Abstract
Objective
To evaluate the results of a prospective multicenter randomized study of adjuvant intraperitoneal 5-fluorouracil (5-FU) administered during 6 days shortly after resection of stages II and III colon cancers.
Summary Background Data
Systemic adjuvant chemotherapy improves the survival of patients with stage III colon cancer receiving treatment for 6 months. Intraperitoneal chemotherapy theoretically combines peritoneal and hepatic effects.
Methods
After resection, 267 patients were randomized into two groups. Patients in group 1 (n = 133) underwent resection followed by intraperitoneal administration of 5-FU (0.6 g/m2/day) for 6 days (day 4 to day 10). These patients also received intravenous 5-FU (1 g) during surgery. Patients in group 2 underwent resection alone (n = 134).
Results
In group 1, 103 patients received the total dose, 18 received a partial dose as a result of technical or tolerance problems, and 12 did not receive the chemotherapy. Rates of surgical death and complications were similar in both groups. Tolerance to treatment was excellent or fair in 97% of the patients and poor in 3%. After a median follow-up of 58 months, 5-year overall survival rates were 74% in group 1 and 69% in group 2; disease-free survival rates were 68% and 62%, respectively. Survival curves were superimposed until 3 years after treatment and began diverging thereafter. Among patients receiving the full treatment, the 5-year disease-free survival rate was improved in the treatment group in patients with stage II cancers but was unchanged in patients with stage III cancers.
Conclusions
Chemotherapy with intraperitoneal 5-FU administered during a short period after surgery was well tolerated but was not sufficient to reduce the risk of death significantly. However, it reduced the risk of recurrence in stage II cancers. These results suggest that it should be associated with systemic chemotherapy to reduce both local and distant recurrences.
Patients with stage II and III colon cancers are considered at high risk of tumor recurrence and constitute the target population for adjuvant therapy. Systemic postoperative chemotherapy has been shown to be effective in increasing survival rates in patients with stage III colon cancer. 1,2
The recognition of predominant patterns of spread, especially the fact that 50% of recurrences are hepatic metastases and that 20% to 50% occur in the peritoneum, 3 has provided the impetus for a series of adjuvant locoregional approaches using portal vein infusion or intraperitoneal perfusion of 5-fluorouracil (5-FU). The main advantage of locoregional chemotherapy is that it achieves high regional and hepatic concentrations of drug, whereas systemic passage and thus toxicity are reduced because of the high extraction by the liver.
Dissemination of colorectal cancer to the liver through the portal vein makes the liver one of the major sites of recurrence after colorectal cancer resection, accounting for 25% to 50% of recurrences. 4 As a consequence, the first attempts at adjuvant regional therapy in colorectal cancer used the portal route, even though the vascularization of micrometastases remains controversial. 5 Despite promising initial results, 6 several studies of adjuvant intraportal chemotherapy produced controversial results. 7–10 Moreover, a meta-analysis 11 of 10 trials comprising 3,500 patients has shown that intraportal adjuvant chemotherapy offered a limited benefit. More recently, a large study comprising 1,235 patients demonstrated that low-dose intraportal 5-FU could not improve overall and disease-free survival rates or reduce the occurrence of liver metastases. 12
There has been little experience with adjuvant intraperitoneal chemotherapy. 13–15 It can theoretically add a local action to the liver effect and thus prevent both liver and peritoneal recurrences. We report in this article early and long-term results of a multicenter prospective randomized study comparing resection and early postoperative adjuvant intraperitoneal chemotherapy with 5-FU versus surgery alone in patients undergoing resection of stage II and III colon cancers. This is the largest series of this type. It is likely to remain unique: phase III trials now cannot have a control arm with surgical resection alone because systemic adjuvant chemotherapy has become the standard treatment.
METHODS
Patients
Patients with resectable T3N0M0 (stage II) or N+M0 (stage III) colon cancer were randomized to receive either surgery plus adjuvant intraperitoneal chemotherapy (group 1) or surgery alone (group 2).
The presurgical workup included physical examination, chest x-rays, ultrasound or CT scan of the abdomen, carcinoembryonic antigen serum level determination, white and red blood cell counts, platelet count, prothrombin time, and determinations of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase.
Patients were randomized at surgery after the surgeon had opened the abdomen and confirmed that the tumor was resectable and not associated with distant metastases. Inclusion in the study was confirmed after the pathologic report was received and the tumor stage was confirmed. Patients with stage I tumor were not confirmed in the study and did not receive adjuvant intraperitoneal chemotherapy.
Inclusion criteria were completely resected stages II and III adenocarcinoma of the colon and informed consent of the patient. There was no age limit for inclusion. Exclusion criteria were procedure requiring a stoma; ischemic heart disease, cardiac failure, or acute and chronic liver disease; preexisting or concomitant neoplasms (with the exception of in situ carcinoma of the cervix uteri and adequately treated basal or squamous carcinoma of the skin); and anemia (hemoglobin < 100 g/L), leukopenia (white cell count < 3.0 × 109/L), or thrombopenia (platelet count < 100 × 109/L). The study followed ethical rules in effect in France.
Surgical Technique
After it was confirmed that there were no distant metastases and curative surgery could be performed, the tumor and adjacent mesocolon and lymph nodes were resected with adequate clearance, and the anastomosis was performed. In group 1, because it was impossible to use the intraperitoneal route at that time, the patients were given 1 g 5-FU intravenously during surgery so that chemotherapy could be started as early as possible. Before closure of the wall, a 12F silicon catheter (Laboratoires Vygon, Ecouen, France) was inserted through a zigzag course in the abdominal wall into the peritoneal cavity. The tip of the catheter was positioned opposite the anastomosis. In patients in group 2, the abdomen was closed.
Intraperitoneal Chemotherapy
As soon as it was deemed likely that they had no postoperative complications, had passed gas, and had a temperature of 38°C or less, patients in group 1 were given 0.6 g/m2/day 5-FU intraperitoneally. The drug was diluted in 1.5 L peritoneal dialysis fluid and delivered using an infusion pump during a 3-hour period in the morning for 6 days. The fluid was left in the cavity until the next infusion. The catheter was removed after the last infusion, and the patient was discharged the day after.
Monitoring and Appraisal of Tolerance
Tolerance to intraperitoneal 5-FU was reviewed daily by clinical monitoring, particularly during the infusion. The tolerance to the treatment was graded as excellent in patients remaining asymptomatic until the complete treatment was administered. Tolerance was graded as poor in patients who had symptoms suggestive of potentially life-threatening complications that could not be related to the surgical procedure. In all other cases, tolerance was graded as fair. After completion of the treatment, several biologic variables were recorded (white and red cell counts, platelet count, prothrombin time, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase) and compared with preoperative values. Treatment was interrupted if sepsis, anastomotic leak, or a temperature of more than 38°C occurred.
Follow-Up
Patients were followed up after treatment by clinical examination, carcinoembryonic antigen level, chest x-rays, and ultrasound or CT scan of the abdomen. Colonoscopy or barium enema was done 1 year after surgery, then repeated every 2 years, depending on the presence of polyps. In case of recurrence, the choice of the treatment was left to the surgeon, and the patient remained in the study for analysis.
Statistical Methods
The Student t test and the chi-square test were used for statistical analysis of the results. Survival, including surgical deaths, was calculated from the date of surgery to the date of the last visit or death. Survival without recurrence was calculated from the date of surgery to the date of diagnosis of tumor recurrence. Considering the possible mechanism of action of the treatment, an analysis of survival without recurrence to the liver or to the peritoneum was made. Survival analysis was performed by computing survival curves according to the Kaplan-Meier method. Survival curves were compared between the two groups using the log-rank test. Survival curves were first compared based on the intention to treat; then, survival analysis and comparisons were made according to the treatment actually received.
RESULTS
Patients
Of the 350 inclusions initially planned, 317 patients had treatment randomly assigned to them from December 1986 to March 1991. The trial was prematurely closed at this date because the option of no adjuvant treatment after resection of stage III colon cancer was no longer ethical.
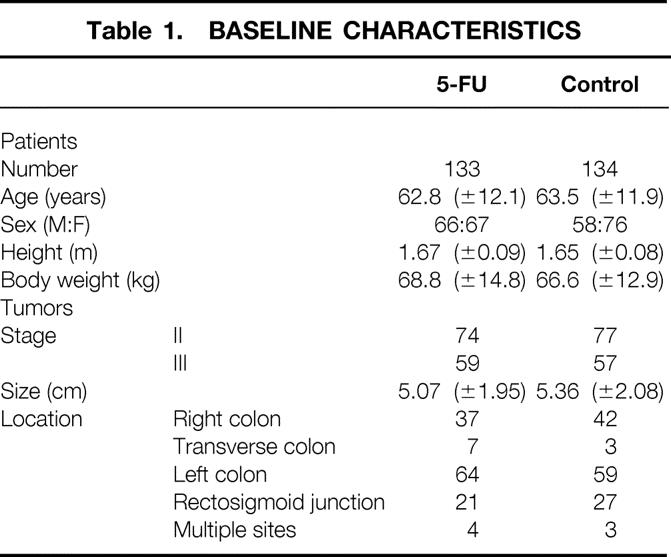
Of the 317 randomized patients, 267 patients who underwent surgery for a colon carcinoma were included in this study between December 1986 and March 1991 in nine centers. Fifty other patients had been initially randomized but were not eligible because of inadequate pathologic status (stage I cancers or distant metastases [n = 39], incomplete resection of tumor [n = 1], other malignancies [n = 6]), benign lesions (n = 2), need for a stoma (n = 1), and other cancer less than 5 years before inclusion (n = 1). Of the 267 randomized patients, 133 were in group 1 and 134 were in group 2. The groups did not differ significantly with regard to patient characteristics or tumor features (Table 1). Symptoms, preoperative biochemical tests, carcinoembryonic antigen level, and surgical procedures were similar in the two groups (data not shown). The median follow-up times were 58 months (range 0–123) in group 1 and 56 months (range 2–125) in group 2.
Table 1. BASELINE CHARACTERISTICS
Postoperative Course
Two hundred twenty-five patients had an uneventful postoperative course, 107/133 in group 1 (80.5%) and 118/134 in group 2 (88%) (df 1; chi-square = 2.91;P = .09). The surgical death rate was 1.5% in group 1 (2/133) and 0% in group 2 (df 1; chi-square = 2.03;P = .15). Deaths were due to pulmonary embolism and cardiac arrhythmia and were not directly related to the treatment of colon cancer. Reversible complications (Table 2) occurred in 24 patients in group 1 (18%) and in 16 patients in group 2 (12%) (df 1; chi-square = 2.10;P = .15).
Table 2. COMPLICATIONS
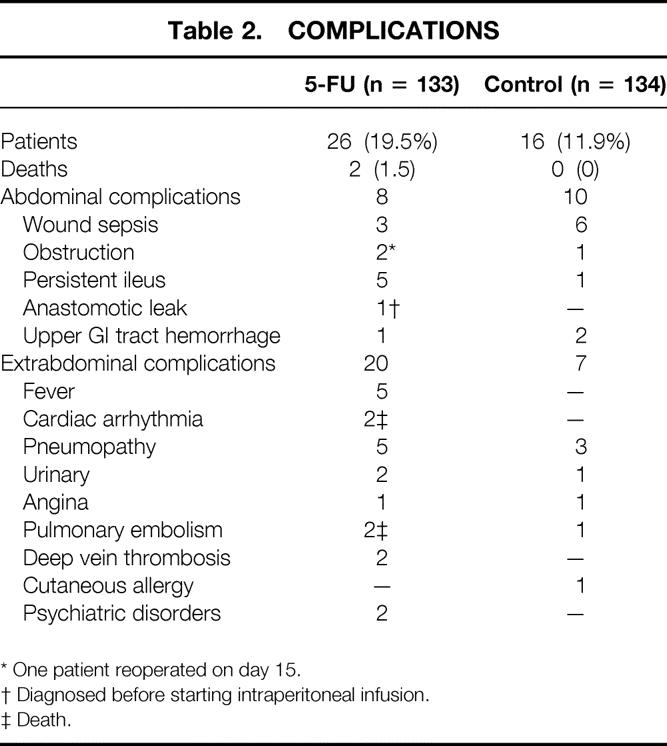
* One patient reoperated on day 15.
† Diagnosed before starting intraperitoneal infusion.
‡ Death.
Intraoperative Intravenous Infusion of 5-FU
Intravenous 5-FU was given during surgery to 132/133 patients in group 1 and by mistake to 2/134 patients in group 2. The mean duration of the infusion was 91.9 minutes ± 47.4 (range 30–240). A moderate and reversible decrease in arterial pressure was observed in three patients.
Intraperitoneal Infusion of 5-FU
The intraperitoneal infusion started between 4 and 14 days after surgery (mean 5.8 ± 1.9). Twelve of the 133 patients in the 5-FU group (9%) did not receive the intraperitoneal infusion because of obstruction or accidental withdrawal of the catheter (n = 5), postoperative complications (n = 6), or mistake (n = 1). One hundred twenty-one patients (91%) received a mean dose of 5.7 g ± 1.5 of intraperitoneal 5-FU. The mean amount of 5-FU given in 103 patients who received the scheduled dose was 6.2 g ± 6.3, whereas 18 patients received a partial dose ranging from 0.09 to 6.0 g (mean 2.9 ± 2.0). The total scheduled dose of 5-FU was not administered in these patients as a result of leaks around the catheter or through the midline incision (n = 10), less-than-perfect tolerance to infusion (n = 5), or postoperative complications (n = 7). Overall, technical problems directly related to the catheter occurred in 13 patients (10%).
Tolerance
Tolerance to treatment was excellent in 99 of 121 patients receiving intraperitoneal 5-FU (81.8%). Tolerance was considered fair in 18 (14.9%) as a result of abdominal pain and distention (n = 12), persistent ileus (n = 3), nausea and vomiting (n = 5), and diarrhea (n = 2). Tolerance was considered poor in 4 (3.3%) as a result of reversible arterial hypotension, chills without sepsis, angina pectoris, and intestinal obstruction. In three of these patients, the treatment was discontinued. No patient in whom tolerance was considered fair as a result of moderate abdominal distension during infusion had treatment discontinued. In 2 of the 103 patients who received the total dose of drug, the infusion was temporarily discontinued because of mild abdominal discomfort and persistent ileus.
The variations of the recorded biochemical parameters were similar in both groups, except the mean decrease in white cell count was statistically more important in group 1 (Table 3).
Table 3. BIOLOGIC PARAMETERS (VARIATION FROM BASELINE)
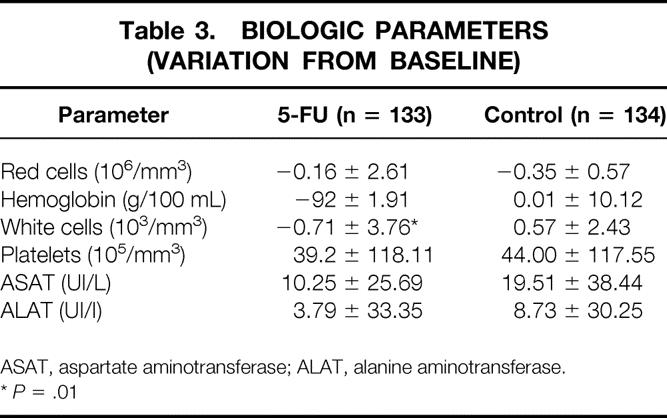
ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase.
*P = .01
Long-Term Results
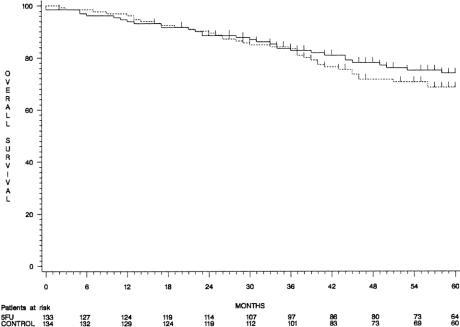
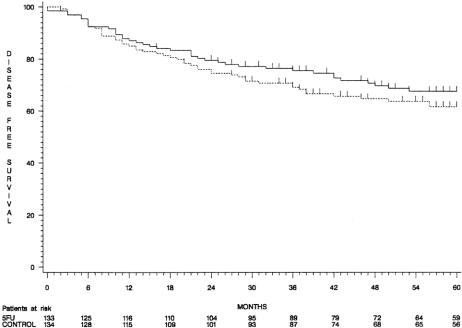
When considering the intention to treat, although 5-year overall and disease-free survival rates were higher in group 1 than in group 2 (74% ± 4% vs. 69% ± 4% and 68% ± 4% vs. 62% ± 4%, respectively), the difference did not reach statistical significance (P = .30 and P = .26, respectively) (Figs. 1 and 2). Overall survival curves were superimposed until 3 years and diverged thereafter. The comparisons were similar in patients with stage II and stage III tumors considered separately (88% ± 4% vs. 79% ± 5% and 57% ± 7% vs. 55% ± 7%).
Figure 1. Overall survival curves (group 1, continuous line; group 2, dotted line).
Figure 2. Disease-free survival curves (group 1, continuous line; group 2, dotted line).
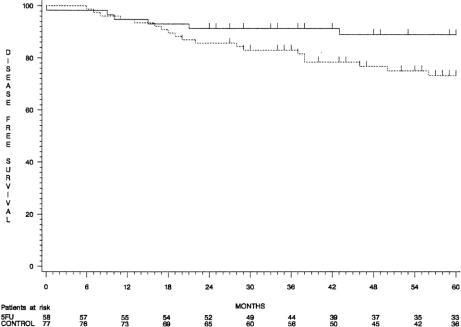
When patients who had received the full dose of intraperitoneal 5-FU were compared with those in group 2, the 5-year disease-free survival rate was significantly higher in the treated group in patients with stage II cancer (89% ± 4% and 73% ± 5%, respectively;P = .05) (Fig. 3), but it was unchanged in patients with stage III tumors. Tumor recurrences (Table 4) were observed in 33 patients (24.8%) in the 5-FU group and in 42 (31.3%) in the control group.
Figure 3. Disease-free survival curves of patients with stage II colon cancer (group 1, continuous line; group 2, dotted line).
Table 4. SITES OF TUMOR RECURRENCE
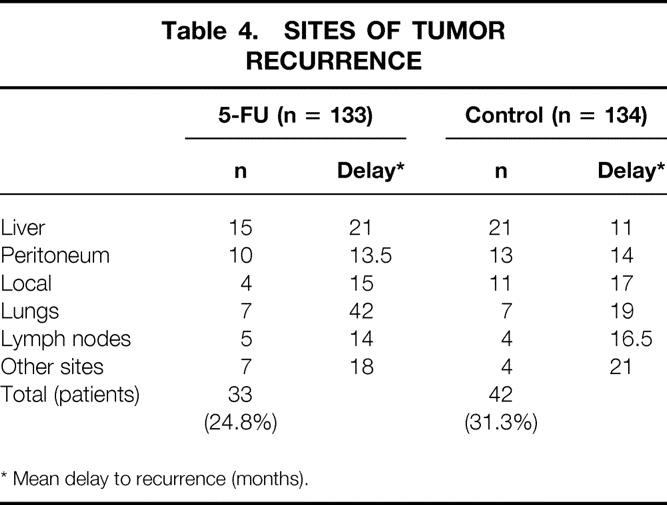
* Mean delay to recurrence (months).
DISCUSSION
Two hundred sixty-seven patients with stage II and stage III colon cancers were included in this randomized trial comparing surgery plus early postoperative adjuvant intraperitoneal 5-FU with surgery alone. Ninety-one percent of the patients in the treated group received early postoperative infusion of 5-FU. Tolerance to intraperitoneal 5-FU administered early after resection was good. The treatment was simple and safe and did not compromise the healing of recent colonic anastomoses. A trend toward improvement in overall and disease-free survival rates was observed after a delay of 3 years after the administration of intraperitoneal 5-FU. A statistically significant reduction in the recurrence rate, particularly in the liver and the peritoneum, was observed in patients with stage II cancers who had received the treatment.
Tolerance to Intraperitoneal Chemotherapy
The present multicenter series, which includes a large number of patients, first demonstrates that intraperitoneal chemotherapy with 5-FU administered during 6 days shortly after surgery is feasible and safe. Complication rates were not increased when compared with the control group, and observed complications did not appear to be related directly to the treatment.
We had demonstrated in an experimental study that healing of recent colonic anastomoses in the rat was not impaired by early postoperative intraperitoneal administration of 5-FU. 16 These data were confirmed in humans in the present study, after others involving smaller cohorts of patients. 13–15 In the present trial, intraperitoneal chemotherapy was not started until the fourth postoperative day so that we could be reasonably sure that there was no problem with the anastomosis. No signs of peritoneal toxicity were observed. Chemical peritonitis has been reported mainly in patients with advanced disease receiving intraperitoneal cisplatin combined with high doses of 5-FU 17 for long periods; this does not seem to be a problem when 5-FU is administered for a short period.
Tolerance to intraperitoneal 5-FU was excellent or fair in 97% of the patients receiving the treatment. The relation of the treatment to events reported as signs of poor tolerance is not obvious. On the whole, adverse effects of intraperitoneal drug administration are mild and reversible. Drug administration was discontinued in only three of our patients; others have reported that treatment had to be modified because of impaired tolerance, mainly abdominal pain and distention, in 20% to 30% of patients. 14,18 Abdominal distention and pain have been reported in 7% to 10% of patients 14,17,18 when large volumes of fluid are infused. Nausea and vomiting are present in 4% to 25% of the patients. Diarrhea has been more frequently observed in patients with advanced disease 17 than in adjuvant trials. 14,18 Prolonged ileus seems more common after intraperitoneal 5-FU administration than after intraperitoneal placebo. 14
In this series, systemic toxicity was limited to a mild and transient decrease in the white cell count. Hematologic toxicity, granulocytopenia, and thrombocytopenia have been mainly observed after prolonged administration of intraperitoneal 5-FU, either alone 17 or combined with intraperitoneal leucovorin and intravenous infusion. 18 No liver toxicity has been reported. 19
With experience, the risk of technical problems, such as leaks around the catheter or through the wound or obstruction or retrieval of the catheter, can be reduced. Leaks were observed in 7.5% of patients in the present study; in another study, 14 they were found in 20% to 50% of patients despite infusion of smaller volumes. Abdominal closure must be watertight and the intraperitoneal catheter must cross the wall obliquely and must be correctly fastened.
Long-Term Results
This is the only study that evaluates the long-term effects of intraperitoneal chemotherapy with 5-FU administered early after surgery for a short period and as the sole adjuvant treatment. Other published studies have reported the results of prolonged intraperitoneal 5-FU infusion delayed after surgery 19 or prolonged intravenous and intraperitoneal drug combinations. 15
The reduction in liver and locoregional recurrence rates observed in our study supports the hypothesis that intraperitoneal infusion combines locoregional and hepatic effects. Unlike the intraportal route, intraperitoneal chemotherapy may theoretically prevent relapse at the site of resection or on the peritoneum; such recurrences were present in 66.7% and 54.6%, respectively, in a necropsy series. 4 Theoretically, the intraperitoneal route combines the effect of intraportal chemotherapy on the liver with a direct effect on the peritoneum and the resection site. 20 This was observed in rats: intraperitoneal 5-FU administration after resection of a chemically induced colon carcinoma prevented liver metastases and peritoneal carcinomatosis. 21 In patients, delayed and prolonged intraperitoneal 5-FU administration was compared with intravenous 5-FU administration; it was found to reduce the peritoneal recurrence rate but did not influence survival. 19
After intraperitoneal administration, the peritoneal concentration of 5-FU can be as high as two to three logs greater than that observed in the plasma. 17,22 Because of the peritoneal–plasma barrier, large molecules, such as many chemotherapeutic agents, take longer to clear from the peritoneal cavity than smaller ones, prolonging contact between drug and tumor cells. 23,24 However, the limited penetration of the drug into the serosa makes intraperitoneal chemotherapy more suitable for adjuvant chemotherapy than for treatment of established carcinomatosis. In humans, approximately 60% of the 5-FU administered intraperitoneally is delivered to the portal circulation; from there, the drug is extracted by the liver at a rate of approximately 90%. 22 These characteristics allow a reduction in the drug concentration in plasma, thus reducing the risk of systemic toxicity, whereas high locoregional concentrations of the drug can be achieved, combining direct and liver antitumor effects. Although measurable systemic concentrations of drugs and related grade 3 toxicity have been observed after intraperitoneal administration of floxuridine and leucovorin, 13 the intraperitoneal route permitted the use of 5-FU doses as high as 1.5 times those administered intravenously, without toxicity. 19
Large volumes of fluid are necessary to achieve a good distribution of the drug. 22 Graf et al 14 confirmed by single photon emission computed tomography in five of their patients that a volume of 500 mL was not sufficient to obtain a wide distribution of fluid. The washout effect of large volumes of fluid is probably important. It may decrease fibrin accumulation and adhesions, particularly if the fluid is left in the cavity, eliminating tumor cells before they fix within scar tissues. The elimination of platelets, white blood cells, and monocytes may also diminish the production of tumor growth factor associated with the wound healing process.
Adjuvant treatment of colorectal carcinoma, administered shortly after resection of tumor, appears attractive because micrometastases are more sensitive to a given drug because of a shorter cell cycle time, better accessibility to drugs, and a smaller chance of harboring resistance. 25,26
The perioperative period appears crucial regarding the host’s defenses against the growth of tumor cells. The development of detectable liver metastases from “dormant” metastatic cells has been demonstrated in a rat model of liver resection. 27 Laparotomy per se was shown to enhance the growth of intraperitoneal tumor implants in mice. 28 Further, although spillage of tumor cells, either through the portal vein or from the involved peritoneal serosa, can occur before surgery, perioperative mobilization of the tumor and surgical dissection probably play a crucial role in tumor dissemination. 29,30 The adjuvant situation in which no obvious residual tumor is present probably represents the best indication for intraperitoneal chemotherapy as a part of multimodal treatment of colorectal cancer. 31
In theory, postoperative chemotherapy should be started as soon as possible after surgery to obtain the best anticancer effect. 32 When this study started, participating surgeons preferred to start the administration 4 days later to reduce the risk of administering intraperitoneal chemotherapy to patients with an intraabdominal complication of surgery. For this reason, intravenous 5-FU was administered during surgery. It is now clear that postoperative chemotherapy with 5-FU is safe and could be administered immediately after surgery. Yu et al 33 have shown that intraperitoneal chemotherapy can be administered just after gastrectomy.
The reduction of the recurrence rate observed in this study was limited to patients with stage II tumors receiving the treatment. This is probably due to the fact that intraperitoneal chemotherapy has a largely regional effect that is probably insufficient in patients with stage III tumors, who have more diffuse disease. In these patients, it seems logical to combine the intraperitoneal and systemic routes of administration. A combination of intraperitoneal and intravenous 5-FU plus leucovorin reduced the occurrence of locoregional and distant sites of initial relapse in patients with stage III tumors. 15
As was observed with intraportal administration of 5-FU, 7,11,34 the effect of intraperitoneal 5-FU was observed only after several years. Systemic chemotherapy is directed toward distant sites of recurrence, and its effects are observed earlier, within the first 3 years after resection of stage III colon cancers. Locoregional chemotherapy is likely to act in patients in whom the disease is still confined to the abdomen. This hypothesis is supported by the results of this study, in which improvement was observed only in patients with stage II cancers. Because systemic and intraperitoneal routes of administration of adjuvant chemotherapy appear to have different targets and different times of effect, they appear well suited for combined use.
This is the first trial demonstrating a benefit of adjuvant chemotherapy in patients with node-negative colon cancer. This short treatment was not sufficient in patients with node-positive colon cancers, who should benefit from a combination of locoregional and systemic chemotherapy (e.g., in the recent EORTC trial 40911, in which 1,850 patients have been randomized).
Footnotes
Correspondence: Bernard Nordlinger, MD, Chirurgie Digestive et Oncologique, Hôpital Ambroise Paré, 9, Avenue Charles de Gaulle, F/92104 Boulogne Cedex, France.
Partially funded by a grant from Produits Roche, Laboratoires Pharmaceutiques, Neuilly sur Seine, France.
Accepted for publication November 10, 1999.
References
- 1. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer: International Multicentre Pooled Analysis of Colon Cancer Trials investigators (IMPACT). Lancet 1995; 345:939–944. [PubMed] [Google Scholar]
- 2.Moertel CG, Fleming TR, McDonald JS, et al. Levamisole and fluorouracil for surgical adjuvant therapy of colon carcinoma. N Engl J Med 1990; 322:352–358. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol 1989; 16(Suppl 6):83–97. [PubMed] [Google Scholar]
- 4.Gilbert JM, Jeffrey I, Evans M, et al. Sites of recurrent tumour after “curative” colorectal surgery: implications for adjuvant therapy. Br J Surg 1984; 71:203–205. [DOI] [PubMed] [Google Scholar]
- 5.Archer SG, Gray BN. Vascularization of small liver metastases. Br J Surg 1989; 76:545–548. [DOI] [PubMed] [Google Scholar]
- 6.Taylor I, Machin D, Mullee M, et al. A randomized controlled trial of adjuvant portal vein cytotoxic perfusion in colorectal cancer. Br J Surg 1985; 72:359–363. [DOI] [PubMed] [Google Scholar]
- 7.Fielding LP, Hittinger R, Grace RH, et al. Randomised controlled trial of adjuvant chemotherapy by portal-vein perfusion after curative resection for colorectal adenocarcinoma. Lancet 1992; 340:502–506. [DOI] [PubMed] [Google Scholar]
- 8.Wolmark N, Rockette H, Petrelli N, et al. Long-term results of the efficacy of perioperative portal vein infusion of 5-FU for treatment of colon cancer. Proc ASCO 1994; 13:194. [Google Scholar]
- 9.Swiss Group for Clinical Cancer Research (SAKK). Long-term results of single course of adjuvant intraportal chemotherapy for colorectal cancer. Lancet 1995; 345:349–353. [PubMed] [Google Scholar]
- 10.Wereldsma JCJ, Bruggink EDM, Meijer WS, et al. Adjuvant portal liver infusion in colorectal cancer with 5-fluorouracil/heparin versus urokinase versus control. Results of a prospective randomized clinical trial (Colorectal Adenocarcinoma Trial I). Cancer 1990; 65:425–432. [DOI] [PubMed] [Google Scholar]
- 11.Liver Infusion Meta-analysis Group. Portal vein chemotherapy for colorectal cancer: a meta-analysis of 4000 patients in 10 studies. J Natl Cancer Inst 1997; 89:497–505. [PubMed] [Google Scholar]
- 12.Rougier P, Sahmoud T, Nitti D, et al. Adjuvant portal-vein infusion of fluorouracil and heparin in colorectal cancer: a randomised trial. Lancet 1998; 351:1677–1681. [DOI] [PubMed] [Google Scholar]
- 13.Kelsen DP, Saltz L, Cohen AM, et al. A phase I trial of immediate postoperative intraperitoneal floxuridine and leucovorin plus systemic 5-fluorouracil and levamisole after resection of high-risk colon cancer. Cancer 1994; 74:2224–2233. [DOI] [PubMed] [Google Scholar]
- 14.Graf W, Westlin JE, Pahlman L, et al. Adjuvant intraperitoneal 5-fluorouracil and intravenous leucovorin after colorectal cancer surgery: a randomized phase II placebo-controlled study. Int J Colorectal Dis 1994; 9:35–39. [DOI] [PubMed] [Google Scholar]
- 15.Scheithauer W, Kornek GV, Marczell A, et al. Combined intravenous and intraperitoneal chemotherapy with fluorouracil + leucovorin vs fluorouracil + levamisole for adjuvant therapy of resected colon carcinoma. Br J Cancer 1998; 77:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillan K, Nordlinger B, Ballet F, et al. The healing of colonic anastomoses after early intraperitoneal chemotherapy: an experimental study in rats. J Surg Res 1988; 44:166–171. [DOI] [PubMed] [Google Scholar]
- 17.Schilsky RL, Choi KE, Grayhack J, et al. Phase I clinical and pharmacologic study of intraperitoneal cisplatin and fluorouracil in patients with advanced intraabdominal cancer. J Clin Oncol 1990; 8:2054–2061. [DOI] [PubMed] [Google Scholar]
- 18.Scheithauer W, Kornek G, Rosen H, et al. Combined intraperitoneal plus intravenous chemotherapy after curative resection for colonic adenocarcinoma. Eur J Cancer 1995; 31:1981–1986. [DOI] [PubMed] [Google Scholar]
- 19.Sugarbaker PH, Gianola FJ, Speyer JL, et al. Prospective randomized trial of intravenous vs. intraperitoneal 5-FU in patients with advanced primary colon or rectal cancer. Semin Oncol 1985; 12(Suppl 4):101–111. [PubMed] [Google Scholar]
- 20.Gyves J. Pharmacology of intraperitoneal infusion 5-fluorouracil and mitomycin-C. Semin Oncol 1985; 12(Suppl 4):29–32. [PubMed] [Google Scholar]
- 21.Nordlinger B, Panis Y, Puts JP, et al. Experimental model of colon cancer: recurrences after surgery alone or associated with intraperitoneal 5-fluorouracil chemotherapy. Dis Colon Rectum 1991; 34:658–663. [DOI] [PubMed] [Google Scholar]
- 22.Speyer JL. The rationale behind intraperitoneal chemotherapy in gastrointestinal malignancies. Semin Oncol 1985; 12:23–28. [PubMed] [Google Scholar]
- 23.Sugarbaker PH, Graves T, DeBruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res 1990; 50:5790–5794. [PubMed] [Google Scholar]
- 24.Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978; 62:1–11. [PubMed] [Google Scholar]
- 25.Norton L, Simon R. Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep 1977; 61:1307–1317. [PubMed] [Google Scholar]
- 26.Salmon SE. Kinetics of minimal residual disease. Recent Results Cancer Res 1979; 67:5–15. [PubMed] [Google Scholar]
- 27.Panis Y, Ribeiro J, Chretien Y, et al. Dormant liver metastases: an experimental study. Br J Surg 1992; 79:221–223. [DOI] [PubMed] [Google Scholar]
- 28.Eggermont AM, Steller EP, Sugarbaker PH. Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery 1987; 102:71–78. [PubMed] [Google Scholar]
- 29.Solomon MJ, Egan M, Roberts RA, et al. Incidence of free colorectal cancer cells on the peritoneal surface. Dis Colon Rectum 1997; 40:1294–1298. [DOI] [PubMed] [Google Scholar]
- 30.Wiggers T, Jeekel J, Arends JW, et al. No-touch isolation technique in colon cancer: a controlled prospective trial. Br J Surg 1988; 75:409–415. [DOI] [PubMed] [Google Scholar]
- 31.Los G, McVie JG. Experimental and clinical status of intraperitoneal chemotherapy. Eur J Cancer 1990; 26:755–762. [DOI] [PubMed] [Google Scholar]
- 32.Jacquet P, Stuart OA, Dalton R, et al. Effect of intraperitoneal chemotherapy and fibrinolytic therapy on tumor implantation in wound sites. J Surg Oncol 1996; 62:128–134. [DOI] [PubMed] [Google Scholar]
- 33.Yu W, Whang I, Suh I, et al. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg 1998; 228:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolmark N, Rockette H, Wickerham DL, et al. Adjuvant therapy of Dukes’ A, B, and C adenocarcinoma of the colon with portal-vein fluorouracil hepatic infusion: preliminary results of National Surgical Adjuvant Breast and Bowel Project protocol C-02. J Clin Oncol 1990; 8:1466–1475. [DOI] [PubMed] [Google Scholar]