Abstract
Objective
To examine the presence and extent of apoptosis as well as the affected cell types in human lung tissue before, during, and after transplantation.
Summary Background Data
Apoptosis has been described in various human and animal models of ischemia-reperfusion injury, including heart, liver, and kidney, but not in lungs. Therefore, the presence of apoptosis and its role in human lungs after transplantation is not clear.
Methods
Lung tissue biopsies were obtained from 20 consecutive human lungs for transplantation after cold ischemic preservation (1–5 hours), after warm ischemia time (during implantation), and 30, 60, and 120 minutes after graft reperfusion. To detect and quantify apoptosis, fluorescent in situ end labeling of DNA fragments (TUNEL assay) was used. Electron microscopy was performed to verify the morphologic changes consistent with apoptosis and to identify the cell types, which were lost by apoptosis.
Results
Almost no evidence of apoptosis was found in specimens after immediate cold and warm ischemic periods. Significant increases in the numbers of cells undergoing apoptosis were observed after graft reperfusion in a time-dependent manner. The mean fraction of apoptotic cells at 30, 60, and 120 minutes after graft reperfusion were 16.6%, 22.1%, and 34.9% of total cells, respectively. Most of the apoptotic cells appeared to be alveolar type II pneumocytes, as confirmed by electron microscopy.
Conclusions
Programmed cell death (apoptosis) appears to be a significant type of cell loss in human lungs after transplantation, and this may contribute to ischemia-reperfusion injury during the early phase of graft reperfusion. This cell loss might be responsible for severe organ dysfunction, which is seen in 20% of patients after lung transplantation. Therefore, this work is of importance to surgeons for the future development of interventions to prevent cell death in transplantation.
Since the first successful human lung transplantation, reported by Cooper in Toronto in 1983, 1 this procedure has become standard treatment for end-stage lung diseases such as cystic fibrosis, emphysema, primary pulmonary hypertension, idiopathic pulmonary fibrosis, and others. 2 As with other forms of organ transplantation, there remains a significant shortage of available donor organs. Human lung transplantation is further limited, however, by the fact that only approximately 20% of lungs from potential donors are suitable for transplantation due to factors such as trauma, pulmonary edema, and aspiration. 3,4 After lung transplantation, severe life-threatening graft dysfunction occurs in up to 30% of patients, related to ischemia/reperfusion (I/R) injury. 5 This injury is due primarily to mechanisms that increase pulmonary vascular permeability and cause alveolar epithelial cell damage. 6 Currently, there is no histologic I/R injury scoring system available to quantify the degree of injury during preservation and reperfusion in transplanted organs. Edema, inflammation, neutrophil migration, intraparenchymal hemorrhage, and necrosis have all been accepted as histologic markers of injury after organ transplantation. 7,8
When sufficient injury is inflicted on a cell, there are two major types of cell death that can occur: necrosis and apoptosis. Necrosis is a form of irreversible cell death accompanied by the loss of cell membrane integrity and ion pump damage. This results in cell swelling, lysis, and release of intracellular enzymes and lysozymes, followed by neutrophil migration, inflammation, and edema. 9 In clinical and experimental work, it has been found that the degree of necrosis in transplanted organs does not necessarily parallel the posttransplant physiologic organ function. 10,11 Another form of cell death, apoptosis, an area of intense clinical and scientific investigation, is thought to be a process of programmed cell death. As observed by electron microscopy, apoptotic cell death is morphologically characterized by overall cellular condensation, shrinkage, and plasma membrane blebbing. 9 Nuclear changes are characterized by chromatin margination and nuclear condensation followed by segmentation and DNA fragmentation. Finally, the apoptotic cell is broken up into smaller membrane-bound apoptotic bodies that are usually phagocytosed by macrophages. 9 An important morphologic distinction from necrosis lies in the fact that apoptotic cell death lacks inflammation. 12
There are strong indications that apoptosis plays an important regulatory role in various disease processes such as cancer, 13,14 autoimmune diseases, 15,16 and others. 17–21 Increasing evidence suggests that alterations in the genetic control of cell survival and elimination seem to be important regulators in these disease states. Of particular importance in organ transplantation, apoptosis has recently been reported in several animal models to occur after I/R injury to the kidney, retina, brain, heart, liver, and adrenal gland. 22–27
Many laboratory studies have shown a relation between organ injury, including I/R injury, and apoptosis induction, whereas it is unknown whether a more significant injury triggers necrosis and a milder injury initiates apoptotic cell death. 28–32 It has been reported that apoptosis appears in rabbit cardiomyocytes after ischemia followed by reperfusion, but it is not detectable after ischemia alone. 23 These findings are of particular interest to transplantation medicine and surgery.
At present, little is known about the relation between I/R injury and the induction of apoptosis after human organ transplantation. Isolated reports of a descriptive nature are available regarding apoptosis in human liver, 33 kidney, pancreas, 34 and heart transplantation, 35 whereas apoptosis after human lung transplantation has not been previously described. Given that lung preservation differs from all other organs in that lungs stored inflated with oxygen undergo aerobic, ischemic, hypothermic preservation 36,37 (in contrast to other organs, which undergo anaerobic, ischemic, hypothermic preservation), the nature of the injury at the cellular and subcellular level could indeed be different. Consequently, the aims of this study were to examine apoptosis after human lung transplantation and to investigate the relation between I/R injury and the induction of apoptosis. Further, we explored the potential of apoptosis as a marker for severity of I/R injury and its relation to organ function after human lung transplantation. We also report the time course of the induction of apoptosis after graft reperfusion, including quantification of apoptosis and the cell types affected in human lung allografts before, during, and after transplantation.
PATIENTS AND METHODS
Lung tissue was obtained from 20 consecutive patients undergoing lung transplantation. Consent for biopsy to be taken from the transplanted lung as part of our study of I/R injury was obtained. The study was reviewed and approved by the Ethics Review Committee of The Toronto General Hospital. The indications for transplant were emphysema in six patients (30%), cystic fibrosis in five (25%), idiopathic pulmonary fibrosis in four (20%), bronchiectasis in two (10%), and primary pulmonary hypertension, lymphangioleiomyomatosis, and bronchiolitis obliterans in one patient each (5% each). Samples from lungs were taken by stapled resection of a small wedge of lung at the end of cold ischemia time at 4°C, which ranged from 1 to 5 hours, after warm ischemia (i.e., during graft implantation; samples were taken at 60 minutes if the implantation time exceeded this period of time) and after graft reperfusion (at 30, 60, and 120 minutes). If the donor lung was volume-reduced to fit into the recipient, larger tissue specimens were available. Six patients (30%) underwent transplantation with cardiopulmonary bypass support.
This study was undertaken during the period in which our lung transplant program switched from Euro-Collins solution to low-potassium dextran glucose solution for clinical lung preservation. Euro-Collins was used in seven patients (35%), low-potassium dextran glucose solution (Perfadex; Biophausia, Uppsala, Sweden) in the other 13 (65%). Prostaglandin E1 (500 μg/L) was used as an adjunct to the lung preservation technique in all patients. 38 All donors received 2 g methylprednisolone before organ donation.
In 19 patients, bilateral lung transplantation was performed; in the remaining patient, who had idiopathic pulmonary fibrosis, a single lung was transplanted, as described by Cooper and Patterson, 39 with the modification of telescoped bronchial anastomoses and a completely everting, endothelium-to-endothelium, atrial anastomosis, which is our current standard technique.
Lung Specimens
Lung biopsies, which were obtained after cold ischemic preservation, warm ischemic implantation time, and at 30, 60, and 120 minutes after graft reperfusion, were snap-frozen in liquid nitrogen and stored at −70°C. For in situ apoptosis detection, tissue segments were thawed and fixed in 10% buffered formalin. We have found this technique to provide reliable results comparable to direct formalin fixation (data not shown). Specimens were then embedded in paraffin blocks and cut in 4-μm sections to avoid overlapping of cells on microscopic evaluation.
In Situ TUNEL Assay
For in situ apoptosis detection, the ApopTag Kit (Oncor, Gaithersburg, MD) was used according to the manufacturer’s instructions. This has been shown to be the most sensitive method for quantitative apoptosis detection available at present. 40
The method used is based on the biochemical property of terminal deoxynucleotidyl transferase (TdT), which catalyzes a template-independent addition of deoxyribonucleotide triphosphate to the 3′-OH ends of double- or single-stranded DNA. 40 After completing the protocol, slides were photographed at low magnification (×100) on a color slide film using a fluorescent microscope with a 520-nm filter (for propidium iodide staining) and a 590-nm filter (for fluorescence staining). To localize apoptotic cells, randomly selected specimens of lung tissue taken at 120 minutes after reperfusion were photographed under the fluorescent light microscope at high magnification (×400).
Apoptotic cells were counted from four randomly chosen fields per slide. Therefore, propidium iodide nuclear staining (red) represents the total number of cells per field; these were counted first, followed by apoptotic cell counts (yellow to bright green) in the same fields. Results for apoptotic cells are given as a percentage of total propidium iodide-stained cells. Two individuals masked to the identity of the specific specimens performed the counting.
Electron Microscopy
Lung biopsy samples similar to those obtained for light microscopy were studied ultrastructurally. Small pieces of fresh tissue were immersed in universal fixative (1% glutaryl-aldehyde, 4% paraformaldehyde, pH 7.4) immediately after biopsy, postfixed in 2% osmium tetroxide, dehydrated in graded acetons, and embedded in an Epon-Araldite mixture (Fisher Scientific Corp., Toronto, Ontario, Canada). Selected blocks were thin-sectioned, mounted on copper grids, and contrasted with uranyl acetate and lead citrate. The grids were examined in a Philips 201 electron microscope (N.V. Philips, Gloeilampenfarbrieken, Eindhoven, The Netherlands).
Statistical Analysis
Statistical analyses were performed using SigmaStat version 3.0 (Jandel Scientific, San Rafael, CA), comparing the percentage of apoptotic cells in tissue samples taken at the different time points using a one-way analysis of variance. Post hoc analysis was performed using the Student-Newman-Keul test. Differences were considered significant at P < .05. For evaluation of a correlation between clinical outcome and the amount of apoptotic cells 60 and 120 minutes after reperfusion, the Pearson product moment test was used.
RESULTS
Patient Profile
Donor and recipient patient profiles are summarized in Tables 1 and 2. The surgical (30-day) death rate was 10% (2/20). The mean total ischemic time was 267 minutes (range 178–430) and the mean warm ischemia time was 61 minutes (range 45–83).
Table 1. DONOR PROFILE
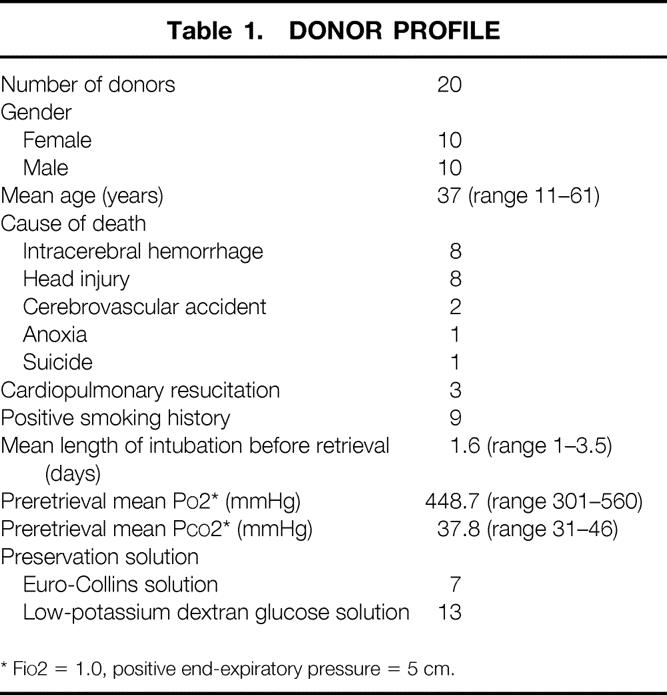
* FiO2 = 1.0, positive end-expiratory pressure = 5 cm.
Table 2. RECIPIENT PROFILE
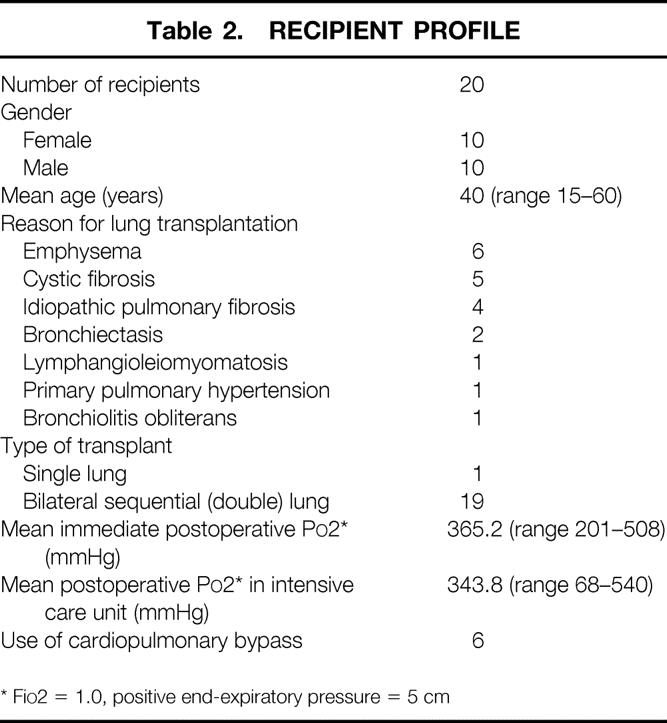
* FiO2 = 1.0, positive end-expiratory pressure = 5 cm
In Situ TUNEL Staining
Results of apoptosis staining showed minimal to no apoptosis in specimens obtained after cold and warm ischemia, independent of the length of ischemic time. At short (<2 hours), intermediate (2–4 hours), or long (4–5 hours) cold ischemic intervals, little if any appreciable apoptosis was detected (Fig. 1). After a subsequent period of warm ischemia (the implantation period), lung tissue samples continued to demonstrate only minimal numbers of apoptotic cells. After graft reperfusion, however, lung tissue samples started to show a significant (P < .001) increase in the percentage of cells undergoing apoptosis. These increased numbers of apoptotic cells were evident as early as 30 minutes after reperfusion of the transplanted lung, when the mean percentage of apoptotic cells was 16.7 ± 2.2%. These numbers continued to increase at 60 minutes as well as 120 minutes after graft reperfusion (Fig. 2) , with mean apoptotic cell percentages of 21.7 ± 6.2% and 33.6 ± 4.0%, respectively (Fig. 3). Photomicrographs taken at high magnification (×400) demonstrated that apoptotic cells were mainly localized to the alveolar epithelial lining, but a few apoptotic cells were also seen in the interstitium (Fig. 4).
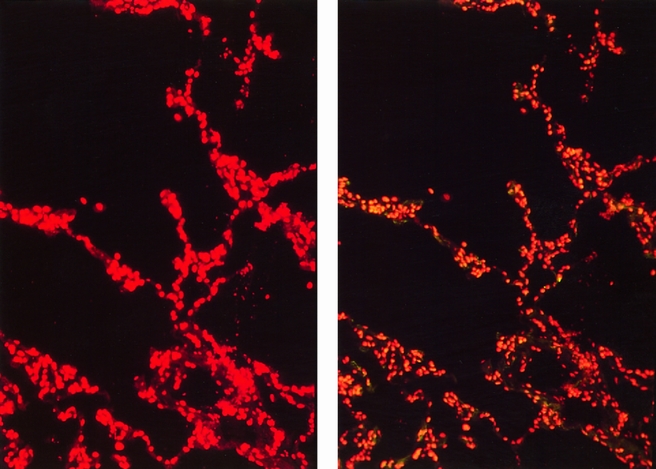
Figure 1. TUNEL immunofluorescence staining showed absence of apoptotic cells in lung tissue after cold ischemic preservation. Lung biopsy taken 3 hours after cold preservation period. (Right) There is no reactivity for apoptosis as shown in Figure 2. Red propidium iodide nuclear staining of the same microscopy slide area (left) shows total number of cells. (Magnification ×100)
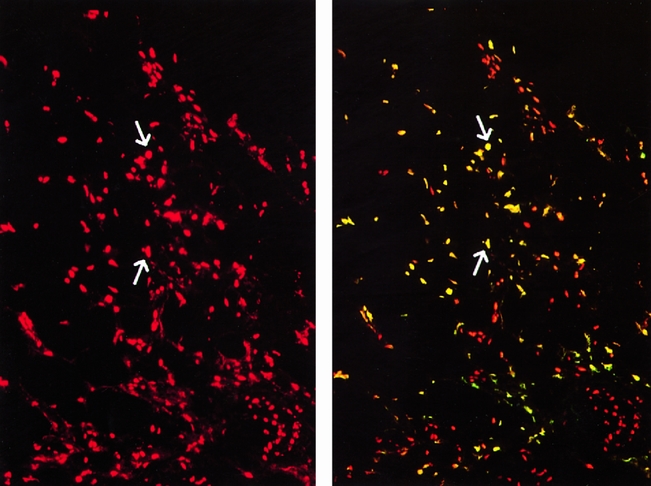
Figure 2. Presence of apoptotic cells in lung tissue 120 minutes after reperfusion. Representative picture of the TUNEL immunofluorescence staining (bright yellowish) of apoptotic cells in lung tissue taken 120 minutes after graft reperfusion after transplantation. The propidium iodide staining (red) can be used to identify nuclei (total number of cells) in the same area of tissue section in comparison to immunofluorescent stained apoptotic cells (arrows). (Magnification ×100)
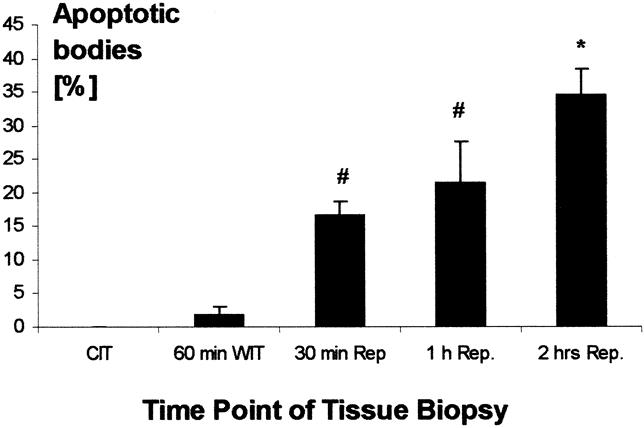
Figure 3. Time course of the induction of apoptosis in human lungs before, during, and after transplantation. CIT, cold ischemic time (preservation time); WIT, warm ischemic time (implantation time); Rep, after organ reperfusion. Data are presented as mean ± SD from different samples. P < .001 as determined by one-way analysis of variance. *P < .05 versus all other groups. #P < .05 versus cold ischemic time and 60 minutes warm ischemic time, as determined by post hoc analysis using the Student-Newman-Keul test.
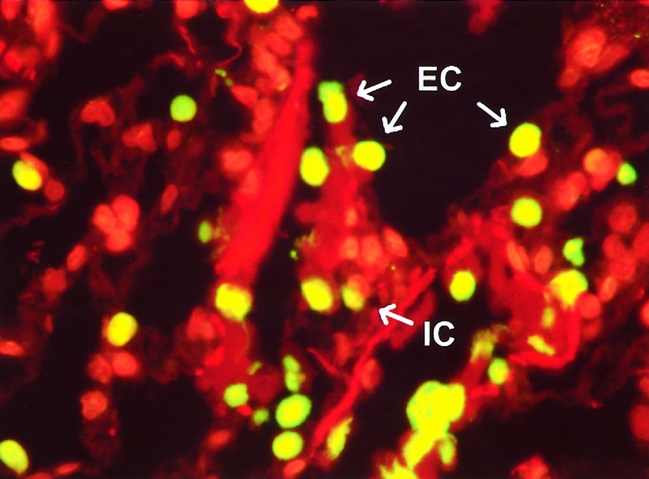
Figure 4. Representative view at higher magnification of lung biopsy taken 2 hours after graft reperfusion. Specimen was stained using the TUNEL technique. Most of the bright-yellow apoptotic cells were identified as epithelial lining cells (EC). A few cells were interstitial cells (IC). (Magnification ×400)
Electron Microscopy
Ultrastructural evaluation of lung biopsy samples after cold ischemic preservation showed well-preserved lung architecture (Fig. 5). Type II pneumocytes showed no evidence of apoptosis, and the vascular spaces showed intact endothelial lining cells. Minimal or no edema fluid was seen within the alveolar spaces. However, ultrastructural examination after 120 minutes of reperfusion showed lung tissue with extensive nuclear and cytoplasmic changes and also increased phagocytic activity of apoptotic cells. Clearing of the cytoplasmic matrix accompanied by shrinkage of the nucleus was seen. Phagocytosed apoptotic cells and remnants in an alveolar and interstitial location were observed at 120 minutes after perfusion, when edematous fluid also appeared in the alveolar spaces. Of note was the absence of inflammatory cells in the lungs, even though vigorous apoptotic activity was occurring.
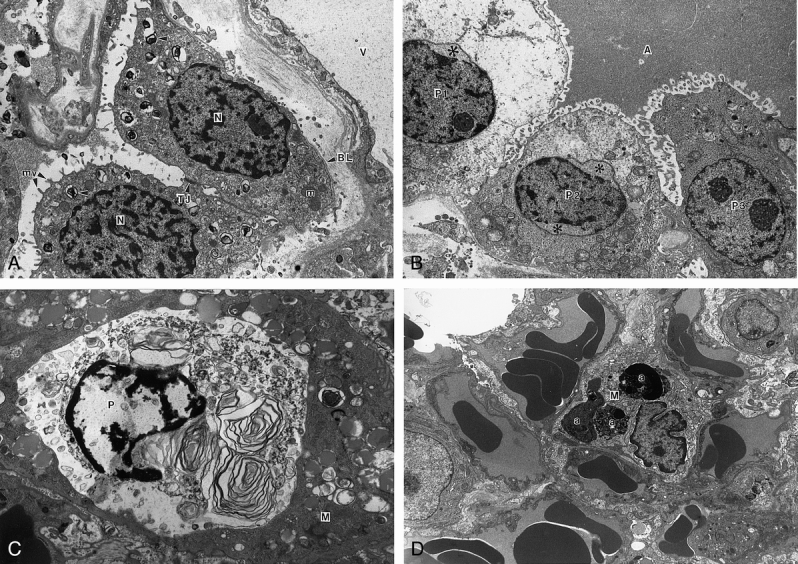
Figure 5. (A) Electron micrograph of lung after cold ischemic preservation. Portions of type II pneumocytes and a blood vessel (V) are shown. The nucleus (N) and cytoplasm of the pneumocytes are within normal limits. Lamellar bodies (arrowheads), mitochondria (m), tight junction (TJ), microvilli (mv), and basal lamina (BL) profiling these epithelial cells are shown. Note intact endothelium lining the blood vessel. (Magnification ×12,175) (B) Electron micrograph of lung after cold and warm ischemia and reperfusion for 120 minutes. Alveolar space and portions of three type II pneumocytes are shown. The type II pneumocyte (P1) shows extensive cytoplasmic swelling and vacuolation. There is blebbing of the nuclear membrane with nuclear shrinkage, leaving a clear empty space (asterisk). The type II pneumocyte in the middle (P2) shows less cytoplasmic vacuolation but more extensive nuclear shrinkage and nuclear membrane blebbing (asterisks). The third type II pneumocyte (P3) shows a relatively normal nucleus and cytoplasmic organelles including mitochondria, rough endoplasmic reticulum, and lamellar bodies. The alveolar space contains edema fluid (A). (Magnification ×7,800) (C) Electron micrograph of lung after cold and warm ischemia and reperfusion for 120 minutes. Apoptotic type II pneumocyte (P) phagocytosed by an alveolar macrophage (M). Note the condensation of nuclear chromatin and cytoplasmic degenerative changes in this pneumocyte. (Magnification ×11,920) (D) Electron micrograph of lung after cold and warm ischemia and reperfusion for 120 minutes. An interstitial macrophage (M) with nuclear fragments of several apoptotic bodies (a). These electron-dense bodies are membrane-bound with pleomorphic shape and variable size. Several blood vessels show intact endothelial lining. Note the absence of an inflammatory cell infiltrate. (Magnification ×6,330)
Clinical Observations
The degree of apoptosis did not significantly correlate with short-term clinical outcome measures such as PO2 after reperfusion, first PO2 in the intensive care unit, 30-day death rate, and ventilation time after surgery. Further, differences in the total ischemia time of the lungs, preservation solution, or ventilation time of the donors before organ retrieval did not significantly correlate with the degree of apoptosis at any of the time periods.
DISCUSSION
Apoptosis has been studied in various organs and disease entities such as cancer, 41 I/R injury, 33,42 and organ rejection. 43 This specific form of cell death has yet to be described in the setting of human lung preservation and transplantation. Also, not only the phenomenon of apoptosis itself, but also its induction during the time course of retrieval, preservation, and transplantation, as well as its dependence on total ischemia time have not been previously described. This is highly relevant to further considerations regarding the extension of ischemic times for organ preservation in our attempts to increase the number and quality of available donor organs.
Our findings of apoptosis in human lung transplantation raise several important points. First, cold ischemic preservation of the lungs before transplantation does not seem to induce apoptosis. This was true for short (1–2 hours), intermediate (2–4 hours), and long (4–6 hours) periods of cold ischemia. This is consistent with previous studies in liver and intestinal I/R injury in animal models. 28,42 This phenomenon may result from decreased cellular metabolism secondary to the hypothermic (4°C) preservation. Second, we found that apoptosis remained at low levels after cold ischemia and during the interval of warm ischemia necessary for graft implantation. This is partly consistent with previous studies of warm ischemia. 26,44 Third, we found a significant increase in apoptosis after reperfusion of the transplanted lung. This increase was noted as early as 30 minutes after reperfusion, and a trend toward increased levels of apoptosis over time was noted during the 120 minutes after reperfusion. This is consistent with research in human liver tissue, which describes a similar rise in apoptosis after liver reperfusion. 33 Our results further confirm the finding described in intestinal I/R injury that apoptosis increases significantly only after reperfusion and is virtually undetectable during cold ischemic preservation. 44
Graft reperfusion is a process associated with an acute increase in the production of oxygen free radicals and a significant accumulation of intracellular calcium. Both of these phenomena are potent inducers of apoptosis. 41 This is in keeping with the theory that oxidative stress related to an abundance of reactive oxygen species and metabolites may induce apoptosis by direct DNA damage, oxidation of lipid membranes, or upregulation of regulatory “apoptosis genes.”45 This hypothesis would explain the acute increases noted in apoptotic cell numbers (mostly type II pneumocytes) in the human lung samples in our study after reperfusion.
The fact that we did not find a significant correlation between apoptosis and clinical physiologic function of the transplanted lungs should not viewed as conclusive, given that the sample size was small and the variation of disease severity and etiology in our patients contributed to significant heterogeneity, which may affect the ultimate outcome related to the implanted lung. Therefore, animal studies under rigorously controlled conditions are required to answer questions about the relation between the amount of apoptosis observed and physiologic function after lung transplantation. These studies are ongoing in our laboratory.
Severe oxidative injury, such as prolonged ischemia, has been known to cause cell death by necrosis. Recently, it has been shown that I/R injury can induce apoptosis as well. 46,47 The finding that apoptosis is induced in the human lung transplantation setting, with such significantly elevated levels after graft reperfusion, is intriguing. Clearly, further investigation is required to define the implications of apoptosis in this setting. This may indeed represent a marker of cell injury resulting from ischemic preservation and the oxidative stress of reperfusion, heralding a poorer posttransplant prognosis. Alternatively, apoptosis may represent a host-protective form of selective cell death, inevitable, to a certain degree, after the stresses imposed by the transplantation process. In a sense, “controlled” cellular senescence may be favored over the “destructive” and inflammatory cellular death seen in necrosis, which results in the local and systemic release of injurious and noxious intracellular components and proinflammatory cytokines such as tumor necrosis factor alpha. Further, because apoptosis is a genetically controlled, programmed cell death, it might indeed be possible to modify this type of cell death in transplanted organs by interventions such as gene therapy. Necrotic cell death may be more difficult to influence.
Apoptosis could also be viewed as an adaptive mechanism that aids the body in its response to a significant injury. This view of the process hypothesizes that a high index of apoptosis in a transplanted organ indicates that organ’s ability to eliminate injured cells and avoid the more destructive process of necrosis. This theory views apoptotic cell death not negatively as a loss of organ tissue and function, but positively as an important part of the process of tissue healing from reperfusion injury. This is similar to the field of fetal organogenesis, where it has been demonstrated that apoptosis is an important aspect of the remodeling of an effective pulmonary alveolar–capillary interface in fetal lungs during middle to late gestation. 48
Future clinical and experimental investigations are needed to evaluate the role of apoptosis as a marker of the extent of reperfusion injury and the resultant condition of the organ after preservation and transplantation. It also remains to be determined when the process of apoptosis peaks, and its rate of recovery. Details regarding the specific cell types affected and their relative proportions also need to be ascertained. Further, the relation between the early index of apoptosis and the development of chronic obliterative bronchiolitis, which occurs in approximately 50% of patients after lung transplantation, needs to be studied.
In summary, we have found that in human lung transplantation, up to 34% of pulmonary cells undergo apoptotic cell death after reperfusion. Cold, aerobic, ischemic preservation up to 5 hours did not appear to induce apoptosis; nor did a period of warm ischemia before implantation. Clearly, further work is required to determine the implication of this magnitude of apoptotic cell death on ultimate organ function. Patients obviously did well in the short term, but the ability of the organ to recover from this type of injury may indeed have significant implications regarding long-term lung function after transplantation and the development of bronchiolitis obliterans.
Footnotes
Correspondence: S. Keshavjee, MD, Division of Thoracic Surgery, The Toronto General Hospital, 200 Elizabeth Street, EN 10–224, Toronto, Ontario, Canada M5G 2C4.
Supported by a grant from the National Sanitarium Association of Canada.
Accepted for publication June 17, 1999.
References
- 1.Toronto Lung Transplant Gorup. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986; 314:1140–1145. [DOI] [PubMed] [Google Scholar]
- 2.Patterson GA. Indications: unilateral, bilateral, heart-lung, and lobar transplant procedures. Clin Chest Med 1997; 18:225–230. [DOI] [PubMed] [Google Scholar]
- 3.Egan TM, Boychuk JE, Rosato K, Cooper JD. Whence the lungs? A study to assess suitability of donor lungs for transplantation. Transplantation 1992; 53:420–422. [DOI] [PubMed] [Google Scholar]
- 4.Sundaresan S, Semenkovich J, Ochoa L, et al. Successful outcome of lung transplantation is not compromised by use of marginal donor lungs. J Thorac Cardiovasc Surg 1995; 109:1075–1080. [DOI] [PubMed] [Google Scholar]
- 5.Haydock DA, Trulock EP, Kaiser LR, et al. Management of dysfunction in the transplanted lung: experience with 7 clinical cases. Washington University Lung Transplant Group. Ann Thorac Surg 1992; 53:635–641. [DOI] [PubMed] [Google Scholar]
- 6.Novick RJ, Gehman KE, Ali IS, Lee J. Lung preservation: the importance of endothelial and alveolar type II cell integrity. Ann Thorac Surg 1996; 62 (1):302–314. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JD, Vreim CE. Biology of lung preservation for transplantation. Am Rev Respir Dis 1992; 146:803–807. [DOI] [PubMed] [Google Scholar]
- 8.Yousem SA, Duncan SR, Griffith BP. Interstitial and airspace granulation tissue reactions in lung transplant recipients. Am J Surg Pathol 1992; 16:877–884. [DOI] [PubMed] [Google Scholar]
- 9.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 1995; 146 (1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 10.Lazar N, Dallos G, Nemes B, et al. Experimental investigation of preservation injury in animal kidneys after reperfusion with Euro-Collins. Acta Chir Lung 1997; 36:192–194. [PubMed] [Google Scholar]
- 11.Allen KJ, Rand EB, Hart J, Whitington PF. Prognostic implications of centrilobular necrosis in pediatric liver transplant recipients. Transplantation 1998; 65:692–698. [DOI] [PubMed] [Google Scholar]
- 12.Gerschenson LE, Rotello RJ. Apoptosis: a different type of cell death. FASEB J 1992; 6:2450–2455. [DOI] [PubMed] [Google Scholar]
- 13.Sheng H, Shao J, Morrow JD, et al. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 1998; 58:362–366. [PubMed] [Google Scholar]
- 14.Staunton MJ, Gaffney EF. Apoptosis: basic concepts and potential significance in human cancer. Arch Pathol Lab Med 1998; 122:310–319. [PubMed] [Google Scholar]
- 15.Chung JH, Kwon OS, Eun HC, et al. Apoptosis in the pathogenesis of cutaneous lupus erythematosus. Am J Dermatopathol 1993; 20:233–241. [DOI] [PubMed] [Google Scholar]
- 16.Beutler B, Bazzoni F. TNF, apoptosis and autoimmunity: a common thread? Blood Cells Mol Dis 1998; 24:216–230. [DOI] [PubMed] [Google Scholar]
- 17.Mallat Z, Tedgui A, Fontaliran F, et al. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med 1996; 335:1190–1196. [DOI] [PubMed] [Google Scholar]
- 18.Narula J, Haider N, Virmani R, et al. Apoptosis in myocytes in end-stage heart failure. N Engl J Med 1996; 335:1182–1189. [DOI] [PubMed] [Google Scholar]
- 19.Olivetti G, Abbi R, Quaini F, et al. Apoptosis in the failing human heart. N Engl J Med 1997; 336:1131–1141. [DOI] [PubMed] [Google Scholar]
- 20.Kazufumi M, Sonoko N, Masanori K, et al. Expression on bcl-protein and APO-1 (Fas antigen) in the lung tissue from patients with idiopathic pulmonary fibrosis. Microsc Res Tech 1997; 38:480–487. [DOI] [PubMed] [Google Scholar]
- 21.Woo D. Apoptosis and loss of renal tissue in polycystic kidney diseases. N Engl J Med 1995; 333:18–25. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Chopp M, Jiang N, et al. Induction of DNA fragmentation after 10 to 120 minutes of focal cerebral ischemia in rats. Stroke 1995; 26:1252–1258. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb RA, Burleson KO, Kloner RA, et al. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 1994; 94:1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchi ER. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp Eye Res 1992; 55:605–613. [DOI] [PubMed] [Google Scholar]
- 25.Schumer M, Colombel MC, Sawczuk IS, et al. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol 1992; 140:831–838. [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki H, Matsuno T, Nakagawa K, Tanaka N. Induction of apoptosis during the early phase of reperfusion after rat liver ischemia. Acta Med Okayama 1997; 51:305–312. [DOI] [PubMed] [Google Scholar]
- 27.Didenko VV, Wang X, Yang L, Hornsby PJ. Expression of p21(WAF1/CIP1/SDI1) and p53 in apoptotic cells in the adrenal cortex and induction by ischemia/reperfusion injury. J Clin Invest 1996; 97:1723–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology 1998; 27:1652–1660. [DOI] [PubMed] [Google Scholar]
- 29.DeMeester SL, Buchman TG, Qiu Y, et al. Heat shock induces IkappaB-alpha and prevents stress-induced endothelial cell apoptosis. Arch Surg 1997; 132:1283–1288. [DOI] [PubMed] [Google Scholar]
- 30.Clemens JA, Stephenson DT, Smalstig EB, et al. Global ischemia activates nuclear factor-kappa B in forebrain neurons of rats. Stroke 1997; 28:1073–1081. [DOI] [PubMed] [Google Scholar]
- 31.Bielawska AE, Shapiro JP, Jiang L, et al. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am J Pathol 1997; 151:1257–1263. [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoyama I, Hayashi S, Kobayashi T, et al. Hepatocyte apoptosis and cytosolic calcium dynamics in ischemic injury. Transplant Proc 1997; 29:3514. [DOI] [PubMed] [Google Scholar]
- 33.Borghi-Scoazec G, Scoazec JY, Durand F, et al. Apoptosis after ischemia-reperfusion in human liver allografts. Liver Transpl Surg 1997; 3:407–415. [DOI] [PubMed] [Google Scholar]
- 34.Boonstra JG, Wever PC, Laterveer JC, et al. Apoptosis of acinar cells in pancreas allograft rejection. Transplantation 1997; 64:1211–1213. [DOI] [PubMed] [Google Scholar]
- 35.Shaddy RE. Apoptosis in heart transplantation. Coron Artery Dis 1997; 8:617–621. [DOI] [PubMed] [Google Scholar]
- 36.Date H, Matsumura A, Manchester JK, et al. Changes in alveolar oxygen and carbon dioxide concentration and oxygen consumption during lung preservation. The maintenance of aerobic metabolism during lung preservation. J Thorac Cardiovasc Surg 1993; 105:492–501. [PubMed] [Google Scholar]
- 37.Keshavjee SH, Yamazaki F, Yokomise H, et al. The role of dextran 40 and potassium in extended hypothermic lung preservation for transplantation. J Thorac Cardiovasc Surg 1992; 103:314–325. [PubMed] [Google Scholar]
- 38.Puskas JD, Cardoso PF, Mayer E, et al. Equivalent eighteen-hour lung preservation with low-potassium dextran or Euro-Collins solution after prostaglandin E1 infusion. J Thorac Cardiovasc Surg 1992; 104:83–89. [PubMed] [Google Scholar]
- 39.Patterson GA, Cooper JD. Lung transplantation. In: Pearson FG, Deslauriers J, Ginsberg RJ, et al, eds. Thoracic Surgery, Vol. 1. New York: Churchill Livingstone; 1995: 931–959.
- 40.Bardales RH, Xie SS, Hsu SM. In situ DNA fragmentation assay for detection of apoptosis in paraffin-embedded tissue sections. Technical considerations. Am J Clin Pathol 1997; 107:332–336. [DOI] [PubMed] [Google Scholar]
- 41.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267:1456–1462. [DOI] [PubMed] [Google Scholar]
- 42.Shah KA, Shurey S, Green CJ. Apoptosis after intestinal ischemia-reperfusion injury: a morphological study. Transplantation 1997; 64:1393–1397. [DOI] [PubMed] [Google Scholar]
- 43.Laguens RP, Meckert PM, Martino JS, et al. Identification of programmed cell death (apoptosis) in situ by means of specific labeling of nuclear DNA fragments in heart biopsy samples during acute rejection episodes. J Heart Lung Transplant 1996; 15:911–918. [PubMed] [Google Scholar]
- 44.Shah KA, Shurey S, Green CJ. Characterization of apoptosis in intestinal ischaemia-reperfusion injury—a light and electron microscopic study. Int J Exp Pathol 1997; 78:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buttke TM, Sandstrom PA. Oxidative stress as a mediatory of apoptosis. Immunol Today 1994; 15:7–10. [DOI] [PubMed] [Google Scholar]
- 46.Renolleau S, Aggoun-Zouaoui D, Ben-Ari Y, Charriaut-Marlangue C. A model of transient unilateral focal ischemia with reperfusion in the P7 neonatal rat: morphological changes indicative of apoptosis. Stroke 1998; 29:1454–1461. [DOI] [PubMed] [Google Scholar]
- 47.Ikeda H, Suzuki Y, Suzuki M, et al. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut 1998; 42:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scavo LM, Ertsey R, Chapin CJ, et al. Apoptosis in the development of rat and human fetal lungs. Am J Respir Cell Mol Biol 1998; 18 (1):21–31. [DOI] [PubMed] [Google Scholar]


