Abstract
Objective
To evaluate the results of local excision alone for the treatment of rectal cancer, applying strict selection criteria.
Background Data
Several retrospective studies have demonstrated that tumor control in properly selected patients with rectal cancer treated locally is comparable to that observed after radical surgery. Although there is a consensus regarding the need for patient selection for local excision, the specific criteria vary among centers.
Methods
The authors reviewed 82 patients with T1 (n = 55) and T2 (n = 27) rectal cancer treated with transanal excision only during a 10-year period. At pathologic examination, all tumors were localized to the rectal wall, had negative excision margins, were well or moderately differentiated, and had no blood or lymphatic vessel invasion, nor a mucinous component. End points were local and distant tumor recurrence and patient survival.
Results
Ten of the 55 patients with T1 tumors (18%) and 10 of the 27 patients with T2 tumors (37%) had recurrence at 54 months of follow-up. Average time to recurrence was 18 months in both groups. Seventeen of the 20 patients with local recurrence underwent salvage surgery. The survival rate was 98% for patients with T1 tumors and 89% for patients with T2 tumors. Preoperative staging by endorectal ultrasound did not influence local recurrence or tumor-specific survival.
Conclusion
Local excision of early rectal cancer, even in the ideal candidate, is followed by a much higher recurrence rate than previously reported. Although most patients in whom local recurrence develops can be salvaged by radical resection, the long-term outcome remains unknown.
Radical en bloc excision of the rectum and mesorectum, either by abdominoperineal or low anterior resection, is the standard against which other operations for rectal cancer are measured. 1,2 However, these radical procedures are associated with significant complications, death, and sometimes distressing functional consequences for the patient.
Unfortunately, many patients surgically treated for rectal cancer may not benefit from the potential advantages of such radical procedures. 3 Some patients with seemingly localized tumors eventually die of disseminated disease that was present but occult at their initial operation. Others who are diagnosed early could theoretically be cured by less radical operations. It is this latter group that has encouraged surgeons to develop alternatives to radical resection in patients with early rectal cancer. 4
Because local excision alone can cure only tumors confined to the bowel wall, accurate preoperative staging is critical for proper patient selection. The introduction of new imaging technology such as endorectal ultrasound (ERUS) and magnetic resonance imaging, both capable of delineating the separate layers of the rectal wall and identifying enlarged mesorectal lymph nodes, has increased the interest in local therapy of early rectal cancers.
Several retrospective studies have demonstrated that tumor control in properly selected patients with rectal cancer treated locally is comparable to that observed after radical surgery. Although there is a consensus regarding the selection criteria for local excision, the specific criteria vary among centers. In addition, because patient populations are heterogeneous and individual surgeon adherence to the stated selection criteria varies, reported series may not be entirely comparable. In this report, we applied strict selection criteria and a well-defined surgical technique to evaluate the results of local excision alone for the treatment of rectal cancer. We also evaluated the impact of preoperative staging by ERUS on the clinical outcome after local excision of rectal cancer.
PATIENTS AND METHODS
Based on a review of our computerized database of 1,367 patients with rectal cancer treated at the University of Minnesota and affiliated hospitals between January 1987 and December 1996, we found a total of 171 patients who underwent transanal excision of the primary lesion. Of these patients, 89 were excluded from analysis because the tumor was excised by snare polypectomy alone (n = 16) or penetrated the perirectal fat (n = 7), the resection margins were positive (n = 19), adverse histologic features were present (poorly differentiated, blood or lymphatic vessel invasion, or mucinous components) (n = 26), or the patient received adjuvant chemoradiation therapy (n = 21). The remaining 82 patients, 6% of the entire group of patients with rectal cancer, form the basis of this study. All these patients were treated with transanal excision with curative intent as the only form of treatment. All tumors were localized to the rectal wall at pathologic examination, had negative excision margins, and were well or moderately differentiated, without blood or lymphatic vessel invasion, and without mucinous components.
All patients underwent a full colonoscopy to exclude synchronous lesions. Chest x-ray and abdominal and pelvic computed tomography scans were obtained to exclude metastatic disease and local extrarectal invasion. The location of the tumor was measured at the time of diagnosis as the distance from the anal verge to the distal tumor margin. Preoperative ERUS was performed in 59 patients.
All tumors were removed by transanal excision. Patients underwent full mechanical and antibiotic bowel preparation. The surgery was usually performed under general anesthesia, with the patient positioned in the prone jackknife position. The lesions were removed using electrocautery to perform a full-thickness excision with approximately a 1-cm margin of normal rectal wall. The excised specimen was pinned out in the operating room and the resection margins were inked before slides were cut. Primary closure was always performed using a single layer of interrupted polyglycolic suture. A proctoscopic examination was performed at the end of the procedure to ensure that the rectal lumen was not compromised. Tumor size was measured as the longest diameter of the tumor on the resected fresh specimen.
Follow-up in our database includes information obtained from periodic visits to our clinic, as well as information included in the tumor registries of the hospitals that comply with the American College of Surgeons National Cancer Database. In the past 5 years, patients with rectal cancer have been followed according to a protocol in which ERUS is performed every 4 months for the first 3 years after surgery and every 6 months for the next 2 years.
The end points of this study were local and distant tumor recurrence and patient survival. A t test and Pearson’s chi-square test were used to compare age, follow-up, and tumor stage in patients with and without preoperative ERUS. Survival curves were estimated using the Kaplan-Meier method and were compared using the log-rank test.
RESULTS
The study included 82 patients with T1 (n = 55) or T2 (n = 27) tumors. The average age of the patients was 67.6 years, similar to the average age of the entire rectal cancer group (65.4 years). There were 44 men and 38 women. The average tumor diameter was 2.9 cm; this was similar in both T1 and T2 tumors (2.8 vs. 3 cm, respectively). The average distance of the tumor from the anal verge was 6.2 cm; this was similar for T1 (6.4 cm) and T2 (5.8 cm) tumors. The average length of follow-up was 54 months; follow-up was slightly longer for patients with T2 than for T1 tumors (58 vs. 52 months, respectively).
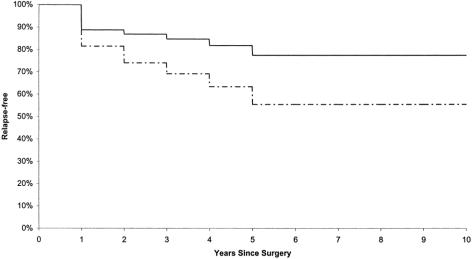
At 54 months of follow-up, 10 of the 55 patients with T1 tumors (18%) and 10 of the 27 patients with T2 tumors (37%) had a recurrence, for an overall recurrence rate of 24% (Fig. 1). The average time to recurrence was 18 months in both groups.
Figure 1. Relapse-free interval among stage I rectal cancer patients treated by transanal excision. Solid line, T1; dashed line, T2.
In the T1 group, nine patients had a local tumor recurrence, one had distant metastasis, and one had both local and distant recurrence. Six of the local recurrences involved the mucosa at the excision site and were diagnosed by proctoscopy. In the remaining four patients, the recurrence was extrarectal and was diagnosed by ERUS. Two patients with local recurrence were treated with radiation therapy alone, and eight patients underwent subsequent surgical treatment: local excision (n = 4), abdominoperineal resection (n = 3), and abdominoperineal resection with a simultaneous resection of a liver metastasis (n = 1). One patient died of cancer (2%). One patient had an apparently complete response to radiation therapy. The rest remained disease-free. Nine patients died of unrelated causes without evidence of disease.
In the T2 group, eight patients had an isolated local recurrence, and two had both local and distant recurrence. The recurrence involved the mucosa in eight patients and was exclusively extrarectal in two. Nine T2 patients had subsequent surgery: low anterior resection (n = 6), abdominoperineal resection (n = 2), and abdominoperineal resection with simultaneous resection of a liver metastasis (n = 1). Two patients died of cancer, and one remained alive with evidence of disease. Eight patients died of other causes without evidence of cancer.
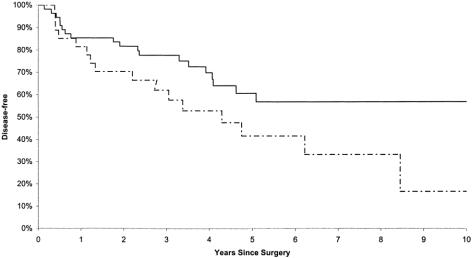
At an average of 54 months (4.5 years) of follow-up, 77% of the T1 patients and 55% of the T2 patients were alive without evidence of disease (Fig. 2).
Figure 2. Disease-free survival among stage I rectal cancer patients treated by transanal excision. Solid line, T1; dashed line, T2.
Although we have increasingly relied on disease stage determined by ERUS to select patients for local excision, some of the patients surgically treated earlier in this series were not staged by ultrasound before surgery. These patients were older and were followed up for a longer period than in the group staged by ERUS, but the distribution by stage between these groups and the length of follow-up were almost identical. The overall accuracy of ERUS for staging these early rectal cancers was only 59%; the accuracy was higher for T2 tumors than for T1 tumors (Table 1). Recurrence and survival rates were not influenced by whether the patient had ERUS before surgery (Table 2).
Table 1. COMPARISON OF ULTRASOUND T STAGE AND PATHOLOGY STAGE IN PATIENTS STAGED WITH PREOPERATIVE ENDORECTAL ULTRASOUND
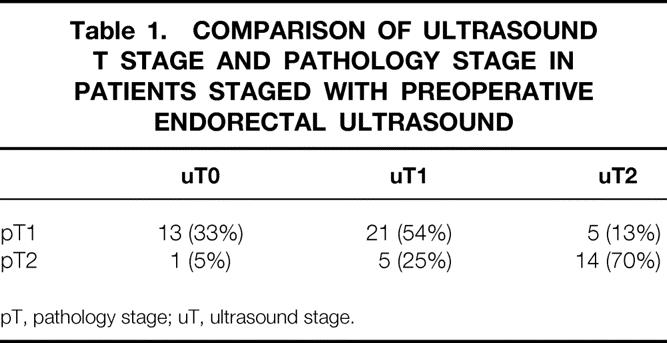
pT, pathology stage; uT, ultrasound stage.
Table 2. RESULTS AFTER TRANSANAL EXCISION OF EARLY RECTAL CANCER IN PATIENTS WITH AND WITHOUT PREOPERATIVE ENDORECTAL ULTRASOUND
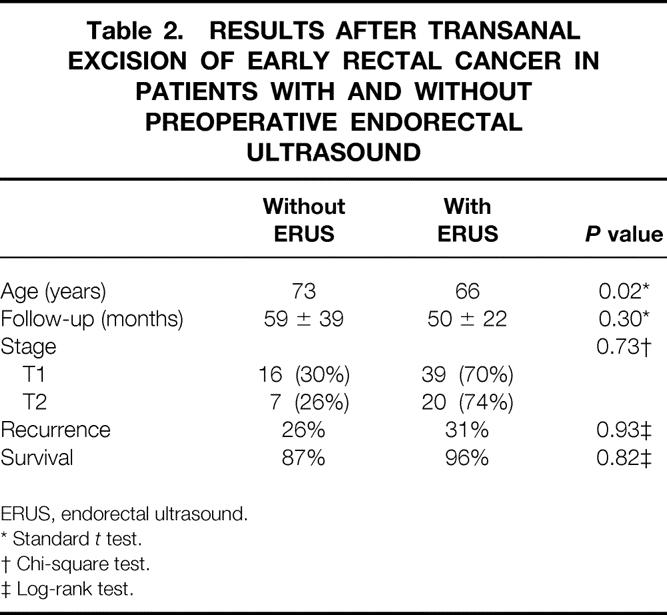
ERUS, endorectal ultrasound.
* Standard t test.
† Chi-square test.
‡ Log-rank test.
DISCUSSION
Radical resection is the mainstay of treatment for rectal cancer. However, the death rate after a radical proctectomy ranges from 2.3% to 3.2%, and postoperative complications develop in 30% to 46% of patients. 5,6 Permanent urinary and sexual dysfunctions are common sequelae of radical proctectomy. 7–9 Complications related to the perineal wound occur in 16% of patients undergoing abdominoperineal resection, and an additional 11% have stoma-related complications. Even patients who do not require a permanent colostomy frequently report urgency, tenesmus, and fecal incontinence. 10,11
Local excision of rectal cancer has been accepted for years as an alternative to radical resection for patients unfit to undergo major surgery. Broad acceptance of local excision as the primary treatment of fit patients with rectal cancer has been limited by concerns about the adequacy of identification and treatment of mesorectal nodal metastases. However, encouraging early results with the local treatment of rectal cancers and the development of new diagnostic technology that provides accurate preoperative staging have increased the interest in this therapeutic alternative.
The results of local treatment of rectal cancer are difficult to interpret because the literature is dominated by retrospective analyses of heterogeneous groups of patients. There is almost uniform agreement that only well- or moderately differentiated T1 and T2 tumors without blood vessel or lymphatic invasion or mucinous components should be treated by local excision for curative intent. Unfortunately, many series include patients with undifferentiated tumors, tumors penetrating the perirectal fat, tumors with questionable or even positive resection margins, patients operated on by different approaches, and even patients undergoing palliative surgery. 3,12–20 Specific reference is not always made to lymphatic and blood vessel invasion, and the role of salvage surgery after failed local excision is not always clearly stated.
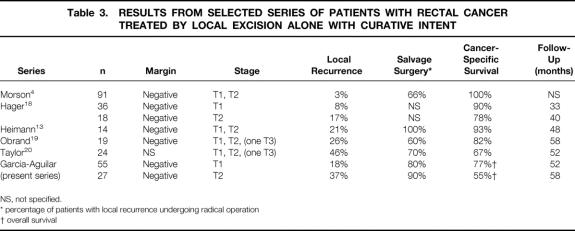
Although most series report recurrence rates of less than 11% for T1 tumors and less than 25% for T2 tumors, 12–19 Taylor et al 20 recently reported a 46% local recurrence rate for T1 and T2 tumors treated by local excision alone (Table 3) . We do not know why the recurrence rate in our series, which included only tumors with favorable histology and negative resection margins, was higher than that in series with less stringent exclusion criteria. These differences may in part reflect variation in the duration or completeness of follow-up.
Table 3. RESULTS FROM SELECTED SERIES OF PATIENTS WITH RECTAL CANCER TREATED BY LOCAL EXCISION ALONE WITH CURATIVE INTENT
NS, not specified.
* percentage of patients with local recurrence undergoing radical operation
† overall survival
Local recurrence after local excision can be due either to microscopic residual disease left at the primary tumor site or persistence of tumor cells in the mesorectal lymph nodes. In most series, the incidence of local recurrence after local excision of rectal cancer parallels the expected incidence of lymph node metastasis, suggesting that tumor failure may occur in the mesorectal lymph nodes. 21–23 The risk of lymph node metastasis in rectal cancer is directly related to tumor penetration of the bowel wall. However, tumor characteristics such as poor differentiation, blood vessel and lymphatic invasion, and the presence of mucinous components are also associated with a higher incidence of lymph node metastasis. Our series did not include tumors with these unfavorable histologic features, and therefore the incidence of lymph node metastasis should be lower than that reported in other series that included unselected patients. This, however, was not reflected by a lower rate of local recurrence in our series.
In our series, most local recurrences occurred at the local excision site and were diagnosed by proctoscopic examination and biopsy. Although we cannot exclude that some of these recurrences may have started in the perirectal nodes and secondarily infiltrated the rectal wall, they most likely represent residual tumor at the previous excision site.
One of the main concerns with the local excision of rectal cancer is the possibility of leaving metastatic lymph nodes in the mesorectum. The accuracy of ERUS in detecting lymph node metastasis ranges from 60% to 80%, and therefore preoperative staging by ERUS does not eliminate this possibility. However, our results suggest that mural recurrences at the tumor excision site are more common than nodal recurrences.
Most patients with local recurrence underwent salvage surgery with curative intent. Although most patients are disease-free after the second procedure, it is unclear whether delayed radical surgery after failure of local excision is therapeutically equivalent to radical surgery alone performed at the time of initial diagnosis. Follow-up after salvage surgery was relatively short in this series, and evaluation of long-term outcome is needed to address this question. However, our disease-free survival rate compares favorably with that reported in two recent studies of patients with early rectal cancer treated by radical surgery. Although each of these studies reported a 12% local recurrence rate for stage I rectal cancers treated by radical resection 6,24 (half the recurrence rate found in our patients), their actuarial 5-year cancer-specific survival rates of 88% and 90% did not differ significantly from that found in our series of local excision.
For the preoperative staging of rectal cancer, ERUS has become increasingly accepted as the method of choice. The aim of this study was not to determine the accuracy of ERUS in the staging of rectal cancer, because patients were selected based on the pathology stage. However, our results demonstrated the difficulty in distinguishing between tumors limited to the submucosa (T1) and tumors invading the muscularis propria (T2). ERUS is more accurate in distinguishing tumors localized to the rectal wall, potential candidates for local excision, from tumors penetrating the perirectal fat, which require radical resection and adjuvant chemoradiation.
The ability to detect local recurrences early may be one of the requirements for a good long-term outcome after local excision of rectal cancer. In our series, most of the recurrences involved the mucosa and were diagnosed by proctoscopy and biopsy. However, a diagnostic modality that can image the perirectal tissue is required to diagnose nodal recurrences. Evidence in the literature suggests that ERUS may be useful in the early detection of locally recurrent rectal cancer. 25 We therefore designed a follow-up protocol in which proctoscopic examination and ERUS are done every 4 months for 3 years after surgery, and every 6 months for an additional 2 years. Half of the local recurrences were diagnosed at the scheduled follow-up visit, and all of the patients underwent radical surgery. Although these data are preliminary and inconclusive, we believe they support the need for careful periodic proctoscopy and ERUS follow-up after local excision to detect recurrence at the earliest possible time.
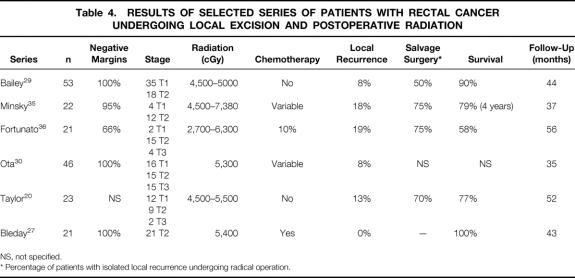
Several authors have tried to extrapolate the beneficial effects of chemoradiation therapy on local recurrence of rectal cancers treated by radical surgery compared with patients treated by local excision. Similar to reports of local excision alone, most of these series are retrospective and heterogeneous in terms of patient selection, tumor stage and histology, type of excision performed, details of adjuvant therapy, and length of follow-up (Table 4) . 26–36 Recurrence rates ranged from 0% to 24% and overall survival rates from 58% to 100%. Two studies in which patients were followed up prospectively 27,30 reported local recurrence rates of 8% with disease-free survival rates of 96% and 86% at 40 and 36 months of follow-up. However, both these series included patients with T3 tumors, which represent the majority of local recurrences. In the combined experience of these series, only one local recurrence occurred in the 36 T2 patients (3%) treated by local excision with adjuvant chemoradiation therapy. These results suggest that postoperative chemoradiation therapy reduces the incidence of local recurrence after local excision of T2 rectal cancers. Longer follow-up will be necessary to determine the long-term outcome.
Table 4. RESULTS OF SELECTED SERIES OF PATIENTS WITH RECTAL CANCER UNDERGOING LOCAL EXCISION AND POSTOPERATIVE RADIATION
NS, not specified.
* Percentage of patients with isolated local recurrence undergoing radical operation.
A prospective multiinstitutional trial to evaluate the efficacy of local excision and postoperative chemoradiation was initiated by the Cancer and Leukemia Group B in 1989. 37 The enrolled patients had mobile uT1-uT2 N0 M0 cancers that were less than 4 cm in diameter, involved less than 40% of the circumference of the rectum, and were located within 10 cm of the anal verge. The trial closed in 1995 with 180 patients accrued. Of the 164 patients who underwent full-thickness local excision, 48 were ineligible because of involved margins, size larger than 4 cm, or stage higher than T2 or lower than T1. Sixty T1 patients were treated by local excision alone, and 52 T2 patients were treated by full-thickness local excision followed by chemoradiation. After 24 months of follow-up, four patients had died of malignant disease. Two patients with isolated local recurrence who underwent rescue radical surgery were alive at the time of the study report, bringing the local recurrence rate to 5.3%. Although the follow-up was short, the preliminary results of this study were better than average, particularly given that it included patients with unfavorable histology.
Decreased rates of death and complications and improved functional outcomes are the hypothetical advantages of local excision over radical surgery for rectal cancer. However, disease-free survival and overall survival rates remain the main criteria by which this surgical treatment of rectal cancer must be evaluated. Local excision of early rectal cancer, even in the ideal candidate, is followed by a much higher recurrence rate than previously reported. Although most patients with local recurrence can be salvaged by radical resection, the long-term outcome remains unknown. Although our results demonstrate 5-year survival rates after local excision similar to those reported with radical surgery, we find our local recurrence rates of 18% for T1 tumors and 37% for T2 tumors alarming. At present, it seems imprudent to excise T2 rectal cancers locally without adjuvant chemoradiation. Although a randomized controlled trial would be the ideal method by which to compare radical surgery with local excision plus adjuvant chemotherapy, the similar 5-year survival rates observed using these two therapies suggests that a very large trial, perhaps too large to be practical, would be necessary to prove a difference in results.
Acknowledgments
The authors thank Deb Caldwell for data collection and management, Robin L. Bliss for statistical analysis, and Molly Schultz for editorial and administrative support.
Footnotes
Correspondence: Julio Garcia-Aguilar, MD, Division of Colon and Rectal Surgery, Box 450 Mayo Bldg., 420 Delaware St. SE, Minneapolis, MN 55455.
Accepted for publication August 24, 1999.
References
- 1.MacFarlane JK, Ryall RDH, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; 341:457–460. [DOI] [PubMed] [Google Scholar]
- 2.Enker WE, Havenga K, Polyak T, Thaler H, Cranor M. Abdominoperineal resection via total mesorectal excision and autonomic nerve preservation for low rectal cancer. World J Surg 1997; 21:715–720. [DOI] [PubMed] [Google Scholar]
- 3.Biggers OR, Beart RW, Ilstrup DW. Local excision of rectal cancer. Dis Colon Rectum 1986; 29:374–377. [DOI] [PubMed] [Google Scholar]
- 4.Morson BC. Factors influencing the prognosis of early cancer of the rectum. Proc R Soc Med 1966; 59:607–608. [PMC free article] [PubMed] [Google Scholar]
- 5.Longo WE, Virgo KS, Johnson FE, et al. Outcome after proctectomy for rectal cancer in Department of Veterans Affairs Hospitals. Ann Surg 1998; 228:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg D, Paty PB, Picon AI, et al. Stage I rectal cancer: identification of high-risk patients. J Am Coll Surg 1998; 186:574–580. [DOI] [PubMed] [Google Scholar]
- 7.Neal DE, Williams NS, Johnston D. A prospective study of bladder function before and after sphincter-saving resections for low carcinoma of the rectum. Br J Urol 1981; 53:558–564. [DOI] [PubMed] [Google Scholar]
- 8.Williams JT, Slack WW. A prospective study of sexual function after major colorectal surgery. Br J Surg 1980; 67:772–774. [DOI] [PubMed] [Google Scholar]
- 9.Havenga K, Enker WE, McDermott K, Cohen AM, Minsky BD, Guillem J. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg 1996; 182:495–502. [PubMed] [Google Scholar]
- 10.Karnajia ND, Schache DJ, Heald RJ. Function of the distal rectum after low anterior resection for carcinoma. Br J Surg 1992; 79:114–116. [DOI] [PubMed] [Google Scholar]
- 11.Lewis WG, Holdsworth PJ, Stephenson BM, Finan PJ, Johnston D. Role of the rectum on the physiological and clinical results of coloanal and colorectal anastomosis after anterior resection for rectal carcinoma. Br J Surg 1992; 79:1082–1086. [DOI] [PubMed] [Google Scholar]
- 12.Killingback M. Local excision of carcinoma of the rectum: indications. World J Surg 1992; 16:437–446. [DOI] [PubMed] [Google Scholar]
- 13.Heimann TS, Oh C, Steinhagen RM, Greenstein AJ, Perez C, Aufses AH. Surgical treatment of tumors of the distal rectum with sphincter preservation. Ann Surg 1992; 216:432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Cosse JJ, Wond RJ, Quan SHQ, Friedman NB, Sternbers SS. Conservative treatment of distal rectal cancer by local excision. Cancer 1989; 63:219–223. [DOI] [PubMed] [Google Scholar]
- 15.Morson BC, Bussey HJR, Samoorian S. Policy of local excision for early rectal cancer of the colorectum. Gut 1977; 18:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lock MR, Ritchie JK, Hawley PR. Reappraisal of radical local excision for carcinoma of the rectum. Br J Surg 1993; 80:928–929. [DOI] [PubMed] [Google Scholar]
- 17.Read DR, Sokil S, Ruiz-Salas G. Transanal local excision of rectal cancer. Int J Colorect Dis 1995; 10:73–76. [DOI] [PubMed] [Google Scholar]
- 18.Hager TH, Gall FP, Hermanek P. Local excision of cancer of the rectum. Dis Colon Rectum 1983; 26:149–151. [DOI] [PubMed] [Google Scholar]
- 19.Obrand DI, Gordon PH. Results of local excision for rectal carcinoma. Can J Surg 1996; 39:463–468. [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg 1998; 175:360–363. [DOI] [PubMed] [Google Scholar]
- 21.Zenni GC, Abraham K, Harford FJ, Potocki DM, Herman C, Dobrin PB. Characteristics of rectal carcinomas that predict the presence of lymph node metastases: implications for patient selection for local therapy. J Surg Oncol 1998; 67:99–103. [DOI] [PubMed] [Google Scholar]
- 22.Brodsky JT, Richard GK, Cohen AM, Minsky BD. Variables correlated with the risk of lymph node metastasis in early rectal cancer. Cancer 1992; 69:322–326. [DOI] [PubMed] [Google Scholar]
- 23.Sitzler PJ, Seow-Choen F, Ho YH, Leong APK. Lymph node involvement and tumor depth in rectal cancers: an analysis of 805 patients. Dis Colon Rectum 1997; 40:1472–1476. [DOI] [PubMed] [Google Scholar]
- 24.Zaheer S, Pemberton JH, Farouk R, Dozois RR, Wolff BG, Ilstrup D. Surgical treatment of adenocarcinoma of the rectum. Ann Surg 1998; 227:800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez JM, McC Mortensen NJ, Takeuchi N, Smilgin Humphreys MM. Endoluminal ultrasonography in the follow-up of patients with rectal cancer. Br J Surg 1994; 81:692–694. [DOI] [PubMed] [Google Scholar]
- 26.Willet CG, Compton CC, Shellito PC, Efrid JT. Selection factors for local excision or abdominoperineal resection of early stage rectal cancer. Cancer 1994; 73:2716–2720. [DOI] [PubMed] [Google Scholar]
- 27.Bleday R, Breen E, Jessup JM, Burgess A, Sentovich SM, Steele G. Prospective evaluation of local excision for small rectal cancers. Dis Colon Rectum 1997; 40:388–392. [DOI] [PubMed] [Google Scholar]
- 28.Mendenhall WM, Rout WR, Vauthey JN, Haigh LS, Zlotecki RA, Copeland EM. Conservative treatment of rectal adenocarcinomas with endocavitary irradiation or wide local excision and postoperative irradiation. J Clin Oncol 1997; 15:3241–3248. [DOI] [PubMed] [Google Scholar]
- 29.Bailey HR, Huval WV, Max E, Smith KW, Butts DR, Zamora LF. Local excision of carcinoma of the rectum for cure. Surgery 1992; 111:555–561. [PubMed] [Google Scholar]
- 30.Ota DM, Skibber J, Rich TA. MD Anderson Cancer Center experience with local excision and multimodality therapy for rectal cancer. Surg Clin North Am 1992; 1:147–152. [Google Scholar]
- 31.Coco C, Magistrelli P, Granone P, Roncolini G, Picciocchi A. Conservative surgery for early cancer of the distal rectum. Dis Colon Rectum 1992; 35:131–136. [DOI] [PubMed] [Google Scholar]
- 32.Rouanet P, Saint Aubert B, Fabre JM, et al. Conservative treatment for low rectal carcinoma by local excision with or without radiotherapy. Br J Surg 1993; 80:1452–1456. [DOI] [PubMed] [Google Scholar]
- 33.Wong CS, Stern H, Cummings BJ. Local excision and post-operative radiation therapy for rectal carcinoma. Int J Radiat Oncol 1993; 25:669–675. [DOI] [PubMed] [Google Scholar]
- 34.Graham RA, Atkins MB, Karp DD, Wazer DE, Hackford AW. Local excision of rectal carcinoma: early results with combined chemoradiation therapy using 5-fluorouracil and leucovorin. Dis Colon Rectum 1994; 37:308–312. [DOI] [PubMed] [Google Scholar]
- 35.Minsky BD, Enker WE, Cohen AM, Lauwers G. Local excision and postoperative radiation therapy for rectal cancer. Am J Clin Oncol 1994; 17:411–416. [DOI] [PubMed] [Google Scholar]
- 36.Fortunato L, Ahmad NR, Yeung RS, et al. Long-term follow-up of local excision and radiation therapy for invasive rectal cancer. Dis Colon Rectum 1995; 38:1193–1199. [DOI] [PubMed] [Google Scholar]
- 37.Weber TK, Petrelli NJ. Local excision for rectal cancer; an uncertain future. Oncology 1998; 12;933–943. [PubMed] [Google Scholar]