Abstract
Objective
To investigate Fas and FasL expression in pancreatic tissues and cultured pancreatic cancer cell lines, and to assess the ability of anti-Fas antibodies to induce apoptosis.
Summary Background Data
Activation of the Fas receptor by Fas-ligand (FasL) results in apoptosis, and dysregulation of this pathway may contribute to abnormal cell proliferation.
Methods
Northern blotting and immunohistochemistry were used to compare Fas and FasL expression in normal and cancerous tissues. DNA 3′-OH end labeling was used to detect apoptotic cells. The effects of Fas activation on cell growth and signaling pathways were investigated in culture.
Results
Pancreatic cancers exhibited increased Fas RNA levels, whereas FasL mRNA levels were similar in both groups. Despite the colocalization of Fas and FasL in the cancer cells, an apoptotic signal was present in approximately 10% of these cells in only 2 of 16 cancer samples. Fas and FasL were coexpressed in all four cell lines, whereas Fas-associated phosphatase 1 was below the level of detection in all cell lines. Only COLO-357 cells underwent apoptosis after Fas activation. Apoptosis was associated with enhanced activation of jun kinase (JNK) and p38 mitogen-activated protein kinase (MAPK). In the presence of actinomycin D, Fas antibody also induced apoptosis in the other three cell lines.
Conclusions
These results suggest that pancreatic cancer cells are resistant to Fas-mediated apoptosis by mechanisms excluding receptor downregulation or Fas-associated phosphatase upregulation and raise the possibility that Fas-mediated apoptosis may be dependent on the activation of the JNK/p38 MAPK pathway in these cells.
Fas (Apo-1/CD95) is a 45-kDa type I membrane protein, and Fas-ligand (FasL) is a 37- to 40-kDa type II membrane protein that belong to the tumor necrosis factor receptor and ligand families. 1,2 Activation of Fas by certain anti-Fas antibodies or by FasL results in apoptotic cell death in susceptible cells. 2,3 Fas–FasL interactions constitute one of the main systems mediating the cytotoxicity of T cells and regulating immune responses, tissue development, and homeostasis. 4 Attenuation of the Fas pathways may cause lymphoproliferative disorders and may accelerate autoimmune diseases, whereas excessive activation of the Fas–FasL system may contribute to pathologic tissue destruction. 4,5 Fas has also been implicated in other functions, such as protection of immune privileged tissues and disposal of cells undergoing genomic alterations. 5 Recent studies have suggested that resistance to apoptosis with loss of Fas function may play an important role in the pathogenesis of several malignancies, including colon and pancreatic cancer. 6–11
Pancreatic ductal adenocarcinoma is a devastating disease with a poor prognosis. 12 It is characterized histologically by the presence of ductal-like cancer cells, extensive stroma formation, and foci of degenerating acinar cells and proliferating small duct cells. 13 Several observations have pointed to the important role of growth factors, oncogenes, and tumor suppressor genes in its pathobiology. Thus, many of these cancers harbor K-ras oncogene 14 and p5315 and DPC416 tumor suppressor gene mutations and frequently overexpress multiple growth factors and their receptors. 17 A recent study demonstrated that cultured human pancreatic cancer cell lines express Fas and FasL but are resistant to Fas-mediated apoptosis. 11 This resistance was said to correlate with the expression of FAP-1, a Fas-associated phosphatase that can block the apoptotic function of Fas. 11
Despite the potential importance of Fas and FasL in the pathobiology of pancreatic cancer, nothing is currently known about Fas and FasL expression in relation to apoptosis in pancreatic cancer tissues. Therefore, in this study we characterized Fas and FasL expression in relation to the presence of apoptotic cells in pancreatic cancer samples. We also investigated the potential of the Fas–FasL system to induce apoptosis in four cultured human pancreatic cancer cell lines. We found that Fas was overexpressed in human pancreatic cancer tissues, where it colocalized in the cancer cells with FasL, but was related with apoptosis in only 2 of 16 cancer samples; that activation of Fas failed to induce apoptosis in most but not all pancreatic cancer cell lines; and that Fas antibody-mediated apoptosis in culture was enhanced in the presence of actinomycin D (actD).
MATERIALS, PATIENTS, AND METHODS
Materials
The following were purchased: ASPC-1, CAPAN-1, and MIA PaCa-2 human pancreatic cancer cells from ATCC (Rockville, MD); human multitissue Northern blot from Clontech (Palo Alto, CA); enhanced chemoluminescence substrate from Pierce (Rockford, IL); p38 MAP Kinase Assay kit and SAPK/JNK Assay kit from New England Biolabs Inc. (Beverly, MA); mouse monoclonal antibodies against human Fas and recombinant human Fas/Fc chimera from R&D Systems Inc. (Minneapolis, MN); anti-CD5 control antibodies from Oncogene Science Inc. (Uniondale, NY); ApopTag in situ apoptosis detection kit from Oncor, Inc. (Gaithersburg, MD); rabbit polyclonal antibodies against FasL from Santa Cruz (Santa Cruz, CA); actD and all other reagents from Sigma Chemical (St. Louis, MO); and mouse monoclonal antibodies against Fas and FasL from Transduction Laboratories (Lexington, KY). COLO-357 human pancreatic cancer cells were a gift from R. Metzgar at Duke University (Durham, NC), and human Fas and FasL cDNAs were a gift from Dr. N. Dean at Isis Pharmaceuticals (Carlsbad, CA).
Patients and Tissue Samples
Normal human pancreatic tissue samples were obtained through a donor program. There were six female and eight male organ donors with a median age of 26 years (range 2–54). Pancreatic adenocarcinoma tissues were obtained from 10 women and 6 men with a median age of 64 years (range 44–77). All patients underwent surgery for pancreatic cancer as recently described. 18 Samples were either immediately frozen on surgical removal in liquid nitrogen and stored at −80°C until RNA extraction or fixed in Bouin’s solution for 18 to 20 hours and embedded in paraffin for histologic analysis. Studies involving human tissues were approved by the Ethics Committee of the University of Ulm, Germany, and the Human Subjects Committee of the University of California, Irvine.
Northern Blot Analysis
Northern blotting was carried out as previously described. 18 Total RNA or poly(A)+ RNA was size-fractionated, electrotransferred to nylon membranes, and hybridized under high stringency conditions with a 0.7-kb Bgl2/EcoR1 fragment of the human Fas cDNA (Genbank accession: E05110), a 0.6-kb EcoR1 fragment of the human FasL cDNA (Genbank accession: D38122), a 493-bp EcoR1/BamH1 fragment of the human FAP-1 cDNA (Genbank accession: L34583), or a 659-bp EcoR1/BamH1 fragment of the human Daxx cDNA (Genbank accession: AF 039136). cDNAs were labeled with [α-32P]dCTP (3,000 Ci/mmol) by random priming before hybridization. A mouse 7S cDNA and a human β-actin cDNA, respectively, were used as loading control.
The 493-bp FAP-1 cDNA fragment, corresponding to nt 477 to 969 of the human FAP-1 cDNA sequence, 19 and the 659-bp Daxx cDNA fragment, corresponding to nt 762 to 1421 of the human Daxx cDNA sequence, 20 were generated by reverse transcriptase–polymerase chain reaction from human placenta RNA. The primers used for the cDNA preparations contained a EcoR1 and BamH1 site, respectively, attached to the 5′-end preceded by a 3-bp overhang: Sense FAP-1, 5′-AGT-GGATCC-GCA-AGCTGTGGAAACACTGA; antisense FAP-1, 5′-GTA-GAATTC-AATTTCCGGTAGCAC-ACCAG; sense Daxx, 5′-AGT-gGATCC-gCAGGA-aAAGGAGTTGGATC; antisense Daxx, 5′-GTA-gAATTC-tGGGATGCCATTCCAATAGG. The fragments were subcloned into pBSK and their authenticity was confirmed by sequencing.
Immunohistochemistry
Paraffin-embedded 4-μm tissue sections were immunostained using the streptavidin–peroxidase technique as previously described. 18 After deparaffinization and blocking endogenous peroxidase activity, the sections were incubated for 15 minutes at 23°C with 10% normal goat serum and for 16 hours at 4°C with the mouse monoclonal antibodies (0.5 μg/mL) against Fas 21,22 and rabbit polyclonal antibodies (0.2 μg/mL) against FasL 6,7,23 that were also used for immunoblotting. Bound antibodies were detected with biotinylated goat antimouse IgG secondary antibodies and goat antirabbit IgG antibodies for Fas and FasL, respectively, and streptavidin–peroxidase complex, using diaminobenzidine tetrahydrochloride as the substrate. Sections were counterstained with Mayer’s hematoxylin. Omission of primary antibodies or incubation in the presence of nonimmunized mouse or rabbit IgG instead of primary antibodies did not yield any immunoreactivity.
In Situ Apoptosis Detection
To identify individual apoptotic cells in tissue sections and cultured cells, the ApopTag in situ apoptosis detection kit that specifically recognizes 3′-OH DNA ends generated by DNA fragmentation was used according to the manufacturer’s directions, as previously described. 24 Briefly, microscope slides of deparaffinized and proteinase K-digested tissue sections or dried and formalin-fixed cultured cells (107/mL) were incubated for 60 minutes at 37°C with terminal deoxynucleotidyl transferase (TdT) enzyme and digoxigenin-dUTP after blocking of endogenous peroxidase activity. Digoxigenin residues catalytically bound to the DNA 3′-OH ends by TdT were detected by antidigoxigenin antibodies conjugated with peroxidase, using diaminobenzidine tetrahydrochloride as the substrate. Sections were counterstained with methyl green. Omission of TdT enzyme did not yield any immunoreactivity. Prostate tissue sections from castrated rats (a gift from Dr. G. Asano at Nippon Medical School, Tokyo, Japan) were used as a positive control. 25
Cell Culture and Growth Assay
ASPC-1 and CAPAN-1 cells were grown in RPMI medium and COLO-357 and MIA PaCa-2 cells in DME medium at 37°C in humidified air with 5% CO2. Media were supplemented with penicillin G (100 U/mL), streptomycin (100 μg/mL), and 8% fetal bovine serum. Cell growth was determined by the 3-(4,5-methylthiazol-2-yl)-2,5-diaphenyltertrazolium bromide (MTT) colorimetric assay as previously described. 18,26 Indicated cells were seeded in 96-well plates and incubated for 24 hours before incubation for 48 hours in the absence or presence of the indicated substrates. The assay was initiated by adding MTT solution to each well at a final concentration of 0.625 μg MTT/mL medium. After 4 hours, the optical density was determined with an enzyme-linked immunosorbent assay plate reader after removal of the medium and dissolving of the dye crystals in acidified isopropanol.
Immunoblot Analysis
Cells were washed twice with ice-cold phosphate-buffered saline and lysed in buffer containing 125 mmol/L Tris (pH 6.8) and 1% sodium dodecyl sulfate (SDS). 27 Cell lysates were subjected to 12% SDS-PAGE and electrotransferred to immobilon-P membranes. After blocking, the membranes were blotted at 23°C for 90 minutes with the indicated primary antibodies and for 60 minutes with corresponding secondary horseradish-conjugated secondary antibodies. Membranes were washed five times in Tris-buffered saline containing 1% Tween-20 for 5 minutes after each incubation step. Bound antibodies were visualized using enhanced chemoluminescence.
Kinase Activity Assays
ERK-1 and ERK-2 (p42/p44) mitogen-activated protein kinase (MAPK) activity was determined as previously described. 27
p38 MAPK activity was determined according to the protocol of the manufacturer. In brief, p38 MAPK was immunoprecipitated with a specific p38 MAPK antibody in whole cell lysates (200 μg/200 μL) and then incubated in kinase buffer containing cold adenosine triphosphate and an ATF-2 fusion protein as substrate. Phosphorylation of ATF-2 at thyrosine 71 by p38 MAPK was then determined by immunoblotting using a phosphospecific ATF-2 antibody.
Jun kinase (JNK) or stress-activated protein kinase (SAPK) activity was determined according to the protocol of the manufacturer. In brief, JNK was precipitated in whole cell lysates (200 μg/200 μL) using an N-terminal c-Jun fusion protein bound to glutathione sepharose and then incubated in kinase buffer containing cold ATP. Phosphorylation of c-Jun was determined at serine 63 by immunoblotting using a phosphospecific c-Jun antibody.
Statistics
Statistical analysis was performed with SigmaStat software (Jandel Scientific, San Raphael, CA). The rank-sum test and Student t test were used when indicated (two-sided). P < .05 was taken as the level of significance.
RESULTS
Northern Blot Analysis in Human Pancreatic Tissues
Northern blot analysis using the human Fas and FasL cDNAs revealed the presence of variable levels of a 3.0-kb Fas mRNA transcript 1 and of a 1.8-kb FasL mRNA transcript 2 in normal pancreatic and in pancreatic cancer samples (Fig. 1). In some of the samples, an additional 1.6-kb FasL mRNA transcript was present, in accord with previous reports of several FasL mRNA transcripts. 2,26 Overall, the Fas mRNA transcript was present in 7 of 14 normal and in 10 of 16 cancer samples; the major FasL mRNA transcript was present in 13 of 14 normal pancreatic samples and in 15 of 16 pancreatic cancer samples. Densitometric analysis of the autoradiographs with normalization to 7S indicated that the median levels of Fas mRNA were significantly increased by 3.6-fold in the cancer samples versus the median level in the normal samples (P = .0057), whereas the median FasL mRNA levels did not differ significantly (Fig. 2). Fas mRNA levels varied maximally 9.1-fold among the normal samples but 86-fold among the cancer samples, indicating that there were large variations in Fas expression in pancreatic cancer. In contrast, FasL mRNA levels varied 5.9-fold among normal samples and 7.7-fold among cancer samples.
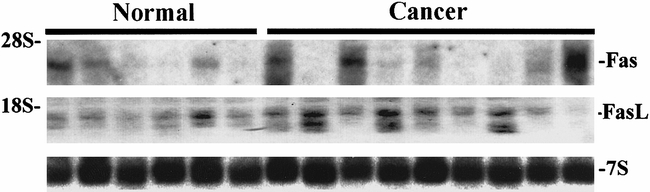
Figure 1. Fas and Fas-ligand (FasL) mRNA expression in human pancreatic tissues. For Northern blot analysis of six normal and nine cancerous samples, total RNA (20 μg/lane) was size-fractionated and electrotransferred onto nylon membranes. After prehybridization and hybridization with [α-P32]dCTP random-primed labeled human Fas and FasL cDNAs (250,000 cpm/mL) and a mouse 7S cDNA (50,000 cpm/mL), membranes were washed under high stringency conditions and exposed at −80°C to Kodak BioMax MS films (Fas, 1 day of exposure; FasL, 3 days of exposure; 7S, 2 hours of exposure). The rRNA markers are indicated on the left.
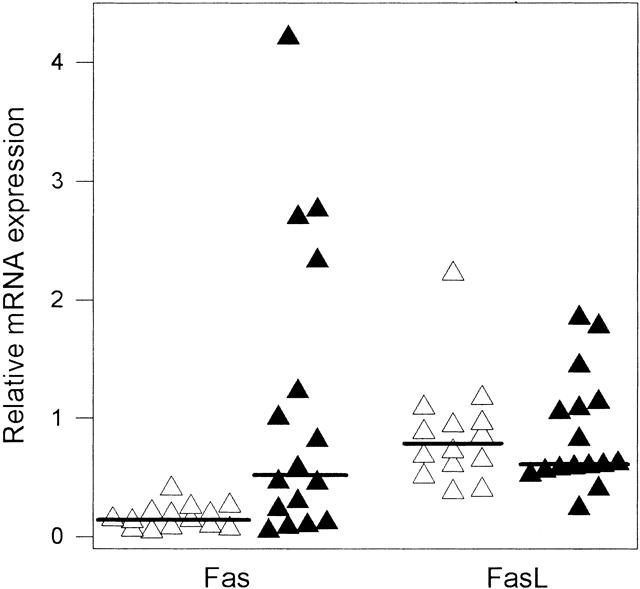
Figure 2. Relative Fas and Fas-ligand (Fas-L) mRNA expression in normal pancreatic samples (open triangles, n = 14) and cancerous pancreatic samples (closed triangles, n = 16). Autoradiographs of Northern blots for Fas, FasL, and 7S were analyzed by densitometry and the level of Fas and FasL expression was calculated in relation to 7S. The median scores (indicated as lines) for Fas in normal and cancerous samples differed significantly (P = .0057).
Immunohistochemistry
To determine the localization of Fas and FasL in the pancreatic tissues, immunohistochemical staining with highly specific antibodies 6,7,21–23 was performed next. In the normal pancreas, faint to moderate Fas and FasL immunoreactivity was present in many ductal cells. Analysis of serial sections indicated that Fas and FasL often colocalized in the ductal cells (Fig. 3). However, although Fas predominantly displayed a membranous staining pattern and was present at low levels in the cytoplasm, FasL was present mainly in the cytoplasm. Faint Fas immunoreactivity was also present in many acinar cells, often exhibiting a membranous distribution. In addition, moderate FasL immunoreactivity was evident in a cytoplasmic pattern in the acinar and islet cells. Analysis of serial sections using DNA 3′-OH end labeling by TdT enzyme indicated that apoptosis was not detectable in the normal pancreas.
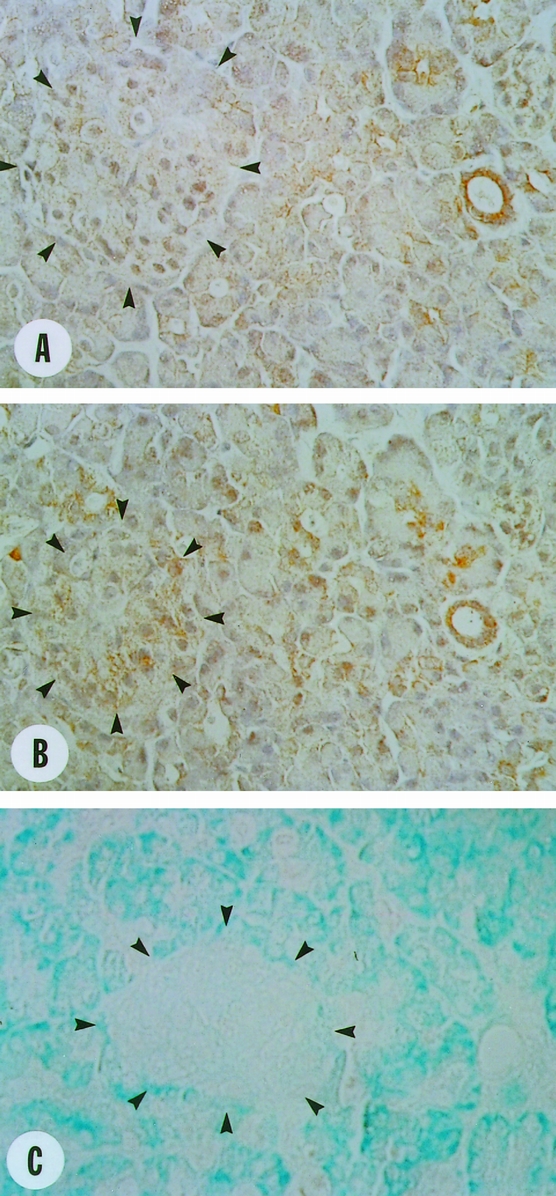
Figure 3. Localization of Fas and Fas-ligand (Fas-L) in the normal pancreas. (A) Faint to moderate membranous and faint cytoplasmic Fas immunoreactivity was observed in many ductal cells and some acinar cells and islet cells (arrowheads). (B) Moderate cytoplasmic and faint membranous FasL immunoreactivity was observed in many ductal cells and some acinar cells and islet cells (arrowheads). (C) Analysis of serial sections revealed that DNA 3′-OH end labeling reactivity was not present in normal acinar and ductal cells. Original magnification ×400.
In the pancreatic cancers, moderate to strong Fas and FasL immunoreactivity was present in many of the ductal-like cancer cells (Fig. 4). Fas and FasL often colocalized in the same cancer cells; in sharp contrast to the findings in the normal pancreas, they often exhibited both cytoplasmic and membranous patterns. Despite the colocalization of Fas and FasL in the cancer cells, analysis of serial sections using DNA 3′-OH end labeling indicated that most of the cancer cells were not undergoing apoptosis. Apoptosis of cancer cells was detected in only 2 of 16 samples investigated in approximately 10% of the cancer cells.
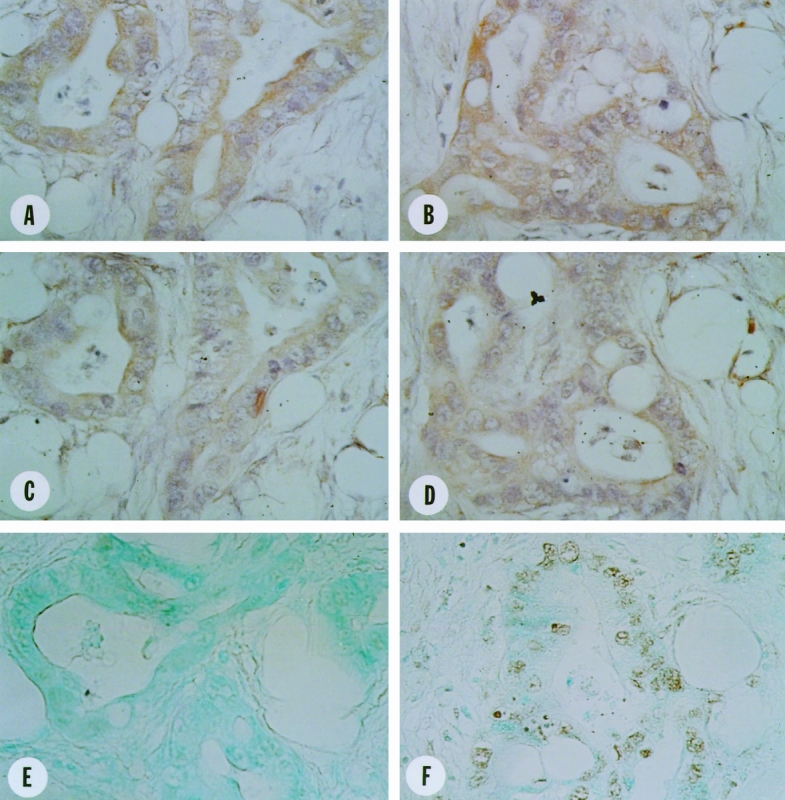
Figure 4. Localization of Fas and Fas-ligand (FasL) in human pancreatic cancer tissues. Moderate to strong Fas (A,B) and FasL (C,D) immunoreactivity was observed in many ductal-like cancer cells. Fas and FasL displayed cytoplasmic and membranous colocalization in the same cancer cells. DNA 3′-OH end labeling reactivity was not present in the cancer cells of most of the cancer tissues (E) but were present in the cancer cells of some cancers (F). Original magnification ×400.
Fas immunoreactivity was only faintly evident in the proliferating ductal cells and atrophic acinar cells in the regions adjacent to the cancer cells (Fig. 5). In contrast, strong FasL immunoreactivity was present in these cells, often exhibiting a distinct granular pattern. Further, both cell types displayed strong DNA 3′-OH end labeling reactivity, which in an analysis of serial sections was especially evident in the cells that coexpressed Fas and FasL. DNA 3′-OH end labeling of prostate tissues from castrated rats was used as a positive control and yielded strong activity.
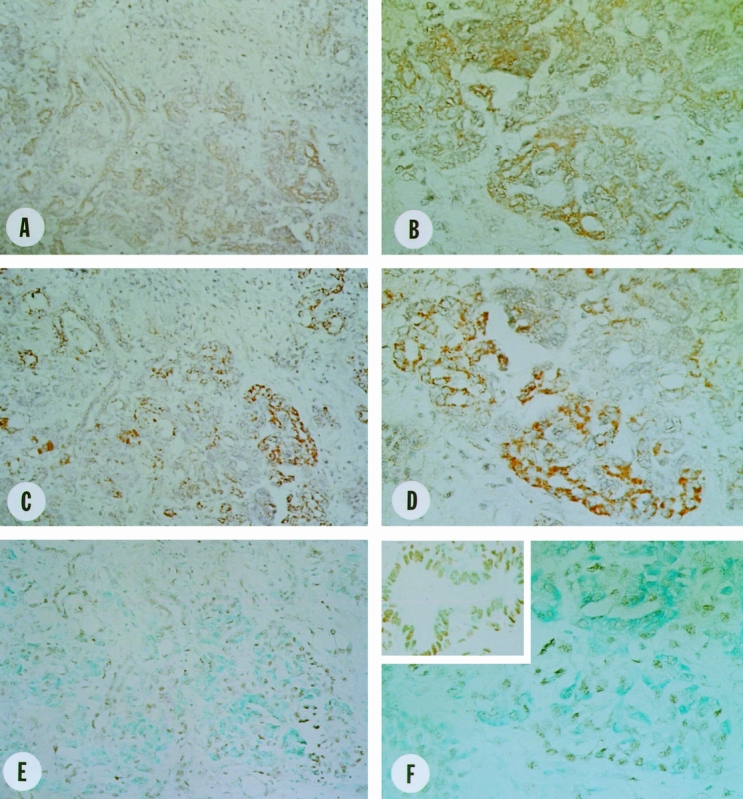
Figure 5. Localization of Fas and Fas-ligand (FasL) in the CP-like lesions of human pancreatic cancer tissues. Faint to moderate membranous Fas (A,B) and strong granular cytoplasmic FasL (C,D) immunoreactivity was observed in many proliferating ductal and atrophic acinar cells. Strong DNA 3′-OH end labeling reactivity was present in the proliferating ductal and atrophic acinar cells (E,F). In the inset in F, castrated rat prostate tissue was used as positive control for DNA 3′-OH end labeling reactivity. Original magnifications: A, C, and E ×200, B, D, and F ×400.
Fas and FasL Expression in Cultured Pancreatic Cells
Fas and FasL expression in ASPC-1, CAPAN-1, COLO-357, and MIA PaCa-2 human pancreatic cancer cells was characterized by immunoblotting using highly specific mouse monoclonal anti-Fas 22 and anti-FasL antibodies 6 and by Northern blotting. A 45-kDa Fas protein and a 3.0-kb Fas mRNA transcript were expressed at various levels in all four pancreatic cancer cell lines (Fig. 6). The highest Fas protein levels were seen in CAPAN-1 cells, whereas the highest Fas mRNA levels were observed in COLO-357 cells, indicating that Fas protein expression did not always correlate with Fas mRNA expression. A 37-kDa FasL protein and a major 1.8-kb and a minor 2.0-kb FasL mRNA transcript were also expressed in all four pancreatic cancer cell lines. FasL protein expression generally correlated with FasL mRNA expression.
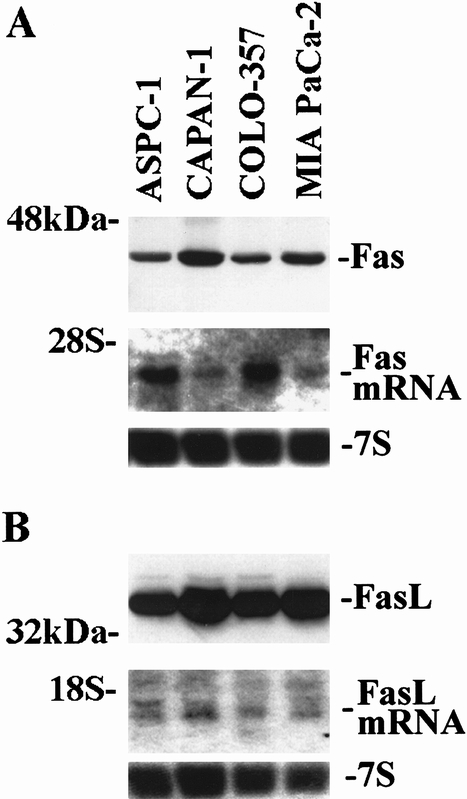
Figure 6. Fas (A) and Fas-ligand (FasL; B) expression in pancreatic cancer cell lines. Immunoblot analysis (upper panels) of total cell lysates (40 μg/lane) was carried out with specific mouse monoclonal antibodies against Fas and FasL (Fas, 30-minute exposures; FasL, 2-minute exposures). Northern blot analysis (middle and lower panels) from total RNA (15 μg/lane) was carried out as described in Figure 1 (Fas, 1 day of exposure; FasL, 2 days of exposure; 7S, 2 hours of exposure).
Effects of Anti-Fas Antibodies on the Growth of Human Pancreatic Cancer Cells
Incubation with increasing concentrations of a mouse monoclonal anti-Fas antibody (up to 10 μg/mL) that can induce apoptosis in susceptible cells 3 did not significantly alter the growth of ASPC-1, CAPAN-1, and MIA PaCa-2 cells (Fig. 7). In contrast, the growth of COLO-357 cells was inhibited by the anti-Fas antibody in a dose-dependent manner, with maximal inhibition of 83% (±2.2% SEM) occurring at 10 μg/mL and an ED50 of 0.07 μg/mL. Under the same conditions, neither 1 μg/mL anti-CD5 control antibody nor 1 μg/mL Fas/Fc chimera altered the growth of any of these cell lines (data not shown).
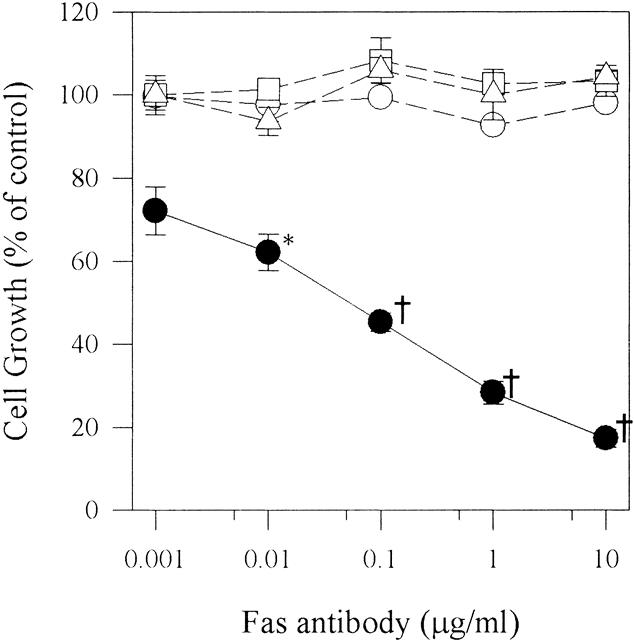
Figure 7. Effect of anti-Fas antibodies on pancreatic cancer cell growth. ASPC-1 (open circles), CAPAN-1 (open squares), COLO-357 (closed circles), and MIA PaCa-2 (open triangles) cells (5,000/well) were plated in 96-well plates and incubated for 24 hours in complete medium and for another 48 hours in complete medium in the absence or presence of increasing concentrations of anti-Fas antibody. Cell growth was then analyzed using the MTT assay. Results are expressed as growth as a percentage of untreated controls and are the means (±SEM) of three separate experiments with quadruplicate determinations of each test point. *P = .0317, †P < .001.
Growth inhibition of COLO-357 cells with 0.1 μg/mL Fas antibody occurred in a time-dependent manner and peaked after 48 hours (Fig. 8). The inhibitory effect was paralleled by the activation of JNK and p38 MAPK. In contrast, ERK-1 and ERK-2 MAPK was not activated in COLO-357 cells in the presence of Fas antibodies (data not shown). Fas antibody was also not able to activate any of the three kinases in the Fas-unresponsive cell lines (data not shown). It has been proposed that Daxx mediates the activation of JNK. 28 Thus, we next determined Daxx mRNA expression by Northern blot analysis using a human Daxx cDNA in the cell lines. Northern blot analysis revealed that a Daxx mRNA transcript was expressed in all cell lines at various levels (Fig. 9).
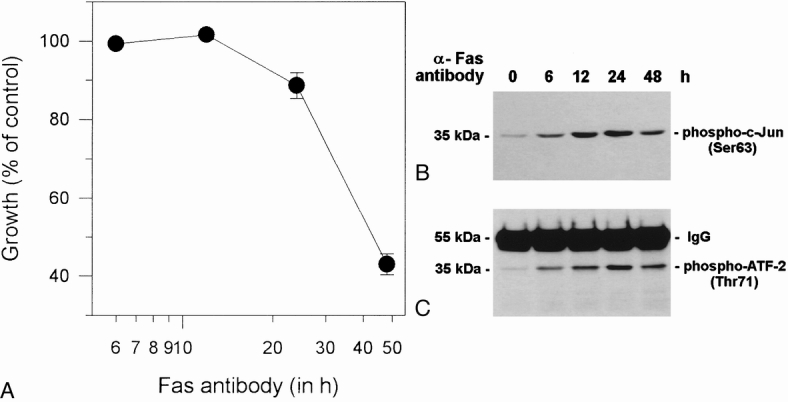
Figure 8. Effect of Fas activation on cell growth, p38 mitogen-activated protein kinase (MAPK), and jun kinase (JNK) in COLO-357 cells. COLO-357 cells were treated with 0.1 μg/mL Fas antibody, as described in Figure 7, for the indicated times. (A) Time course of Fas-induced growth inhibition. The MTT assay was carried out as described in
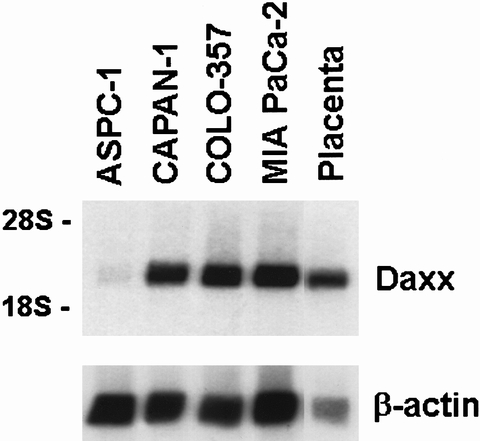
Figure 9. Daxx expression in pancreatic cancer cell lines. Northern blot analysis from poly(A)+ RNA (5 μg/lane) was carried out as described in Figure 1 with a human Daxx cDNA fragment (500,000 CPM/mL) and a human β-actin cDNA (50,000 cpm/mL). Daxx, 12-hour exposure; β-actin, 1-hour exposure.
To confirm specificity of the Fas antibody-induced cell death, cells were incubated with FasL. Similar to the Fas antibody, FasL (100 ng/mL) inhibited the growth of COLO-357 cells by 58.2% (±2.7%), whereas the growth of the other cell lines was not altered (data not shown). Moreover, COLO-357 cells were incubated with anti-Fas antibody in the absence or presence of increasing concentrations of Fas/Fc chimera. The Fas/Fc chimera consist of the extracellular domain of human Fas fused to the Fc region of human IgG1 and can inhibit anti-Fas antibody-induced apoptosis. Anti-Fas antibodies alone (0.1 μg/mL) inhibited the growth of COLO-357 cells by 52% (±5.0%), whereas the presence of the Fas/Fc chimera markedly attenuated the Fas-induced cell death in a dose-dependent manner (Fig. 10). The ED50 of the Fas/Fc chimera to inhibit Fas-mediated cell death was 0.04 μg/mL under the conditions described.
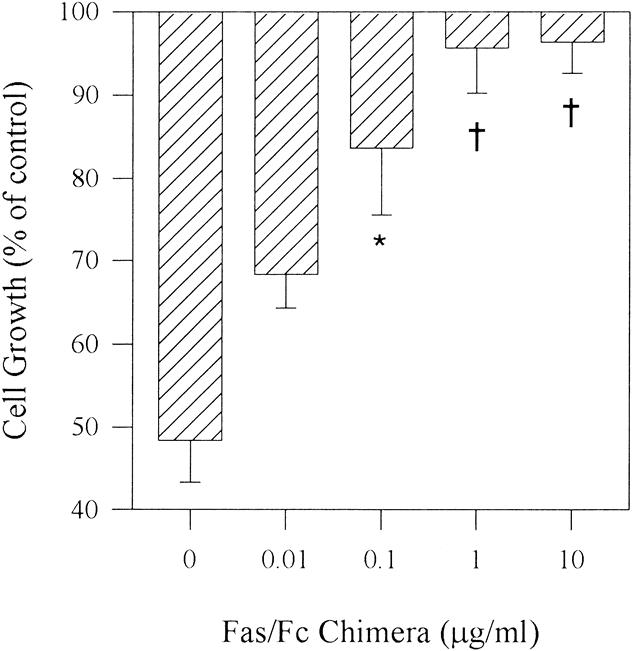
Figure 10. Effect of Fas/Fc chimera on anti-Fas antibody-induced cell death. COLO-357 cells were incubated for 48 hours with or without the anti-Fas antibody (0.1 μg/mL) as described in Figure 7 in the absence or presence of increasing concentrations of Fas/Fc chimera. *P < .003.
Induction of apoptosis in COLO-357 cells by the anti-Fas antibody was confirmed by DNA 3′-OH end labeling. 24 Incubating COLO-357 cells for 24 hours in the presence of the anti-Fas antibody (0.1 μg/mL) resulted in the presence of nuclear DNA 3′-OH reactivity in COLO-357 cells (Fig. 11).
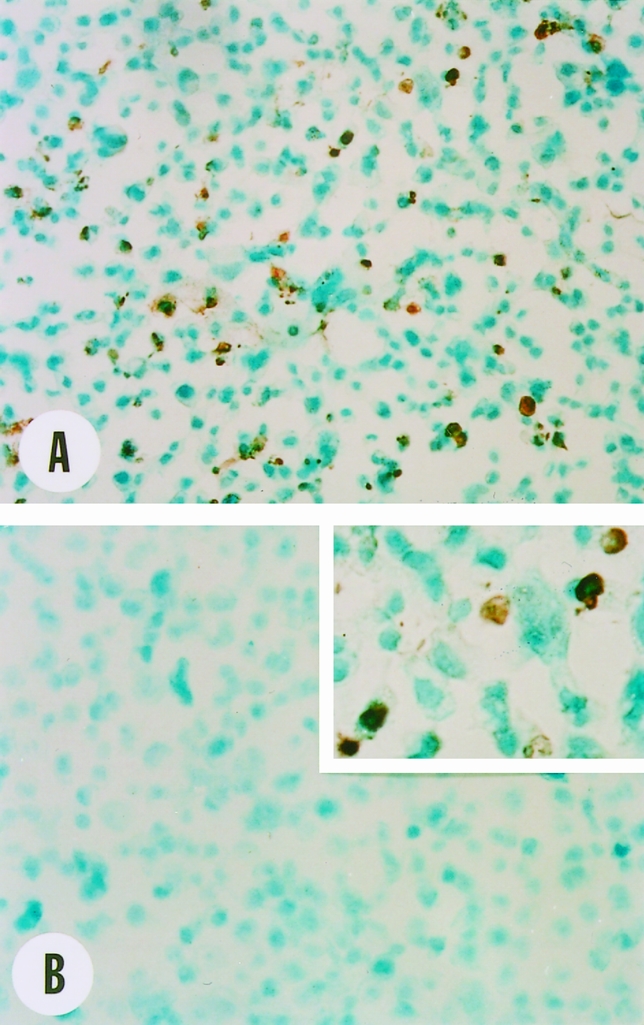
Figure 11. Effect of anti-Fas antibodies on DNA 3′-OH end labeling in COLO-357 cells. COLO-357 cells (5 × 105/well) were seeded in six-well plates and incubated for 24 hours and then for 24 hours in the absence or presence of the anti-Fas antibody (0.1 μg/mL). After collection, cells were fixed, dried onto microscope slides, and subjected to DNA 3′-OH end labeling as described in text. Strong DNA 3′-OH reactivity was present in the nucleus of many cells treated with the anti-Fas antibody (A, B inset). In contrast, DNA 3′-OH reactivity was absent in control cells (B). Original magnifications: ×200, inset ×400.
FAP-1 Expression in Human Pancreatic Cancer Cells
A recent study 11 has suggested that resistance to Fas-induced apoptosis correlated with high FAP-1 mRNA expression in human pancreatic cancer cell lines. By Northern blot analysis of poly(A)+ RNA from human pancreatic cancer cell lines with a human FAP-1 cDNA fragment, 19 FAP-1 mRNA was not detectable in ASPC-1, CAPAN-1, COLO-357, and MIA PaCa-2 cells (data not shown). In contrast, under the same conditions, the 8.5-kb human FAP-1 cDNA fragment was expressed at various levels in human heart, brain, placenta, lung, kidney, and normal pancreas, but not in liver and skeletal muscle (Fig. 12).
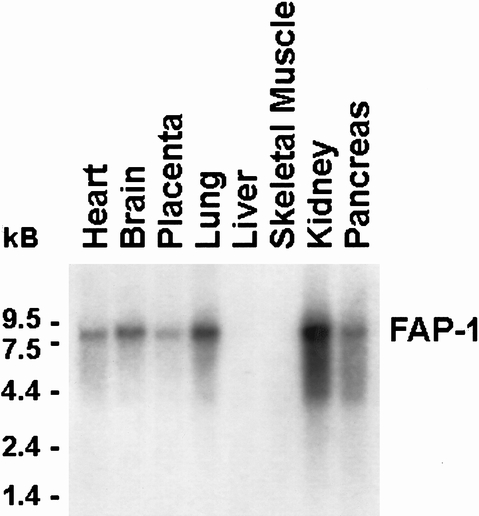
Figure 12. Expression of Fas-associated phosphatase 1 (FAP-1) in various adult human tissues using a multitissue poly(A)+ RNA blot. Northern blot analysis was carried out as described in Figure 1 with a human FAP-1 cDNA fragment (500,000 cpm/mL, exposure 1 day).
Effect of Actinomycin D on Fas-Induced Growth Inhibition
Next, the indicated cell lines were incubated with Fas antibody in the absence or presence of the RNA inhibitor actD. Neither Fas antibody alone (10 μg/mL) nor actD alone (10 ng/mL) significantly altered the growth of ASPC-1 and CAPAN-1 cells (Fig. 13). However, the combination inhibited the growth of ASPC-1 and CAPAN-1 cells by 26% (±5.1%) and 44% (±10.1%), respectively (P < .008). Fas antibody or actD alone inhibited the growth of COLO-357 cells and resulted in additive effects in combination. In MIA PaCa-2, Fas antibody alone did not alter cell growth and actD alone inhibited cell growth by 47% (±8.2%, P < .008), whereas the combination resulted in a significant potentiation of growth inhibition to 72% (±3.6%) compared with actD alone (P = .0231).
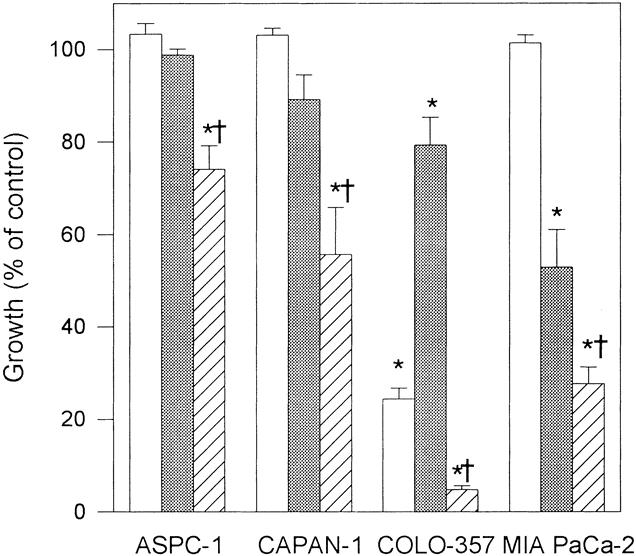
Figure 13. Effect of actinomycin D (actD) on Fas-induced growth inhibition. Indicated cells (5,000/well) were plated in 96-well plates, incubated for 24 hours in complete medium and for another 48 hours in complete medium in the absence or presence of 10 μg/mL anti-Fas antibody alone (open rectangles), 10 ng/mL actD alone (dotted rectangles), or anti-Fas antibody in combination with actD (striped rectangles). Cell growth was then analyzed using the MTT assay. Results are expressed as growth as a percentage of untreated controls and are the means (±SEM) of three separate experiments with quadruplicate determinations of each test point. *P < .008 compared with untreated control, †P < .024 compared with Fas or actD alone.
DISCUSSION
Tissue development is regulated not only by proliferation and differentiation of cells, but also by cell death. Cell death that occurs during tissue turnover is called programmed cell death or apoptosis. 29,30 During apoptosis, the cytoplasm and nucleus condense, get fragmented, and are phagocytosed by adjacent macrophages and granulocytes. 29 This ensures that a cell will not only die, but that the remainder will be rapidly cleared without initiating an inflammatory response. 4 Apoptotic cell death is triggered by various events and also occurs during the death process induced by cytotoxic T cells. 31 The Fas–FasL system is believed to be one of the main apoptotic cell death signaling pathways, 4 and recent evidence suggests that malfunction of the Fas–FasL system may be involved in several malignant diseases. 32–34 Thus, Fas or FasL over- and underexpression have been shown in human lung and colon cancer, renal carcinomas, and several malignant brain tumors. 6–10,35–39
In this study, we demonstrated that Fas was overexpressed in a significant portion of pancreatic cancer samples in comparison with normal pancreatic samples. In contrast, FasL expression, as determined by Northern blotting, was similar in normal and cancerous samples. However, by immunohistochemistry, the ductal-like cancer cells exhibited moderate to strong Fas and FasL immunoreactivity, and the ligand and its receptor often colocalized at the cell membrane and in the cytoplasm, whereas Fas and FasL immunoreactivity was present at low levels in the ductal cells of the normal pancreas. The abundance of Fas protein in the cancer cells is in agreement with the increased expression of Fas mRNA in the pancreatic tumors. Although FasL immunoreactivity was also abundant in these cells in comparison with the normal pancreas, FasL mRNA levels were comparable in the normal and cancer samples. This observation raises the possibility that there is increased recycling or attenuated degradation of FasL protein in pancreatic cancer cells. This may be a consequence of perturbations in regulatory mechanisms that modulate intracellular FasL levels. 32,40
DNA 3′-OH end labeling of serial sections demonstrated that most of the cancer cells did not undergo apoptosis despite expression and colocalization of Fas and FasL. In contrast, colocalization of Fas and FasL in the proliferative ductal and atrophic acinar cells in the chronic pancreatitis-like lesions of the pancreatic cancers was associated with apoptosis. These observations suggest that the regulatory pathways resulting in Fas-mediated apoptosis may be disrupted in most pancreatic cancers. The fact that cultured pancreatic cancer cells also expressed Fas and FasL and that most of them were not susceptible to Fas-mediated cell death supports this hypothesis.
The biochemical and molecular mechanisms that render pancreatic cancer cells resistant to apoptosis mediated by FasL are not known. Our results suggest that the resistance in pancreatic cancer cells was not due to the downregulation of Fas expression, a resistance mechanism that has been observed in other cell systems. 41 It was proposed in a recent study that resistance to Fas-mediated apoptosis in pancreatic cancer cells was due to the overexpression of FAP-1. 11 FAP-1 can block the signal transduction of Fas by interacting with the COOH-terminal three amino acids. 42 However, by Northern blotting, we were not able to detect FAP-1 mRNA expression in pancreatic cancer cell lines, suggesting that FAP-1 may not be a major factor for resistance to Fas-mediated apoptosis.
In other cell systems, several additional mechanisms of Fas resistance have been described. Thus, mutations of the Fas gene in patients with multiple myeloma 43 and in mice with lymphoproliferative disease 4 have been described. Recently, truncated receptors of the tumor necrosis factor receptor family have been described that can participate in receptor formation but subsequently block intracellular death signaling. 44,45 Moreover, overexpression of inhibitors of apoptosis or survival factors, such as NF-κB or Bcl-2, 33 or lack of intracellular apoptotic signal transduction may further contribute to the observed resistance to Fas-dependent apoptosis in pancreatic cancer. This hypothesis is supported by the fact that inhibition of transcription by actD converted susceptibility to Fas-mediated apoptosis in the unresponsive cell lines. In view of the fact that even enucleated susceptible cells undergo apoptosis on Fas activation, 46 our results further suggest that the Fas–FasL system is potentially functional in the resistant cells but is effectively blocked by mechanisms that are dependent on RNA transcription.
Fas engagement with endogenous FasL or FasL presentation by adjacent cells results in the formation of Fas-trimers followed by intracellular recruitment of the Fas-associating proteins FADD or Daxx to the so-called death domain. 4,28,32 The subsequent activation of apoptotic signaling cascades leads to the activation of the caspase cystein protease family that executes cell death. 32 FADD directly activates this cascade by association with FLICE (FADD-like interleukin-1β-converting enzyme), also termed caspase 8. 32 In the case of Daxx, it has been proposed that activation of JNK is involved in the downstream signaling. 28 Our observations indicate that human pancreatic cancer cell lines express Daxx. Further, Fas antibody activated JNK in COLO-357 cells but not in the Fas-resistant cell lines. It is possible, therefore, that Fas-mediated apoptosis in human pancreatic cancer cell lines is mediated by JNK activation.
The role of p38 MAPK activation in Fas-mediated apoptosis has been controversial. It has been demonstrated that activation of Fas results in p38 MAPK activation, but inhibition of p38 MAPK activation did not suppress Fas-induced apoptosis in human white blood cells. 47,48 In other systems, it has been shown by use of specific kinase inhibitors or transfection experiments that p38 MAPK is essential for Fas-mediated apoptosis. 49,50 Nonetheless, we demonstrated that p38 MAPK and JNK were activated in the Fas-responsive cell line but not in the resistant cell lines, and that JNK and p38 MAPK activation correlated with the onset of apoptosis. It is possible, therefore, that JNK and p38 MAPK activation may be important for the induction of apoptosis by the Fas–FasL system in pancreatic cancer cells. However, additional studies need to be carried out to investigate these issues in greater detail.
Recent reports have demonstrated that several tumor cell lines that have become resistant to Fas-induced apoptosis and constitutively express FasL can counterattack cytotoxic T lymphocytes and natural killer cells. 6,39 Therefore, cancer cells that underwent genomic alterations are no longer susceptible to elimination by the activation of the Fas cascade. Together with these findings, our observations suggest that the resistance of cancer cells to Fas-induced apoptosis may provide a growth advantage that not only prevents the cancer cells from undergoing apoptosis, but also enables them to counterattack invading cytotoxic T cells and natural killer cells by expressing FasL. Our findings therefore raise the possibility that restoration of the pathways that activate apoptosis in pancreatic cancer cells may ultimately have a role in the therapy of this disorder.
Acknowledgments
The authors thank Dr. N. Dean at Isis Pharmaceuticals (Carlsbad, CA) for the generous gift of human Fas and FasL cDNAs, Dr. G. Asano at Nippon Medical School (Tokyo, Japan) for providing prostate tissue sections from castrated rats, and Dr. R. Metzgar at Duke University (Durham, NC) for the gift of COLO-357 human pancreatic cancer cells.
Footnotes
Correspondence: M. Korc, MD, Division of Endocrinology, Diabetes and Metabolism, University of California Irvine, Med. Sci. I, C240, Irvine, CA 92697.
Supported by National Institutes of Health Grant CA-40162 (to M. Korc). M. Kornmann was supported in part by a postdoctoral fellowship award (Ko 1716/1-2) from the Deutsche Forschungsgemeinschaft.
Accepted for publication June 7, 1999.
References
- 1.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991; 66:233–243. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993; 75:1169–1178. [DOI] [PubMed] [Google Scholar]
- 3.Cifone MG, De Maria R, Roncaioli P, et al. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med 1994; 180:1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagata N, Golstein P. The Fas death factor. Science 1995; 267:1449–1456. [DOI] [PubMed] [Google Scholar]
- 5.Depraetere V, Golstein P. Fas and other cell death signaling pathways. Sem Immunol 1997; 9:93–107. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996; 184:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiraki X, Tsuji N, Shioda T, et al. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA 1997; 94:6420–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Reyher U, Strater J, Kittstein W, et al. Colon carcinoma cells use different mechanisms to escape CD95-mediated apoptosis. Cancer Res 1998; 58:526–534. [PubMed] [Google Scholar]
- 9.Gutierrez-Steil C, Wrone-Smith T, Sun X, et al. Sunlight-induced basal cell carcinoma tumor cells and ultraviolet-B-irradiated psoriatic plaques express Fas ligand (CD95L). J Clin Invest 1998; 101:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Lorenzo MJ, Gamen S, Etxeberria J, et al. Resistance to apoptosis correlates with a highly proliferative phenotype and loss of Fas and CPP32 (caspase-3) expression in human leukemia cells. Int J Cancer 1998; 75:473–481. [DOI] [PubMed] [Google Scholar]
- 11.Ungefroren H, Voss M, Janssen M, et al. Human pancreatic adenocarcinomas express Fas and Fas ligand yet are resistant to Fas-mediated apoptosis. Cancer Res 1998; 58:1741–1749. [PubMed] [Google Scholar]
- 12.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin 1998; 48:6–29. [DOI] [PubMed] [Google Scholar]
- 13.Oertel JE, Heffess CS, Oertel YC. Pancreas. In: Sternberg SS, ed. Diagnostic Surgical Pathology. New York: Raven; 1989: 1057–1093.
- 14.Almoguera C, Shibata D, Forrester K, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 1988; 53:549–554. [DOI] [PubMed] [Google Scholar]
- 15.Barton CM, Staddon SL, Hughes CM, et al. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer 1991; 64:1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn SA, Schutte M, Hoque ATMS, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996; 271:350–360. [DOI] [PubMed] [Google Scholar]
- 17.Korc M. Role of growth factors in pancreatic cancer. Surg Oncol Clin North Am 1998; 7:25–41. [PubMed] [Google Scholar]
- 18.Kornmann M, Ishiwata T, Beger HG, Korc M. Fibroblast growth factor-5 stimulates mitogenic signaling and is overexpressed in human pancreatic cancer: evidence for autocrine and paracrine actions. Oncogene 1997; 15:1417–1424. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Shinji I, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science 1995; 268:411–415. [DOI] [PubMed] [Google Scholar]
- 20.Kiriakidou M, Driscoll DA, Lopez-Guisa JM, Strauss JF 3d. Cloning and expression of primate Daxx cDNAs and mapping of the human gene to chromosome 6p21.3 in the MHC region. DNA Cell Biol 1997; 16:1289–1298. [DOI] [PubMed] [Google Scholar]
- 21.Rehemutulla A, Hamilton CA, Chinnaiyan AM, Dixit VM. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1). J Biol Chem 1997; 272:25783–25786. [DOI] [PubMed] [Google Scholar]
- 22.Fulda S, Sieverts H, Friesen C, et al. The CD95 (APO-1/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res 1997; 57:3823–3829. [PubMed] [Google Scholar]
- 23.Giordano C, Stassi G, De Maria R, et al. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s Thyroiditis. Science 1997; 275:960–963. [DOI] [PubMed] [Google Scholar]
- 24.Törmänen U, Eerola A-K, Rainio P, et al. Enhanced apoptosis predicts shortened survival in non-small cell lung cancer. Cancer Res 1995; 55:5595–5602. [PubMed] [Google Scholar]
- 25.Wright AS, Thomas LN, Douglas RC, et al. Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J Clin Invest 1996; 98:2558–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng J, Zhou T, Changdan L, et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 1994; 263:1759–1762. [DOI] [PubMed] [Google Scholar]
- 27.Kornmann M, Arber N, Korc M. Inhibition of basal and mitogen-stimulated pancreatic cancer cell growth by cyclin D1 antisense is associated with loss of tumorigenicity and potentiation of cytotoxicity to cisplatinum. J Clin Invest 1998; 101:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 1997; 89:1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol 1980; 68:251–306. [DOI] [PubMed] [Google Scholar]
- 30.Walker NI, Harmon BV, Gobe GC, Kerr JFR. Patterns of cell death. Methods Achiev Exp Pathol 1988; 13:18–54. [PubMed] [Google Scholar]
- 31.Golstein P, Ojcius DM, Young JD. Cell death mechanisms and the immune system. Immunol Rev 1991; 121:29–65. [DOI] [PubMed] [Google Scholar]
- 32.Nagata S. Apoptosis by death factor. Cell 1997; 88:355–365. [DOI] [PubMed] [Google Scholar]
- 33.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267:1456–1462. [DOI] [PubMed] [Google Scholar]
- 34.Wyllie AH. Apoptosis and carcinogenesis. Eur J Cell Biol 1997; 73:189–197. [PubMed] [Google Scholar]
- 35.Hellquist HB, Oleunicka B, Jadner M, et al. Fas receptor is expressed in human lung squamous cell carcinomas, whereas bcl-2 and apoptosis are not pronounced: a preliminary report. Br J Cancer 1997; 76:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horie S, Kano M, Higashihara E, et al. Expression of Fas in renal cell carcinoma. Jap J Clin Oncol 1997; 27:384–388. [DOI] [PubMed] [Google Scholar]
- 37.Tachibana O, Lampe J, Kleihues P, Ohgaki H. Preferential expression of Fas/APO1 (CD95) and apoptotic cell death in perinecrotic cells of glioblastoma multiforme. Acta Neuropathol 1996; 92:431–434. [DOI] [PubMed] [Google Scholar]
- 38.Niehans GA, Brunner T, Frizelle SP, et al. Human lung carcinoma express Fas ligand. Cancer Res 1997; 57:1007–1012. [PubMed] [Google Scholar]
- 39.Walker PR, Saas P, Dietrich PY. Role of Fas ligand (CD95L) in immune escape: the tumor cell strikes back. J Immunol 1997; 158:4521–4524. [PubMed] [Google Scholar]
- 40.Green DR, Ware CF. Fas-ligand: privilege and peril. Proc Natl Acad Sci USA 1997; 94:5986–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strand S, Hofmann WJ, Hug H, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—a mechanism of immune evasion? Nat Med 1996; 2:1361–1366. [DOI] [PubMed] [Google Scholar]
- 42.Yanagisawa J, Takahashi M, Kanki H, et al. The molecular interaction of Fas and FAP-1. A tripeptide blocker of human Fas interaction with FAP-1 promotes Fas-induced apoptosis. J Biol Chem 1997; 272:8539–8545. [DOI] [PubMed] [Google Scholar]
- 43.Landowski TH, Qu N, Buyuksal I, Painter JS, Dalton WS. Mutations in the Fas antigen in patients with multiple myeloma. Blood 1997; 90:4266–4270. [PubMed] [Google Scholar]
- 44.Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997; 277:818–821. [DOI] [PubMed] [Google Scholar]
- 45.Marsters SA, Sheridan JP, Pitti RM, et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol 1997; 7:1003–1006. [DOI] [PubMed] [Google Scholar]
- 46.Schulze-Osthoff K, Walczak H, Droge W, Krammer PH. Cell nucleus and DNA fragmentation are not required for apoptosis. J Cell Biol 1994; 127:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frasch SC, Nick JA, Fadok VA, et al. p38 mitogen-activated protein kinase-dependent and -independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J Biol Chem 1998; 273:8389–8397. [DOI] [PubMed] [Google Scholar]
- 48.Salmon RA, Foltz IN, Young PR, Schrader JW. The p38 mitogen-activated protein kinase is activated by ligation of the T or B lymphocyte antigen receptors, Fas or CD40, but suppression of kinase activity does not inhibit apoptosis induced by antigen receptors. J Immunol 1997; 159:5309–5317. [PubMed] [Google Scholar]
- 49.Brenner B, Koppenhoefer U, Weinstock C, et al. Fas- or ceramide-induced apoptosis is mediated by a Rac1-regulated activation of Jun N-terminal kinase/p38 kinases and GADD153. J Biol Chem 1997; 272:22173–22181. [DOI] [PubMed] [Google Scholar]
- 50.Juo P, Kuo CJ, Reynolds SE, et al. Fas activation of the p38 mitogen-activated protein kinase signalling pathway requires ICE/CED-3 family proteases. Mol Cell Biol 1997; 17:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


