medical treatment with limited symptomatic control. When taken together, no difference in the prevalence of postoperative syndromes exists between the two groups.
Reported success rates for pain relief after pancreaticoduodenectomy demonstrate considerable variation, ranging from 60% to 100%. 12,13,15–17 Many of these series include significant numbers of completion or total pancreatectomies, complicating the evaluation of cephalic pancreatectomy alone. 13,16 We judge our success in this experience to have been 65%, with no significant differences between the standard Whipple and PPPD groups. Our series is different from other published series in the relatively high number of patients with stenosis of the pancreatic duct or previously failed pancreatic procedures. These patients appear to have a lower likelihood of success: 50% for late-stage dorsal duct obstruction and 27% for rescue operations after longitudinal pancreaticojejunostomy. If these patients are excluded, 88% achieved satisfactory pain relief. These data underscore the fact that patient selection has an important impact on the outcome of pancreaticoduodenectomy for chronic pancreatitis.
Evaluation of preoperative and in-hospital patient characteristics by multivariate analysis revealed that pathology in the head of the pancreas was the only independent factor associated with good or fair postoperative pain relief. Radiographically defined pathology in the head of the pancreas, particularly cystic or solid mass lesions, was the strongest predictor of success, and only 6 of 41 patients with such findings did not benefit from pancreaticoduodenectomy. Conversely, only 29% (7/24) of patients with diffuse pancreatic disease and no localized pathology in the head of the gland achieved equivalent pain relief. Other reports have emphasized this factor and have even required localized pathology in the head of the gland to define candidacy for resection. 13,16,37 Similarly, we previously found that the success of distal pancreatectomy for treatment of pain in chronic pancreatitis was highly linked to specific pathology such as pseudocyst in the body or tail of the gland. 6 Other characteristics identified that differentiated between patients with pancreatic pathology and those without included age older than 50 years, duration of symptoms less than 2 years, recent onset of diabetes, and presentation after a single episode of pain.
Poor surgical results were associated with disease duration greater than 10 years, with no demonstrable pathology in the head of the pancreas, or with failed longitudinal pancreaticojejunostomies. In this study, none of the patients with failed modified Puestow operations benefited from pancreatic head resection. A previous study from our group reported successful salvage of failed modified Puestow procedures by pancreaticoduodenectomy in 70% (7/10) of patients. 39 This was a small group of patients in whom outcome assessment may have been difficult due to the widely ranging duration of follow-up (1 month to 11.5 years). Total pancreatectomy may be the only remaining surgical option for these patients. However, total pancreatectomy results in significant complications secondary to obligate pancreatic exocrine and endocrine insufficiency. An increased incidence of peptic ulcer disease has also been observed after this operation. 13 In our estimation, total pancreatectomy seems overly aggressive as an initial procedure for patients with chronic pancreatitis and may be better offered to highly selected patients as a staged operation after the failure of cephalic or distal pancreatectomy.
In summary, the Whipple and PPPD procedures produce similar results with respect to late outcomes such as pain relief, new-onset diabetes, and weight maintenance. They differ, however, in immediate postoperative LOS for the unpredictable but substantial number of patients in whom DGE develops. The Whipple operation may be preferred when cancer is suspected and a short LOS is desirable. Alternatively, PPPD might be the operation of choice for the severely malnourished patient, although our data do not support the theoretical rationale of this procedure. Patient selection can affect the success of pancreaticoduodenectomy in the treatment of chronic pancreatitis. Patients with radiographically detectable complications of the disease concentrated focally in the head of the pancreas are the most likely to benefit from the operation. Others with intractable abdominal pain, longstanding disease, unsuccessful Puestow operations, and no focal findings in the head of the gland have a significantly lower likelihood of a satisfactory outcome.
Footnotes
Correspondence: Andrew L. Warshaw, MD, Dept. of Surgery, WHT 506, Massachusetts General Hospital, Boston, MA 02114.
Supported by the Marshall K. Bartlett, MD, Resident Research Fellowship, Massachusetts General Hospital and Harvard Medical School (to REJ).
Accepted for publication July 23, 1999.
References
- 1.Warshaw AL, Banks PA, Fernandez-del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115:765–776. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw AL. Conservation of pancreatic tissue by combined gastric, biliary, and pancreatic duct drainage for pain from chronic pancreatitis. Am J Surg 1985; 149:563–569. [DOI] [PubMed] [Google Scholar]
- 3.Jalleh RP, Aslam M, Williamson RC. Pancreatic tissue and ductal pressures in chronic pancreatitis. Br J Surg 1991; 78:1235–1237. [DOI] [PubMed] [Google Scholar]
- 4.Prinz RA, Greenlee HB. Pancreatic duct drainage in chronic pancreatitis. Hepatogastroenterology 1990; 37:295–300. [PubMed] [Google Scholar]
- 5.Holmberg JT, Isaksson G, Ihse I. Long-term results of pancreaticojejunostomy in chronic pancreatitis. Surg Gynecol Obstet 1985; 160:339–346. [PubMed] [Google Scholar]
- 6.Rattner DW, Fernandez-del Castillo C, Warshaw AL. Pitfalls of distal pancreatectomy for relief of pain in chronic pancreatitis. Am J Surg 1996; 171:142–145. [DOI] [PubMed] [Google Scholar]
- 7.Sawyer R, Frey CF. Is there still a role for distal pancreatectomy in surgery for chronic pancreatitis? Am J Surg 1994; 168:6–9. [DOI] [PubMed] [Google Scholar]
- 8.Whipple AO. Treatment of carcinoma of the ampulla of Vater. Ann Surg 1935; 102:763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traverso LW, Longmire WPJ. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet 1978; 146:959–962. [PubMed] [Google Scholar]
- 10.Beger HG, Buchler M, Bittner RR, Oettinger W, Roscher R. Duodenum-preserving resection of the head of the pancreas in severe chronic pancreatitis. Early and late results. Ann Surg 1989; 209:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson K. Carcinoma of the ampulla of Vater. Successful radical resection. Br J Surg 1944; 31:368–373. [Google Scholar]
- 12.Gall FP, Gebhardt C, Meister R, Zirngibl H, Schneider MU. Severe chronic cephalic pancreatitis: use of partial duodenopancreatectomy with occlusion of the pancreatic duct in 289 patients. World J Surg 1989; 13:809–816. [DOI] [PubMed] [Google Scholar]
- 13.Traverso LW, Kozarek RA. Pancreatoduodenectomy for chronic pancreatitis: anatomic selection criteria and subsequent long-term outcome analysis. Ann Surg 1997; 226:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barens SA, Lillemoe KD, Kaufman HS, et al. Pancreaticoduodenectomy for benign disease. Am J Surg 1996; 171:131–134. [DOI] [PubMed] [Google Scholar]
- 15.Howard JM, Zhang Z. Pancreaticoduodenectomy (Whipple resection) in the treatment of chronic pancreatitis. World J Surg 1990; 14:77–82. [DOI] [PubMed] [Google Scholar]
- 16.Martin RF, Rossi RL, Leslie KA. Long-term results of pylorus-preserving pancreatoduodenectomy for chronic pancreatitis. Arch Surg 1996; 131:247–252. [DOI] [PubMed] [Google Scholar]
- 17.Stapleton GN, Williamson RC. Proximal pancreatoduodenectomy for chronic pancreatitis. Br J Surg 1996; 83:1433–1440. [DOI] [PubMed] [Google Scholar]
- 18.Frey CF, Pitt HA, Prinz RA. A plea for uniform reporting of patient outcome in chronic pancreatitis. Arch Surg 1996; 131:233–234. [DOI] [PubMed] [Google Scholar]
- 19.Grace PA, Pitt HA, Longmire WP. Pylorus-preserving pancreatoduodenectomy: an overview. Br J Surg 1990; 77:968–974. [DOI] [PubMed] [Google Scholar]
- 20.Whipple AO. The rationale for cancer of the pancreas and ampullary region. Ann Surg 1941; 114:612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLeod RS, Taylor BR, O’Connor BI, et al. Quality of life, nutritional status, and gastrointestinal hormone profile following the Whipple procedure. Am J Surg 1995; 169:179–185. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg 1995; 130:295–299. [DOI] [PubMed] [Google Scholar]
- 23.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997; 226:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traverso LW, Longmire WPJ. Preservation of the pylorus in pancreaticoduodenectomy: a follow-up evaluation. Ann Surg 1980; 192:306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warshaw AL, Torchiana DL. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. Surg Gynecol Obstet 1985; 160:1–4. [PubMed] [Google Scholar]
- 26.Braasch JW, Deziel DJ, Rossi RL, Watkins EJ, Winter PF. Pyloric and gastric-preserving pancreatic resection. Experience with 87 patients. Ann Surg 1986; 204:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt DR, McLean R. Pylorus-preserving pancreatectomy: functional results. Br J Surg 1989; 76:173–176. [DOI] [PubMed] [Google Scholar]
- 28.Itani KM, Coleman RE, Meyers WC, Akwari OE. Pylorus-preserving pancreatoduodenectomy: a clinical and physiologic appraisal. Ann Surg 1986; 204:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAfee MK, van Heerden JA, Adson MA. Is proximal pancreatoduodenectomy with pyloric preservation superior to total pancreatectomy? Surgery 1989; 105:347–351. [PubMed] [Google Scholar]
- 30.Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 1993; 217:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andren-Sandberg A, Kulli C, Wagner M, Friess H, Buchler M. There is no delayed gastric emptying after a pancreatic resection without complications. Pancreas 1998; 17:423. [Google Scholar]
- 32.Traverso LW, Kozarek RA. The Whipple procedure for severe complications of chronic pancreatitis. Arch Surg 1993; 128:1047–1050. [DOI] [PubMed] [Google Scholar]
- 33.Kozuschek W, Reith HB, Waleczek H, Haarmann W, Edelmann M, Sonntag D. A comparison of long-term results of the standard Whipple procedure and the pylorus-preserving pancreatoduodenectomy. J Am Coll Surg 1994; 178:443–453. [PubMed] [Google Scholar]
- 34.Fink AS, DeSouza LR, Mayer EA, Hawkins R, Longmire WPJ. Long-term evaluation of pylorus preservation during pancreaticoduodenectomy. World J Surg 1988; 12:663–670. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi I, Miyachi M, Kanai M, et al. Different gastric emptying of solid and liquid meals after pylorus-preserving pancreatoduodenectomy. Br J Surg 1998; 85:927–930. [DOI] [PubMed] [Google Scholar]
- 36.Muller MW, Friess H, Beger HG, et al. Gastric emptying following pylorus-preserving Whipple and duodenum-preserving pancreatic head resection in patients with chronic pancreatitis. Am J Surg 1997; 173:257–263. [DOI] [PubMed] [Google Scholar]
- 37.Buchler MW, Friess H, Muller MW, Wheatley AM, Beger HG. Randomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis. Am J Surg 1995; 169:65–69. [DOI] [PubMed] [Google Scholar]
- 38.Morel P, Mathey P, Corboud H, Huber O, Egeli RA, Rohner A. Pylorus-preserving duodenopancreatectomy: long-term complications and comparison with the Whipple procedure. World J Surg 1990; 14:642–646. [DOI] [PubMed] [Google Scholar]
- 39.Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage. Arch Surg 1994; 129:374–379. [DOI] [PubMed] [Google Scholar]


