Abstract
Objective
To assess the influence of preoperative portal vein embolization (PVE) on the long-term outcome of liver resection for colorectal metastases.
Summary Background Data
Preoperative PVE of the liver induces hypertrophy of the remnant liver and increases the safety of hepatectomy.
Methods
Thirty patients underwent preoperative PVE and 88 patients did not before resection of four or more liver segments. PVE was performed when the estimated rate of remnant functional liver parenchyma (ERRFLP) assessed by CT scan volumetry was less than 40%.
Results
PVE was feasible in all patients. There were no deaths. The complication rate was 3%. The post-PVE ERRFLP was significantly increased compared with the pre-PVE value. Liver resection was performed after PVE in 19 patients (63%), with surgical death and complication rates of 4% and 7% respectively. PVE increased the number of resections of more than four segments by 19% (17/88). Actuarial survival rates after hepatectomy with or without previous PVE were comparable: 81%, 67%, and 40% versus 88%, 61%, and 38% at 1, 3, and 5 years respectively.
Conclusions
PVE allows more patients with previously unresectable liver tumors to benefit from resection. Long-term survival is comparable to that after resection without PVE.
Curative liver resection of colorectal metastasis is the only treatment offering a chance of long-term survival, which ranges from 25% to 40% at 5 years. 1–5 However, it can be performed in only 10% of patients. 5,6 Our goal is to increase the number of patients who can benefit from liver resection. When characteristics such as location, volume, multinodularity, or extrahepatic disease make tumor unresectable, we have shown that curative resection can be achieved in some patients after downstaging by effective chemotherapy. 7 When a tumor is technically resectable, resection can still be contraindicated if the future remnant liver is too small, risking severe postoperative liver failure. 8 For this group of patients, preoperative portal vein embolization (PVE) of the liver has been proposed to induce ipsilateral atrophy and contralateral compensatory hypertrophy of the remnant liver, thus preventing postoperative liver failure. The rationale for this approach relies on the experimental and clinical experience of almost 100 years. 9,10 Makuuchi et al 11 reported that fatal liver failure did not occur after major liver resection for cancer when the portal vein of the resected liver was obstructed. Since 1982, they have been performing preoperative PVE to “initiate compensatory hypertrophy of the future remnant liver.”11
The aim of the present study is to assess the place of preoperative PVE in the multimodal approach to large resections of colorectal liver metastases and its effect on patient outcome.
PATIENTS AND METHODS
Patients
From September 1990 to September 1998, 30 patients with technically resectable colorectal liver metastases requiring a resection of four or more segments were deemed ineligible for liver resection because the remnant liver was too small. PVE was proposed for these consecutive 30 patients, who represent the study population.
During the same period, 88 patients underwent a liver resection of four or more Couinaud’s segments 12 for colorectal liver metastases in our department. The resections extended to four, five, and six segments in 58, 21, and 9 patients, respectively. Sixty-seven of these 88 patients (76%) received preoperative chemotherapy based on our protocol, reported previously. 7,13–15 None of these 88 patients underwent preoperative PVE, and none had postoperative liver failure related to a remnant liver that was too small.
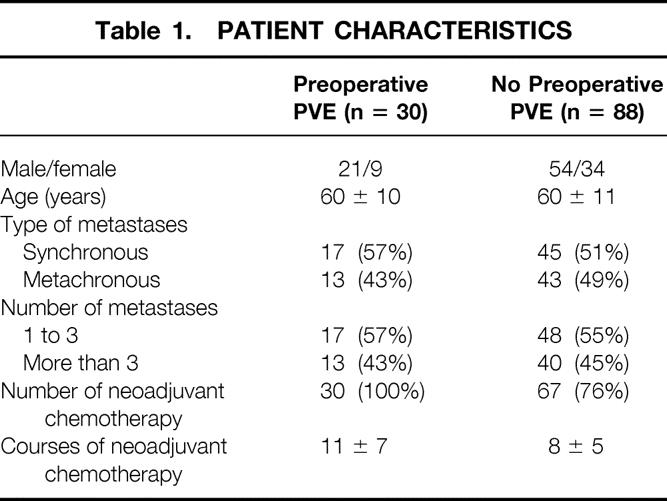
The groups with and without PVE before resection of four or more segments were comparable in terms of sex, age, number and type of metastases (synchronous vs. metachronous), and number of courses of neoadjuvant chemotherapy. Patient characteristics are shown in Table 1.
Table 1. PATIENT CHARACTERISTICS
Systemic Chemotherapy
All 30 PVE patients received an average of 11 ± 7 courses of chemotherapy before PVE. Patients received chemotherapy systematically to treat synchronous metastases (17 patients) or to diminish the tumor volume for metachronous metastases (13 patients), based on our protocol. 7,13–15 In brief, patients received chronomodulated intravenous infusions through an implanted venous access port (Port-a-Cath, Pharmacia, Uppsala, Sweden) of 5-fluorouracil (700–1,200 mg/m2/day), folinic acid (300 mg/m2/day), and oxaliplatin (25 mg/m2/day). Courses lasted 4 to 5 days and were repeated every 2 to 3 weeks. The drugs were administered in ambulatory patients using a time/dose-programmed multichannel pump (Intelliject, Aguettant, Lyon, France) in accord with the pattern recently published. 7 Neoadjuvant chemotherapy was stopped before PVE to encourage regeneration of the future remnant liver parenchyma.
Planned Hepatectomy
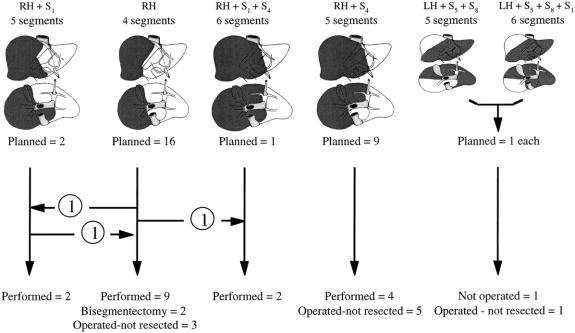
Based on the morphologic evaluation before PVE, all 30 patients were found to need a resection of at least four liver segments. 12,16 The planned hepatectomy was right-sided in 28 patients (93%) and left-sided in 2 (7%). The classification of planned hepatectomies according to the number of segments is shown in Figure 1.
Figure 1. Relation between planned hepatectomy (darkened on anterior and inferior views of the liver) before portal vein embolization (above) and actual management (below) in 30 patients. RH, right hepatectomy; LH, left hepatectomy; Sx, segment number x (according to Couinaud 12).
PVE
The main criterion for PVE was that resection of a large part of the functioning liver parenchyma was technically feasible but contraindicated because the remnant liver would be too small, with its associated risk of postoperative liver failure, the prime cause of death after hepatectomy. 17,18 The decision to proceed with PVE was made only after careful evaluation of CT scan volumetry. PVE was performed systematically when the estimated rate of remnant functional liver parenchyma (ERRFLP) was 40% or less.
The technique of percutaneous PVE was reported in detail elsewhere. 19 In summary, after selectively catheterizing a small portal vein branch contralateral (29 patients) or ipsilateral (1 patient) to the tumor under ultrasound guidance, control venous portography was performed under fluoroscopy, and a guidewire was placed into the main portal branch ipsilateral to the tumor. Embolization was then performed with a mixture of enbucrilate (Histoacryl, Braun, Melsungen Laboratories, Melsungen, Germany) and lipiodol (Lipiodol Ultrafluide, Guerbet, Aulnay-sous-Bois, France). The catheter was then removed while injecting 2 mL fibrin glue (Tissucol, Immuno AG, Vienna, Austria) into the needle tract under ultrasound control.
Follow-Up
Liver function tests including total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and prothrombin time were performed before PVE, daily for 5 days thereafter, and before surgery. CT scan volumetric measurements were performed before PVE and before surgery.
CT Scan Volumetric Measurements
CT estimation of liver volume in vivo is applicable for clinical use because there is a close linear relation between the CT-estimated volumes and actual volumes. 20–23 CT scans of the liver were obtained using a Siemens Somaton model HiQ (Siemens, Paris, France). Serial transverse scans at 1-cm intervals from the dome of the liver to the most inferior part of the organ were obtained, with enhancement by intravenous bolus injection of contrast, and with the patient suspending respiration in expiration. Each slice of the liver was traced with a cursor, and the corresponding area was calculated by computer. The middle hepatic vein and gallbladder were used as landmarks to define the borders between the right and left livers. Segment 4 volume was measured with the middle hepatic vein and the umbilical portion of the left portal vein as landmarks. The total volumes measured (whole liver volume, tumor volume, and remnant liver volume) were calculated by multiplying the area of each part by the interval thickness and by adding all the interval volumes of each part.
The ERRFLP was systematically assessed before PVE and before surgery for patients undergoing surgery and at approximately 4 weeks for those not undergoing surgery. ERRFLP was calculated as follows:MATH 1 (volume of entire liver − tumor volume)
The increase in percentage of remnant liver volume was calculated as follows:MATH 2
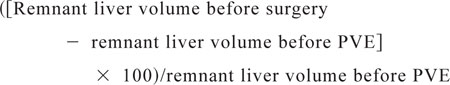 |
Decision to Perform Surgery With the Intention of Curative Hepatectomy
All CT scan investigations were reviewed by the same two radiologists experienced in hepatic imaging. Hepatic resection was reconsidered when hypertrophy of the future remnant liver was considered to have reached a plateau on repeated CT scans.
Liver Resection
The surgical technique for liver resection in our unit has been described previously. 16 A careful search of the abdominal cavity is made for recurrent local disease, extrahepatic metastases, and peritoneal seeding. Frozen-section examination is performed for any suspicious tissue and systematically for at least one lymph node of the hepatic pedicle. A complete examination of the liver is performed by palpation and by intraoperative ultrasonography to confirm the number and size of the lesions, to define their relation with the intrahepatic vascular structures, and to look for occult liver metastases. Parenchymal dissection is performed using the ultrasonic dissector (CUSA, Cavitron Ultrasonic Aspirator, Valley Lab, Boulder, CO), and resections were performed provided a tumor-free margin of 1 cm could be obtained.
Data Analysis
Results are given as mean ± standard deviation unless stated otherwise. Statistical analysis was performed as indicated with a statistical analysis program package (StatView 4.5 software, Abacus Concepts, Inc., Berkeley, CA). Paired Student t tests were used. Survival rates were calculated using the Kaplan-Meier method, and groups were compared with the log-rank test. P < .05 was considered statistically significant.
RESULTS
PVE was feasible in all 30 patients (technical success rate of 100%). Right PVE was performed in 28 patients. The branches to segment 4 (caudate lobe) were not embolized routinely even in patients in whom this segment was to be resected. Left portal vein associated to right anterior portal vein embolization was performed in two patients.
No patients died within 2 months of PVE. In the one patient with an ipsilateral percutaneous approach to the embolized liver, the ipsilateral branch of the hepatic artery was damaged during right portal vein embolization. This led to septic liver necrosis, which was treated by percutaneous drainage (morbidity rate 3%). The patient recovered but refused liver resection. He died of his disease 20 months after PVE.
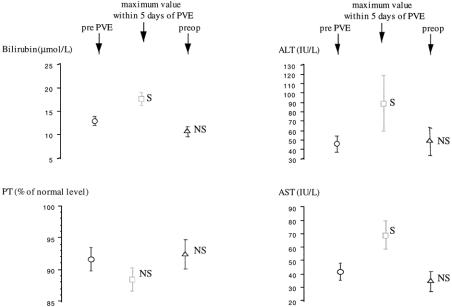
Before PVE, all patients had laboratory values in the normal range. Figure 2 shows that maximum values of total bilirubin and both AST and ALT in the 5 days after PVE were increased compared with pre-PVE values, whereas prothrombin time remained unmodified. Before surgery, all liver function test values had returned to baseline.
Figure 2. Chronologic course of liver biochemical parameters. Data are expressed as mean ± SEM. AST, aspartate aminotransferase; ALT, alanine aminotransferase; PT, prothrombin time; PVE, portal vein embolization; S and NS, significantly (P < .005, paired t test) and not significantly different from value before portal vein embolization.
Before PVE, the ERRFLP was less than 30% in 21 patients (70%), 30% to 35% in 7 patients (23%), and 35% to 40% in 2 patients (7%). The most important result was the significant increase of the ERRFLP, from 26% ± 6% to 37% ± 8% (P < 10−4) (gain 43% ± 14%, range 14–71%, median 42%). Based on the volumetric assessment, all 30 patients were considered to have sufficient liver reserve for the initial planned hepatectomy.
Liver Resection
Figure 1 shows the relation between the planned hepatectomy before PVE and the actual management of the 30 patients. Two patients (6%) did not undergo surgery: one, already mentioned, refused surgery, and the other had contralateral tumoral progression precluding curative resection. Twenty-eight patients (94%) underwent surgery a mean of 63 ± 45 days after PVE (range 21–136 days, median 49 days). At laparotomy, resection was canceled in nine patients (30%) because tumoral extension precluded curative resection. Extrahepatic tumor spread was found in three patients, intrahepatic tumor spread in six patients. In the former patients, tumor deposits confirmed by frozen-section examination were found in the hepatic pedicle (lymph node) and in the peritoneum in one and two patients, respectively. In the latter cases, palpation or ultrasonography of the liver found contralateral vascular invasion in four patients, either to the hilar structures or the confluence of hepatic veins into the vena cava, and contralateral deep nonresectable nodules in two patients.
Nineteen patients (63%) underwent liver resection extended to four liver segments in nine patients, five segments in six patients, and six segments in two patients. Two patients had a resection of only two segments, thus having had an a posteriori unnecessary PVE. One patient underwent a right hepatectomy instead of the planned right hepatectomy plus segment 1. Two patients needed a larger hepatectomy than anticipated: two scheduled right hepatectomies needed to be extended to segment 1 and segments 1 and 4 (see Fig. 1).
Impact of PVE on the Feasibility of Liver Resection
Overall, preoperative PVE allowed resection in 19/30 patients (63%); 17 of these resections involved four or more segments. During the same period, 88 patients with colorectal liver metastases underwent a hepatectomy of four or more segments without preoperative PVE. Thus, during the study period, PVE increased the number of this type of hepatectomy by 19% (17/88).
Postoperative Course
There was one death within 60 days of surgery (3%). This patient underwent PVE after 18 courses of chemotherapy for bilateral colorectal metastases and resection of segment 3 and the left part of segment 2 (two-stage hepatectomy). He died of sepsis and multiple organ failure 45 days after right hepatectomy. The left branch of the portal vein was damaged during the second operation, necessitating blood transfusion (10 units) as well as continuous portal triad clamping for 27 minutes for repair. In addition, the right hepatectomy required five sessions of intermittent portal triad clamping of 15 minutes each. This death was technique-related and not attributable directly to the PVE.
No intraoperative difficulties were encountered in any patient with regard to previous PVE. Two patients had biliary fistulas (surgical complication rate 7%) that healed spontaneously. The nine patients who underwent only exploratory laparotomy and the five patients who had a smaller (n = 3) or a larger (n = 2) resection than planned all had an uneventful postoperative course. There was no case of liver failure among the 28 patients who underwent surgery.
The actuarial survival rates of the 19 patients who underwent resection after PVE were 81%, 67%, and 40% at 1, 3, and 5 years, respectively. This was statistically better than for the 11 patients who did not undergo surgery (n = 2) or who underwent surgery but not resection (n = 9) (actuarial survival rates = 50%, 15%, and 15% at 1, 3, and 5 years, respectively;P = .04, log-rank). The actuarial survival rate of patients who underwent resection after PVE was comparable to that of the 88 patients who underwent resection without PVE (actuarial survival rates = 88%, 61%, and 38% at 1, 3, and 5 years, respectively;P = .9, log-rank), as shown in Figure 3. These results were maintained even considering only the 17 patients who underwent resection of four or more segments after PVE (data not shown).
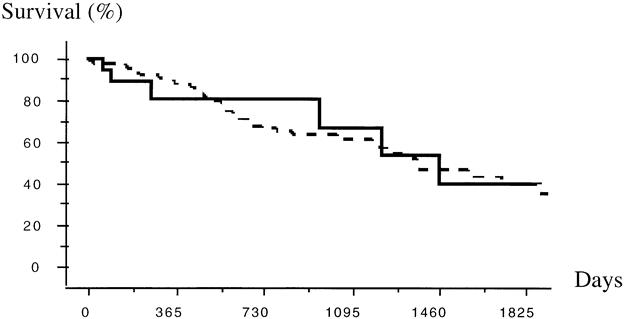
Figure 3. Actuarial survival rates after liver resection of four or more segments (according to Couinaud 12) in 88 patients without (dashed line) and in 19 patients with (solid line) preoperative portal vein embolization. P = .9, log-rank.
DISCUSSION
As a preparation for large hepatic resection, PVE was feasible in all patients of the present series. There were not deaths, and the complication rate was low. By inducing hypertrophy of the remaining liver, PVE allowed resection with curative intent in 19 patients. In 11 patients, resection did not follow PVE, mainly because of tumoral extension (10/11). This series confirms reports on the feasibility of PVE, the delay and extent of induced contralateral hypertrophy, and the subsequent resectability in patients with formerly unresectable tumors because of a potentially insufficient remnant liver. 24–41 This series is the first to report a long-term survival rate comparable to that after primary equivalent resections. These actuarial survival rates are comparable to those reported for resection of colorectal metastases (i.e., 25–40% at 5 years 2–7). Because resection was the only chance of long-term survival, and because of the success of PVE from previous experience, we considered it unethical to design a randomized study in which some patients would not undergo PVE.
We found that PVE increased the feasibility of liver resection of four or more segments by 19%. This indicates that this technique can be used in a significant subset of patients, in whom it introduces the possibility of safe, curative resection. Preoperative PVE in this type of patient was used in 8.6% of patients by Roche et al 40 and 3% of patients by Kawasaki et al. 30 In both of these series, the number of hepatectomies involving four or more segments was not reported.
The discrepancy between the planned and the actual management of our patients is due not only to imaging inaccuracy but also to other variables, including the systematic use of intraoperative frozen-section analysis of liver pedicle lymph nodes and of any suspicious tissue, the systematic use of intraoperative ultrasonography of the liver, and finally the aggressive approach to these patients, resection being the only chance of long-term survival.
Even if it is assumed that an ERRFLP of less than 30% is associated with a high risk of postoperative liver failure, we performed PVE for ERRFLPs of 40% or less. This cutoff rate was chosen because all our patients had received previous massive chemotherapy, which is known to be a major risk factor for postoperative liver failure. 42,43 In fact, in the present series, the ERRFLP before PVE was less than 30% in 70% of the patients and 30% to 40% in 30% of the patients. Imamura et al 41 have performed PVE in 86 of 113 patients (76%) scheduled for hepatectomy leaving less than 45% of liver parenchyma.
The fact that PVE is well tolerated might enlarge its indications in the future. PVE could be used not only for patients with an insufficient remnant liver but also to increase the safety of large liver resections, even if already feasible in terms of remnant liver volume.
We have used cyanoacrylate as the embolizing material because it ensures a definitive obstruction of the portal vein. Embolization with absorbable material in one series had a 50% failure rate, and these patients required repeated embolizations. 26 Tsuge et al 33 and Roche et al 40 have switched from Gelfoam to cyanoacrylate because of the high rate of embolized portal vein repermeation with Gelfoam. The concomitant use of lipiodol in our technique allows visualization of the embolized portal cast on plain radiographs. 19 The significant and transient increase in bilirubin and transaminases after PVE may be explained by extensive peribiliary inflammation and hepatocyte necrosis, as demonstrated by De Baere et al 27 when using cyanoacrylate. Other materials used with success have included gelatin sponge, 26 thrombin, 31 fibrin glue, 34 Gelfoam, 32 or alcohol. 37
The ipsilateral or contralateral approach to the portal vein branch to be embolized is controversial. 29,35 After the one case of septic necrosis of the embolized liver resulting from an iatrogenic arterial branch thrombosis after an ipsilateral portal vein embolization, we have preferred the contralateral approach. Provided the arterial branch of the embolized parenchyma is patent, PVE has been clinically well tolerated in all reported series.
PVE may be considered retrospectively as unnecessary in two of our patients who underwent resection of only two segments. These patients had an uneventful postoperative course. Makuuchi et al 11 described 16 patients undergoing PVE in whom either hepatectomy was canceled or the resected liver part was smaller than the embolized volume. No problem attributable to PVE was reported, as in the present series. A larger-than-planned hepatectomy was performed in two of our patients (see Fig. 1). This was possible because of sufficient hypertrophy of the nonembolized liver parenchyma.
PVE via the ileocolic vein through laparoscopy 33 or laparotomy 11,41 allows the surgeon to assess hepatic or extrahepatic tumor extension that would preclude future curative resection, possibly avoiding unnecessary PVE. In a series of 86 patients with various tumors, Imamura et al 41 identified only two inoperable tumors based on the findings at laparotomy for PVE.
The present series shows that a significant subset of patients a priori excluded from large curative resection may be able to undergo surgery if a multimodal approach is used, including both neoadjuvant chemotherapy, which diminishes or at least limits the tumor burden, and preoperative PVE, which increases the remaining liver volume. 7,43
CONCLUSION
PVE followed by hepatic resection represents a two-step hepatectomy: progressive atrophy of the embolized territory, which triggers compensatory hypertrophy of the future remaining parenchyma, followed by liver resection. This approach preserves sufficient functioning liver parenchyma to prevent postoperative liver failure. By removing the contraindication of an insufficient remnant liver, PVE increases the resectability of colorectal liver metastases with a survival benefit comparable to that obtained with primary liver resection. It can be considered a new neoadjuvant modality in the management of hepatic colorectal metastases.
Footnotes
Correspondence: Daniel Azoulay, MD, PhD, Centre Hépato-Biliaire, Hôpital Paul Brousse, 94804, Villejuif, France.
Accepted for publication October 8, 1999.
References
- 1.Fortner JG, Silva JS, Golbey RB, et al. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. Ann Surg 1984; 199:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adson MA. Resection of liver metastases: when is it worthwhile? World J Surg 1987; 11:511–520. [DOI] [PubMed] [Google Scholar]
- 3.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Surgery 1988; 103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 4.Jaeck D, Bachelier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Br J Surg 1997; 84:977–980. [DOI] [PubMed] [Google Scholar]
- 5.Scheele J, Stangl R, Altendorf-Hofmann A, Paul M. Resection of colorectal metastases. World J Surg 1995; 19:59–71. [DOI] [PubMed] [Google Scholar]
- 6.Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer: biologic perspectives. Ann Surg 1989; 210:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bismuth H, Adam R, Lévi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996; 224:509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boerma EJ. Research into the results of resection of hilar bile duct cancer. Surgery 1990; 108:572–580. [PubMed] [Google Scholar]
- 9.Rous P, Larimore LD. Relation of the portal blood to liver maintenance. J Exp Med 1920; 31:609–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schalm L, Bax HR, Mansens BJ. Atrophy of the liver after occlusion of the bile ducts or portal vein and compensatory hypertrophy of the unoccluded portion and its clinical importance. Gastroenterology 1956; 31:131–154. [PubMed] [Google Scholar]
- 11.Makuuchi M, Le Thai B, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990; 107:521–527. [PubMed] [Google Scholar]
- 12.Couinaud C. Le Foie: Etudes Anatomiques et Chirugicales. Paris: Masson et Cie; 1957.
- 13.Caussanel JP, Lévi F, Brienza S, et al. Phase I trial of 5-day continuous venous infusion of oxaliplatinum at circadian-modulated vs. constant rate. J Nat Cancer Inst 1990; 82:1046–1050. [DOI] [PubMed] [Google Scholar]
- 14.Lévi F, Misset JL, Brienza S, et al. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichanel programmable pump. Cancer 1992; 69:893–900. [DOI] [PubMed] [Google Scholar]
- 15.Lévi F, Zidani R, Misset JL, the International Organization for Cancer Chronotherapy. Randomized multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet 1997; 350:681–686. [DOI] [PubMed] [Google Scholar]
- 16.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg 1982; 6:3–9. [DOI] [PubMed] [Google Scholar]
- 17.Detroz B, Sugarbaker PH, Knol JA, et al. Causes of death in patients undergoing liver surgery. Cancer Treat Res 1994; 69:241–257. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann H, Reichen J. Hepatectomy: preoperative analysis of hepatic function and postoperative liver function [review]. Dig Surg 1998; 15:1–11. [DOI] [PubMed] [Google Scholar]
- 19.Azoulay D, Raccuia JS, Castaing D, Bismuth H. Right portal vein embolization in preparation for major hepatic resection. J Am Coll Surg 1995; 181:267–269. [PubMed] [Google Scholar]
- 20.Heymsfield SB, Fulenwider, Nordlinger B, et al. Accurate measurement of liver, kidney, and spleen volume mass by computerized axial tomography. Ann Intern Med 1979; 90:185–187. [DOI] [PubMed] [Google Scholar]
- 21.Henderson JM, Heymsfield SB, Horowitz J, Kutner MH. Measurement of liver and spleen volume by computed tomography. Radiology 1981; 141:525–527. [DOI] [PubMed] [Google Scholar]
- 22.Van Thiel DH, Hagler NG, Schade RR, et al. In vivo hepatic volume determination using sonography and computed tomography. Gastroenterology 1985; 88:1812–1817. [DOI] [PubMed] [Google Scholar]
- 23.Nagino N, Nimura Y, Kamiya J, et al. Right or left trisegment portal vein embolization for hilar duct carcinoma. Surgery 1995; 117:677–681. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986; 10:803–808. [DOI] [PubMed] [Google Scholar]
- 25.Nakao N, Miura K, Takahashi H, et al. Hepatocellular carcinoma: combined hepatic, arterial, and portal venous embolization. Radiology 1986; 161:303–307. [DOI] [PubMed] [Google Scholar]
- 26.Makuuchi M, Kosuge T, Lygidakis NJ. New possibilities for major liver surgery in patients with Klatskin tumors and primary hepatocellular carcinoma: an old problem revisited. Hepato-Gastroenterology 1991; 38:329–336. [PubMed] [Google Scholar]
- 27.De Baere TD, Roche A, Vavasseur D, et al. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology 1993; 188:73–77. [DOI] [PubMed] [Google Scholar]
- 28.Lee KC, Kinoshita H, Hirohashi K, et al. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg 1993; 17:109–115. [DOI] [PubMed] [Google Scholar]
- 29.Nagino M, Nimura Y, Hayakawa N. Percutaneous transhepatic portal embolization using newly devised catheters: preliminary report. World J Surg 1993; 17:520–-524. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki S, Makuuchi M, Kakazu T, et al. Resection for multiple metastatic liver tumors after portal embolization. Surgery 1994; 115:674–677. [PubMed] [Google Scholar]
- 31.Kawasaki S, Makuuchi M, Miyagawa S, Kakazu T. Radical operation after portal embolization for tumor of the hilar bile duct. J Am Coll Surg 1994; 178:480–486. [PubMed] [Google Scholar]
- 32.Yamakado K, Hirano T, Kato N, et al. Hepatocellular carcinoma: treatment with a combination of transcatheter arterial chemoembolization and transportal ethanol injection. Radiology 1994; 193:75–80. [DOI] [PubMed] [Google Scholar]
- 33.Tsuge H, Mimura H, Kawata N, Orita K. Right portal vein embolization before extended right hepatectomy using laparoscopic catheterization of the ileocolic vein: a prospective study. Surg Laparoscop Endoscop 1994; 4:258–263. [PubMed] [Google Scholar]
- 34.Nagino N, Nimura Y, Kamiya J, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology 1995; 21:434–439. [PubMed] [Google Scholar]
- 35.Nagino M, Nimura Y, Kamiya J, et al. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology 1996; 200:559–563. [DOI] [PubMed] [Google Scholar]
- 36.De Baere T, Roche A, Elias D, et al. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology 1996; 24:1386–1391. [DOI] [PubMed] [Google Scholar]
- 37.Shimamura T, Nakajima Y, Una Y, et al. Efficacy and safety of preoperative percutaneous transhepatic portal embolization with absolute ethanol: a clinical study. Surgery 1997; 81:135–141. [DOI] [PubMed] [Google Scholar]
- 38.Wakabayashi H, Okada S, Maeba T, Maeta H. Effect of preoperative portal vein embolization on major hepatectomy for advanced-stage hepatocellular carcinomas in injured livers: a preliminary report. Surg Today Jpn J Surg 1997; 27:403–410. [DOI] [PubMed] [Google Scholar]
- 39.Elias D, De Baere T, Roche A, et al. Preoperative selective portal vein embolizations are an effective means of extending the indications of major hepatectomy in the normal and injured liver. Hepato Gastroenterol 1998; 45:170–177. [PubMed] [Google Scholar]
- 40.Roche A, Lasser P, De Baere T, Elias D. L’embolisation portale préopératoire: un moyen efficace pour hypertrophier le foie sain et élargir les indications des résections hépatiques. Chirurgie 1998; 123:67–73. [DOI] [PubMed] [Google Scholar]
- 41.Imamura H, Shimada R, Kubota M, et al. Preoperative portal vein embolization: an audit of 84 patients. Hepatology 1999; 29:1099–1105. [DOI] [PubMed] [Google Scholar]
- 42.Didolkar MS, Fitzpatrick JL, Elias EG, et al. Risk factors before hepatectomy, hepatic function after hepatectomy and computed tomographic changes as indicators of mortality from hepatic failure. Surg Gynecol Obstet 1989; 169:17–26. [PubMed] [Google Scholar]
- 43.Elias D, Lasser P, Rougier P, et al. Frequency, technical aspects, results, and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg 1995; 180:213–219. [PubMed] [Google Scholar]