Abstract
Objective
To review the surgical experience with pectus excavatum chest deformities at UCLA Medical Center during a 30-year period.
Background
Pectus excavatum is a relatively common malformation that is often symptomatic; however, children’s physicians often do not refer patients for surgical correction.
Methods
Hospital records from 375 patients who underwent repair of pectus excavatum deformities between 1969 and 1999 were reviewed. Decrease in stamina and endurance during exercise was reported by 67%; 32% had frequent respiratory infections, 8% had chest pain, and 7% had asthma. The mean pectus severity score (width of chest divided by distance between posterior surface of sternum and anterior surface of spine) was 4.65 (normal chest = 2.56). All patients had marked cardiac deviation into the left chest. Repair was performed with subperiosteal resection of the abnormal cartilages, transverse wedge osteotomy of the anterior sternum, and internal support with a steel strut for 6 months. Repair was performed on 177 children before age 11 years; 38 adults with severe symptoms underwent repair.
Results
The mean hospital stay was 3.1 days. With a mean follow-up of 12.6 years, all patients with preoperative respiratory symptoms, exercise limitation, and chest pain experienced improvement. Vital capacity increased 11% (mean) within 9 months in 35 patients evaluated. There were no deaths. Complications included hypertrophic scar formation (35), atelectasis (12), pleural effusion (13), recurrent sternal depression (5), and pericarditis (3). More than 97% had a very good or excellent result.
Conclusion
Pectus excavatum deformities can be repaired with a low rate of complications, a short hospital stay, and excellent long-term physiologic and cosmetic results.
Congenital chest wall deformities have long been considered as curious and essentially unimportant anomalies of thoracic contour. During the past several years, however, pectus malformations have come to be recognized as fairly common and much more symptomatic than previously believed. Pectus excavatum is by far the most common congenital chest deformity, occurring in approximately one in every 700 births (personal communication, March of Dimes Birth Defects Foundation, March 1995), but the recognition of symptoms and the recommendation for surgical correction remain highly controversial: less than 15% of patients undergo surgery. Few medical conditions arouse such opinionated views from physicians as does the discussion regarding whether pectus excavatum is primarily a cosmetic disorder, or whether it causes physiologic impairment and limitations to the patient. The timeliness of this controversy has been accentuated in recent years as the insurance coverage for many medical conditions has been restricted by managed health care.
Compounding the decision of whether to correct pectus excavatum disorders by surgery is the lack of reliable and consistent standard investigative studies that clearly indicate the physiologic limitations on a specific patient by pectus excavatum, or the improvement that occurs after surgery. Because only a few hospitals have compiled a large surgical experience, and because most surgeons perform only a few pectus operations each year using a variety of surgical techniques, the reported results have been inconsistent, further confusing the decision of whether to correct the deformity.
The present report reviews the surgical experience with pectus excavatum chest deformities at UCLA Medical Center during a 30-year period.
PATIENTS AND METHODS
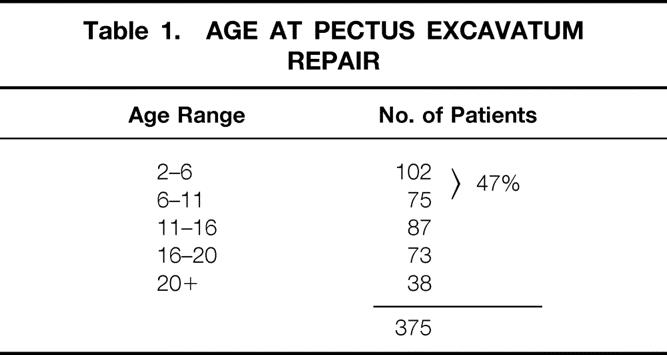
The medical records of all patients who underwent surgical correction of pectus excavatum deformities at UCLA Medical Center from January 1969 through November 1999 were reviewed. Three hundred seventy-five patients were identified. During the same period, an additional 76 patients with pectus carinatum and 4 with Poland’s syndrome underwent surgical repair. There were 298 male patients and 77 female patients (21%). The deformity was evident during the first few months of life in 88% of patients. A family history of pectus deformity was present in 42% of patients. The age at surgery ranged from 2.5 to 53 years (Table 1). Surgical repair was performed on 224 patients during the past 10 years. Three hundred thirty-six of the operations were performed by one surgeon. The period of follow-up has extended from 3 months to 30 years (mean 12.6 years).
Table 1. AGE AT PECTUS EXCAVATUM REPAIR
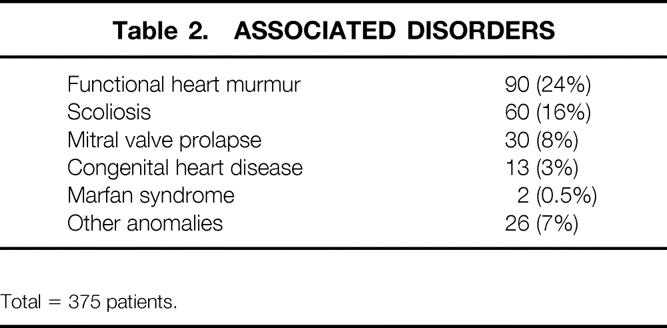
The most common complaint for young patients was related to the unattractive physical appearance of the deformity. Mild to severe exercise limitation with decreased stamina and endurance and inability to keep up with peers in strenuous athletic activities was reported by 251 patients (67%), most frequently in those older than 6 years; it was most severe in those older than 16 years. Many had been able to participate in competitive athletic activities during early adolescence but then found it progressively more difficult to keep up with peers. One hundred twenty patients (32%) had frequent respiratory infections; 26 (7%) had asthmatic symptoms, which were often severe after exercise. Functional heart murmurs were present in 90 patients (24%); however, only 13 patients had cardiac anomalies (Table 2). Two adult patients had mild to moderate angina. Forty-eight of the older patients experienced aching or compression-type pain in the lower anterior chest after exercise, with three having pain even at rest. Sixty patients (16%) had mild or moderate scoliosis; three patients had severe spinal deformities. Two patients had Marfan syndrome. Other anomalies were present in 26 patients (7%).
Table 2. ASSOCIATED DISORDERS
Total = 375 patients.
Almost all patients showed a narrow anterior-posterior diameter of the chest; only two patients were slightly overweight. Thirty-nine of the 55 patients tested had electrocardiographic evidence of right ventricular strain, and 30 (8%) had mitral valve prolapse. Almost all patients had displacement of the heart into the left chest. The mean pectus severity score as determined on chest x-ray or computed tomography scan by measuring the internal width of the lower chest and dividing by the distance between the posterior surface of the sternum and the anterior surface of the spine, was 4.65 (range 3.54–9.50); the normal chest is 2.56 (Fig. 1). 1 There was a tendency for patients with a high pectus severity score to have the most severe symptoms. Approximately 15% of the patients, largely those younger than 6 years, had minimal measurable physiologic impairment.
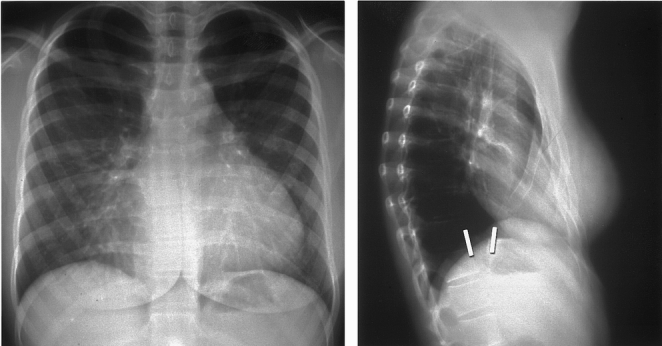
Figure 1. Chest x-rays from a 15-year-old girl with severe symptoms from pectus excavatum. Note the short distance between the posterior surface of the sternum and the anterior surface of the spine. The pectus severity score was 9.5 (normal chest 2.56).
The surgical technique used for each of the 375 patients was a modification of that described by Ravitch 2 and Welch, 3 and has been extensively detailed in previous reports. 4,5 The repair of pectus excavatum includes the following essential features:
1. A transverse curvilinear incision is made midway between nipples and costal margin, extending from mid-nipple line bilaterally.
2. Limited skin flaps are elevated over the pectoralis muscles using needle-point electrocautery to minimize blood loss.
3. The pectoralis muscles are reflected laterally over a short distance from attachments to the sternum and deformed costal cartilages; the abdominal muscles are mobilized from the lower costal cartilages.
4. The perichondrium is incised on the midanterior surface of the lower four to five costal cartilages bilaterally, extending from the costochondral junction to the sternum.
5. Abnormal costal cartilages are resected subperichondrially, carefully preserving the perichondrium.
6. The xiphoid is detached from the sternum.
7. The intercostal muscles and perichondrial sheaths of the resected cartilages are transected from the sternum.
8. The lower retrosternal space is mobilized.
9. The pleura is incised on the right side of the mediastinum and a small chest tube is inserted.
10. A transverse anterior wedge osteotomy of the sternum is made at the level where the sternum depresses posteriorly.
11. The posterior table of the sternum is gently fractured without displacement and then elevated and twisted to the desired position.
12. Nonabsorbable sutures are placed through the anterior table of the sternum across the osteotomy.
13. A stainless-steel (Adkins) strut (Baxter Healthcare Corp., Operating Room Division, McGaw Park, IL) is placed across the lower anterior chest to support the tip of the sternum and is wired to the appropriate rib on each side (fifth or sixth). 6
14. The xiphoid and perichondrial sheaths are sutured back to the sternum. The perichondrial sheaths are closed loosely.
15. The pectoralis and abdominal muscles are sutured together over the sternum.
16. Thorough hemostasis is achieved with needle-point electrocautery, and the wound is copiously irrigated with cefazolin solution.
17. The skin is closed with subcuticular absorbable sutures and Steri-Strips or staples.
The endotracheal tube is removed in the recovery room within 2 hours. The chest tube is removed within 24 hours after surgery. Intravenous cefazolin is given for 3 days, and oral cephalexin is given for 3 additional days. Postoperative pain was remarkably mild in all patients and was controlled with intravenous analgesics for the first 2 postoperative days and by oral medications thereafter. Epidural analgesia was not used.
During the past 15 years, the mean duration of the operation has been 2.7 hours (2.2 hours for patients younger than 12 years, 3.2 hours for older patients). Only the infrequent patient with cardiac anomalies has been placed in an intensive care unit after surgery during the past 18 years. The total hospital stay rarely exceeded 4 days (mean 3.1 days); many children younger than 10 years were discharged within 48 hours. Blood loss rarely exceeded 100 mL (mean 94 mL). The steel strut was removed on an outpatient basis approximately 6 months after repair with the patient under light general anesthesia (mean operating time 22 minutes). The majority of patients returned to school or work within 2 weeks. Full physical activity, including strenuous exercise but not body contact sports, was resumed by almost all patients within 10 weeks.
RESULTS
Each of the 251 patients with preoperative limitation in stamina and endurance with exercise experienced marked improvement within 4 months after surgery, and most were able to participate in vigorous exercise, including running, swimming, hiking, basketball, and tennis, before removal of the sternal bar. Body contact sports, including football, were resumed after removal of the sternal bar. Of the 120 patients with preoperative respiratory symptoms, 115 had a decrease in frequency and severity of pulmonary infections after repair. Twenty-four of the 26 patients with asthmatic symptoms showed clinical improvement after surgery, as evidenced by fewer episodes of wheezing and a 25% to 40% decrease in requirement for medications. Each of the 48 patients with chest pain reported considerable improvement within 3 months. Thirty-three of the 35 patients who underwent preoperative measurement of vital capacity with an incentive spirometer experienced improvement within 6 months (mean improvement 11%). Although objective measurements of physiologic improvement after surgery are not available for all patients, almost all showed a shifting of the heart from the left chest to a normal position on chest radiograph within a few weeks (Figs. 2 and 3). Functional heart murmurs were no longer audible in 74 of the 90 patients.
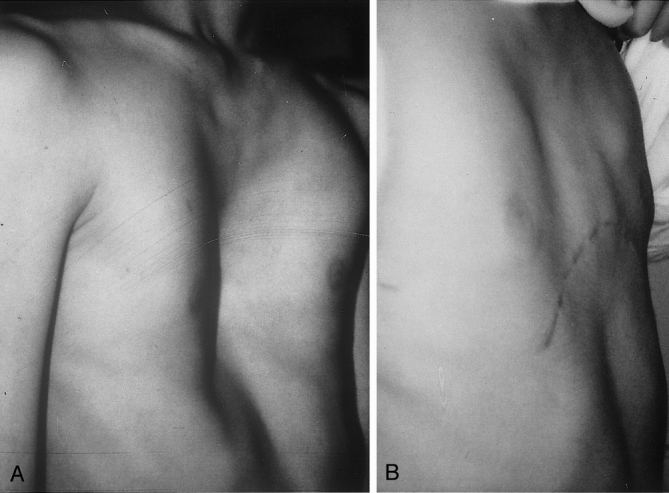
Figure 2. (A) Chest of a 16-year-old boy with symptomatic pectus excavatum. (B) Nine months after surgery.
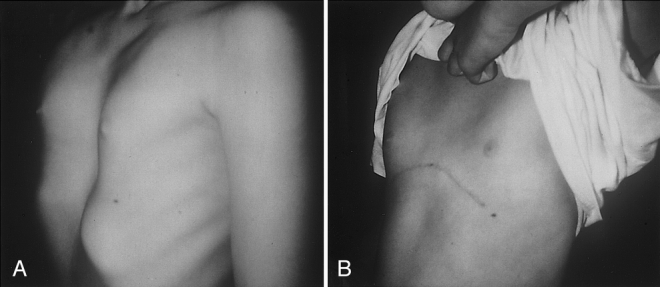
Figure 3. (A) Chest of an 8-year-old girl with symptomatic pectus excavatum. (B) Fourteen months after surgery.
Postoperative complications included wound seroma in 12 patients, pleural effusion in 13 patients, atelectasis or pneumonitis in 12 patients, and unintentional pneumothorax in 6 patients. Pericardial effusion occurred in three older patients with severe depression (severity index > 6.0); pericarditis developed in two of them, and they were given indomethacin. Recurrent depression occurred in five of the early patients in whom a sternal support bar was not used; three of them underwent a secondary repair. Protrusion of the second or third costal cartilage developed in 21 preadolescent patients within 6 years after surgery at the time of pubescent skeletal growth; however, only 5 patients underwent late resection of additional cartilage on an outpatient basis. Mild to moderate hypertrophy of the cutaneous scar occurred in the wounds of 35 patients. It is our current practice to inject triamcinolone solution (10 mg/mL) into the dermis at the time of pectus repair, or into the scar when hypertrophy becomes apparent. Only seven patients reported mild or moderate discomfort from the sternal bar. There were no deaths within 12 months after surgery.
DISCUSSION
Symptoms from pectus excavatum are recognized infrequently during early childhood, apart from an unwillingness to go without a shirt while swimming or to participate in other athletic or social activities. Most patients are therefore advised by well-meaning family physicians or pediatricians that the deformity will improve with age, that it will not affect heart or lung performance, that it is primarily a cosmetic problem, and that surgical repair is dangerous, minimally effective, and unnecessary. It is clear that each of these views is incorrect with our present knowledge of pectus excavatum deformities and the current techniques for surgical repair based on a large clinical experience with long-term follow-up. 4,7 The severe compression of the heart noted in the majority of patients with a pectus severity index of more than 4.4 is clearly more than a cosmetic deformity.
Easy fatigability and decreased stamina and endurance became apparent during early adolescence when the child participated in more vigorous physical activities. Many adolescent patients chose sedentary activities, such as working with computers, rather than athletics. For all patients undergoing repair, the heart was considerably displaced into the left chest and pulmonary expansion during inspiration was confined. Some very competitive children have been able to compensate for the pectus-induced limitations in chest expansion in short-duration athletic activities by wider diaphragmatic excursions, at a greater expense of energy. Phrenic excursions during respiration, particularly while exercising, were often much greater than in normal children to compensate for the limited movements of the bony thorax. Most of the patients had an asthenic habitus, a narrow anterior-posterior chest diameter, and a slouching posture. Deep inspirations accentuated the severity of the depression in almost all patients. None of the patients who were followed up before surgery for more than 3 years showed any evidence of spontaneous regression of the deformity; on the contrary, almost all patients showed a progressive worsening. Younger children commonly had a potbelly appearance, accentuated by flaring of the lowest costal cartilages.
Approximately 35% of patients, most often in the preadolescent age group, had a symmetric depression involving the lower 70% of the sternum. The remaining patients had an asymmetric depression, with the sternum tilted posteriorly on the right and the involved costal cartilages being more depressed on the right, occasionally extending almost up to the clavicle. Some of these patients had protrusion of a few costal cartilages on the left side.
During the past 12 years, we have been consulted by several patients older than 20 years who have severe untreated pectus excavatum deformities with worsening symptoms and limitations, and who are very desirous of having surgical correction. Thirty-eight of these patients underwent successful repair. 8 Although the technical aspects of repair were more tedious than in children, the postoperative recovery and the long-term results have been similar to those in younger patients.
Objective measurements to document the severity of the physical limitations caused by pectus excavatum are often difficult to obtain in children, imprecise, and confusing. No consensus has been achieved during the past few decades regarding the degree of cardiopulmonary impairment that occurs with this common deformity, and how much improvement results after surgical repair. 9 Studies conducted almost three decades ago using cardiac catheterization before and after pectus repair showed significant improvement in stroke volume and cardiac output. 10 Using cycle ergometry testing, Cahill et al 11 showed that children with severe pectus deformities had an abnormally elevated work of breathing, and that there was a significant improvement in maximum oxygen uptake and total exercise time in 16 children tested before and after repair. A comprehensive review of many published reports indicating improvement in cardiac or respiratory function after pectus repair is provided by Shamberger and Welch. 12 Because of the high expense and often invasive nature of the procedures used for physiologic evaluation before and after pectus excavatum repair, we have not favored the routine use of such tests for many years. The severity of the pectus depression can be easily measured by calculating the pectus severity index from computed tomography scans, as recommended by Haller et al. 1 In the present study, 186 patients had the pectus severity index calculated from chest x-rays, which appears to be accurate and less expensive than computed tomography scans.
In our early experience, for children younger than 6 years, we used an internal autologous tissue substernal support by suturing the perichondrium of the fifth ribs from each side together posterior to the sternum. 13 We discontinued this procedure 16 years ago when we observed mild recurrent depression in three patients, one of whom experienced mild constriction of the fifth ribs bilaterally. We have not observed the appearance of the constrictive asphyxiating thoracic dystrophy reported by Haller et al, 14 and more recently by Weber and Kurkchubasche, 15 in any of the 375 patients who underwent surgery.
Although we have used the substernal support bar for adolescent patients for 30 years, we have included the use of the strut for patients of all ages during the past 12 years. The sternal support bar eliminates postoperative flail chest and paradoxical respiration, which reduces pain and permits early ambulation and deeper respiratory excursions, ultimately reducing the length of hospital stay and overall cost. The bar is easily removed and ensures a good long-term results: recurrent depression is extremely uncommon, even when repair is performed at an early age. Costal cartilage regeneration from the perichondrial sheaths is remarkably rapid in children, with the chest becoming very stable within 4 weeks. For adults, the costal cartilages are usually brittle and thick and often must be scooped out from the perichondrial sheaths with a rongeur rather than being stripped out with a smaller elevator. Cartilage regeneration in adults has been slightly slower than in children, although all patients had a stable chest within 8 weeks. Removal of the support bar approximately 6 months after repair has not resulted in recurrent depression in any of the patients. We prefer to perform surgical repair in the preadolescent age group in patients who have moderate symptoms and a high pectus severity index because the operation is technically easier at this age, taking approximately 1 hour less than in adolescents.
Although the surgical technique used in the present review has provided excellent results in more than 97% of patients with a short hospital stay (mean 3.1 days), we are awaiting with enthusiasm the long-term results with the revolutionary new concept of minimally invasive pectus excavatum repair recently described by Nuss et al. 16 The current experience of these authors with 40 patients who underwent bar removal 2 or more years after the repair with relatively short follow-up is very gratifying (personal communication, Kelly RE Jr, Nuss D). Longer follow-up, however, with a larger group of patients progressing through the adolescent period of rapid skeletal growth will be necessary to determine the frequency of complications and the recurrence rate. Whether the minimally invasive technique will be effective in correcting deformities in older patients or in those with combined depression and protrusion deformities remains to be determined. Pain appears to be more severe and prolonged, complications are more frequent and severe, the hospital stay is longer, and the limitation of physical activity longer than for the patients reviewed in the present report.
To obtain optimal results, repair of pectus excavatum requires attention to several technical details, with small alterations made on the basis of different anatomic features in a specific patient. As noted with other major operations, the number of repairs performed by a surgical team may have a close relation with the complication rate and the outcome. 17
This retrospective clinical study confirms that pectus excavatum deformities can be repaired with a low rate of complications and a short hospital stay. The improvement in respiratory symptoms, exercise tolerance, and endurance as well as cosmetic appearance of more than 97% of the patients in this study support the view that symptomatic patients of all ages should undergo repair, preferably during preadolescent years. Routine use of substernal support with minimal preoperative testing has provided excellent long-term clinical results at low cost.
Footnotes
Correspondence: Eric W. Fonkalsrud, MD, Dept. of Surgery, UCLA Medical Center, Los Angeles, CA 90095.
Accepted for publication June 25, 1999.
References
- 1.Haller JA Jr, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987; 22:904–906. [DOI] [PubMed] [Google Scholar]
- 2.Ravitch MM. Operative technique of pectus excavatum repair. Ann Surg 1949; 129:429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welch KJ. Satisfactory surgical correction of pectus excavatum deformity in childhood: a limited opportunity. J Thorac Surg 1958; 36:697–713. [PubMed] [Google Scholar]
- 4.Fonkalsrud EW, Salman T, Guo W, et al. Repair of pectus deformities with sternal support. J Thorac Cardiovasc Surg 1994; 107:37–42. [PubMed] [Google Scholar]
- 5.Fonkalsrud EW. Chest wall deformities. In: Rowe MI, O’Neill JA, Grosfeld JL, Fonkalsrud EW, Coran AG, eds. Essentials of Pediatric Surgery. St. Louis: CV Mosby; 1995: 383–391.
- 6.Adkins PC, Blades B. A stainless steel strut for correction of pectus excavatum. Surg Gynecol Obstet 1961; 113:111–113. [PubMed] [Google Scholar]
- 7.Haller JA Jr, Scherer LR, Turner CS, et al. Evolving management of pectus excavatum based on a single institutional experience of 664 patients. Ann Surg 1989; 209:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonkalsrud EW, Bustorff-Silva J. Repair of pectus excavatum and carinatum in adults. Am J Surg 1999; 177:121–124. [DOI] [PubMed] [Google Scholar]
- 9.Shamberger RC. Congenital chest wall deformities. Curr Prob Surg 1996; 33:469–552. [DOI] [PubMed] [Google Scholar]
- 10.Beiser GD, Epstein SE, Stampfer M, et al. Impairment of cardiac function in patients with pectus excavatum, with improvement after operative correction. N Engl J Med 1972; 287:267–272. [DOI] [PubMed] [Google Scholar]
- 11.Cahill JL, Lees GM, Robertson HT. A summary of preoperative and postoperative cardiorespiratory performance in patients undergoing pectus excavatum and carinatum repair. J Pediatr Surg 1984; 19:430–433. [DOI] [PubMed] [Google Scholar]
- 12.Shamberger RC, Welch KJ. Cardiopulmonary function in pectus excavatum. Surg Gynecol Obstet 1988; 166:383–391. [PubMed] [Google Scholar]
- 13.Fonkalsrud EW, Follette D, Sarwat AK. Pectus excavatum repair using autologous perichondrium for sternal support. Arch Surg 1978; 113:1433–1437. [DOI] [PubMed] [Google Scholar]
- 14.Haller JA, Colombani P, Humphries C, et al. Chest wall constriction after too extensive and too early operations for pectus excavatum. Ann Thorac Surg 1996; 61:1618–1625. [DOI] [PubMed] [Google Scholar]
- 15.Weber TR, Kurkchubasche AG. Operative management of asphyxiating thoracic dystrophy after pectus repair. J Pediatr Surg 1998; 33:262–265. [DOI] [PubMed] [Google Scholar]
- 16.Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998; 33:545–552. [DOI] [PubMed] [Google Scholar]
- 17.Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 1999; 230:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]


