Abstract
Objective
To describe a large single-center experience with hepatic resection for metastatic leiomyosarcoma.
Summary Background Data
Liver resection is the treatment of choice for hepatic metastases from colorectal carcinoma. In contrast, the role of liver resection for hepatic metastases from leiomyosarcoma has not been defined.
Methods
The records of 26 patients who between 1982 and 1996 underwent a total of 34 liver resections for hepatic metastases from leiomyosarcoma were reviewed. There were 23 first, 9 second, and 2 third liver resections. The records were analyzed with regard to survival and predictive factors.
Results
In the 23 first liver resections, there were 15 R0, 3 R1, and 5 R2 resections. Median survival was 32 months after R0 resection and 20.5 months after R1/2 resection. The 5-year survival rate was 13% for all patients and 20% after R0 resection. In 10 patients with extrahepatic tumor at the time of the first liver resection, 6 R0 and 4 R2 resections were achieved. After R0 resection, the median survival was 40 months (range 5–84 months), with a 5-year survival rate of 33%. After repeat liver resection, the median survival was 31 months (range 5–51 months); after R0 resection, median survival was 31 months and after R1/2 resection it was 28 months. There was no 5-year survivor in the overall group after repeat liver resection.
Conclusions
Despite frequent tumor recurrence, the long-term outcome after liver resection for hepatic metastases from leiomyosarcoma is superior to that after chemotherapy and chemoembolization. Although survival after tumor debulking also seems to be more favorable than after nonoperative therapy, these data indicate that only an R0 resection offers the chance of long-term survival. The presence of extrahepatic tumor should not be considered a contraindication to liver resection if complete removal of all tumorous masses appears possible. In selected cases of intrahepatic tumor recurrence, even repeated liver resection might be worthwhile. In view of the poor results of chemoembolization and chemotherapy in hepatic metastases from leiomyosarcoma, liver resection should be attempted whenever possible.
The liver is a common site of metastases from gastrointestinal or retroperitoneal leiomyosarcoma. However, hepatic recurrence is uncommon after curative surgery of primary sarcomas on the trunk or extremities. 1 Because metastases from leiomyosarcoma are usually not sensitive to chemotherapy or chemoembolization, the outcome is often poor, with only short survival. Without treatment, the median survival of patients with liver metastases is no more than 14 months. 2,3
Although in recent years hepatic resection has become a safe procedure, with a surgical death rate of less than 5% in most series, there are few data on liver resection for noncolorectal, nonneuroendocrine metastases. In view of the limited number of therapeutic options for hepatic metastases from leiomyosarcoma, an aggressive surgical approach to this tumor in an attempt to improve the otherwise poor prognosis appears justified. In this report we present our experience with hepatic resection for liver metastases from leiomyosarcoma.
PATIENTS AND METHODS
Between January 1983 and December 1996 in the Klinik für Abdominal- und Transplantationschirurgie of the Hannover Medical School, 34 liver resections were performed in 26 patients with liver metastases from leiomyosarcoma. There were 18 men and 8 women, with a mean age of 54 years (range 23–67 years). The site of the primary tumor was the stomach (n = 8; 31%), small bowel (n = 4; 15%), vena cava (n = 1; 4%), kidney (n = 1; 4%), colon (n = 1; 4%), upper abdomen/stomach (n = 5; 19%), and retroperitoneum (n = 5; 19%).
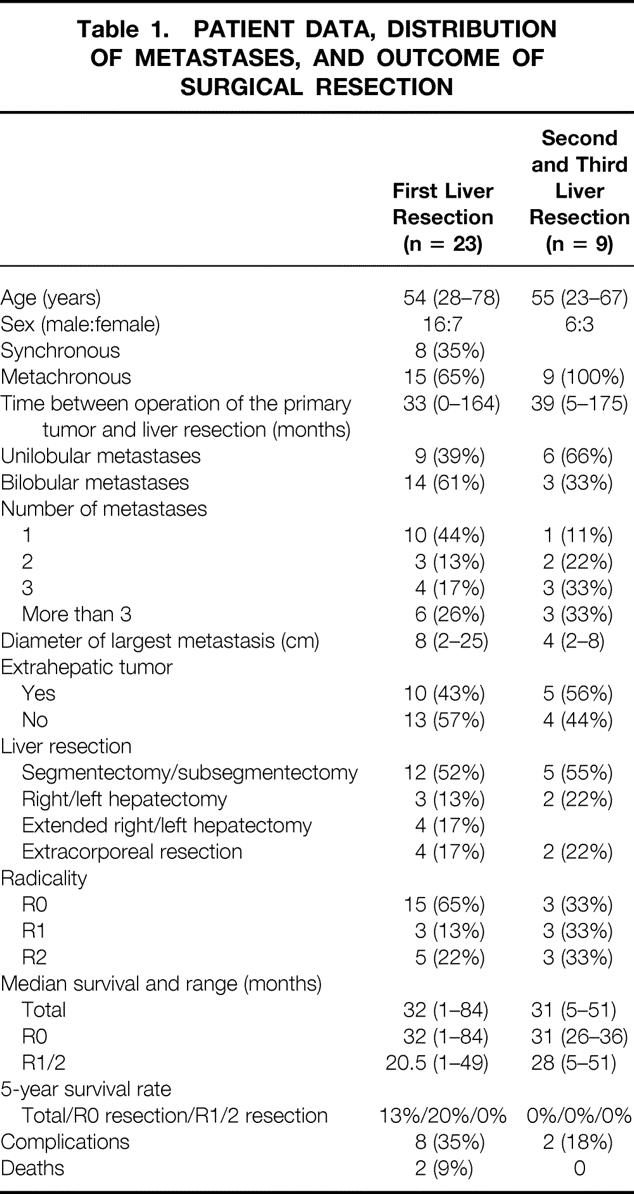
Three patients had already undergone one liver resection before admission to our department. Overall, 23 first liver resections, 9 second, and 2 third resections were performed (Table 1).
Table 1. PATIENT DATA, DISTRIBUTION OF METASTASES, AND OUTCOME OF SURGICAL RESECTION
The patients’ medical records were analyzed for the intraoperative and postoperative course, and follow-up was until December 1998 or death. In all but one patient, the data evaluated were complete. Survival was calculated in accordance with the Kaplan-Meier method, and statistical analysis was performed with the log-rank test.
RESULTS
First Liver Resection
Of the 23 first liver resections, 8 were synchronous (occurrence of metastases within 6 months after the diagnosis of primary tumor) and 15 were metachronous. At the time of the 23 liver resections, extrahepatic tumor was found in 10 patients. The extrahepatic tumor was the primary tumor in five, the primary tumor plus other extrahepatic tumor in three, and a tumor other than the primary in two patients.
Hepatic involvement was unilobular in 9 and bilobular in 14 patients. A solitary metastasis was found in 10 patients. In three patients there were two hepatic metastases, in four patients three, and in six patients more than three. The diameter of the metastasis (in multiple tumors, that of the largest metastasis) was 8 cm (range 2–25 cm).
The 23 first liver resections included 12 segmentectomies or subsegmentectomies or a combination of both, 3 right hepatectomies, 3 extended right hepatectomy, and 1 extended left hepatectomy, as well as 4 extracorporeal (ante situm, ex situ) resections. Table 1 summarizes the patient data, the distribution of metastases, and the outcome of surgical resection.
Second and Third Liver Resection
There were nine second and two third liver resections. In second liver resections, the metastases were unilobular in six and bilobular in three patients. In five of nine patients, the tumor was confined to the liver, and in four patients an additional extrahepatic tumor was found. The diameter of the metastases ranged from 2 to 8 cm (median 4 cm). The nine second liver resections comprised five segmentectomies or subsegmentectomies, two right hepatectomies, and two extracorporeal (ante situm, ex situ) resections (see Table 1).
In both patients undergoing a third liver resection, extrahepatic tumor was diagnosed (lymph node and pulmonary metastases). In both, only a segmental liver resection was performed.
Tumor Grading
Tumor grading showed low-grade leiomyosarcoma (grade I/II) in all but one of the first and second liver resections. Only one patient with liver metastases from a high-grade leiomyosarcoma (grade III/IV) underwent repeat liver resection.
Radicality, Survival, and Tumor Recurrence
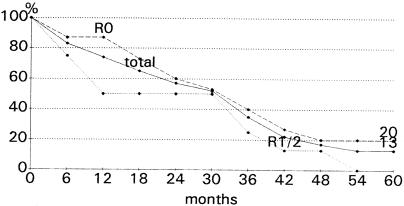
The 23 first liver resections included 15 R0, 3 R1, and 5 R2 resections. R0 resection is defined as complete removal of both liver metastases and probable extrahepatic tumor, R1 resection as resection margins with histologic tumor infiltration, and R2 resection as macroscopic residual tumor after resection. Median survival was 32 months (range 1–84 months) after R0 resection and 20.5 months (range 1–49 months) after R1/2 resection (P = .31; NS) (Fig. 1). The 5-year survival rate was 13% for all patients, and 20% and 0% after R0 and R1/2 resections, respectively (see Table 1).
Figure 1. Survival after first liver resection for hepatic metastases from leiomyosarcoma.
After curative resection of synchronous metastases (n = 4), median survival was 22 months; after metachronous metastases (n = 11), it was 32 months (P = .61; NS).
In second liver resection, there were 3 R0, 3 R1, and 3 R2 resections. Median survival after the second liver resection was 31 months (range 5–51 months). For R0 resection, the median survival was 31 months; for R1/2 resection, it was 28 months. There was no 5-year survivor in the overall group undergoing repeated liver resection (see Table 1). The two third liver resections were both classified as R2 resection, and the patients survived 9 and 14 months.
Overall, three patients survived longer than 5 years. One of these patients died 84 months after the first liver resection of an intrahepatic tumor recurrence; the two other patients were still alive 63 months after surgery. Both of these latter patients had no evidence of disease at last follow-up, but one of them underwent another operation for three intraabdominal tumor nodules in the mesentery and greater omentum. In all the other patients, intrahepatic or extrahepatic tumor recurrence was seen.
Death and Complication Rates
Overall, the surgical complication rate was 29% (10/34). This included transient liver insufficiency (n = 2), lymph fistula (n = 2), bile leakage (n = 1), pneumonia (n = 1), sepsis (n = 1), hemorrhage (n = 1), portal vein thrombosis (n = 1), and pleural effusion (n = 1). After first liver resection, complications occurred in 8 of 23 patients (34%); after second resection, complications occurred in 2 of 9 patients (22%). Complications occurred after ex situ or ante situm resection (n = 4), after extended hepatectomy (n = 2), after right hepatectomy (n = 1), and after segmentectomy (n = 3).
Two patients died in the hospital after liver resection (1 month after surgery), resulting in a surgical death rate of 6%. Both lethal complications occurred after segmentectomy/subsegmentectomy. In one patient, intraoperative hemorrhage required massive transfusion of packed blood cells (5,500 mL). In the other patient, severe postoperative bleeding after an uneventful surgical course required transfusion of 16,000 mL packed blood cells. Causes of death were sepsis and multiorgan failure, in one case probably resulting from a portal vein thrombosis (see Table 1).
The median intraoperative volume of transfused packed blood cells was 1,250 mL (range 0–5,500 mL). In extracorporeal liver resection, the median volume was 2,250 mL (range 0–5,500 mL); for right, left, or extended hepatectomy, the median volume was 1,250 mL (range 500–4,000 mL); and for segmentectomy/subsegmentectomy, the median volume was 1,000 mL (range 0–5,500 mL).
Resection of Extrahepatic Tumor
At the first liver resection, an extrahepatic tumor was found in 10 patients. Table 2 shows the location of the extrahepatic tumor, the radicality of surgery, and patient survival.
Table 2. LOCATION OF EXTRAHEPATIC TUMOR, SURGICAL RADICALITY, AND OUTCOME IN 10 PATIENTS UNDERGOING FIRST LIVER RESECTION FOR METASTASES FROM LEIOMYOSARCOMA
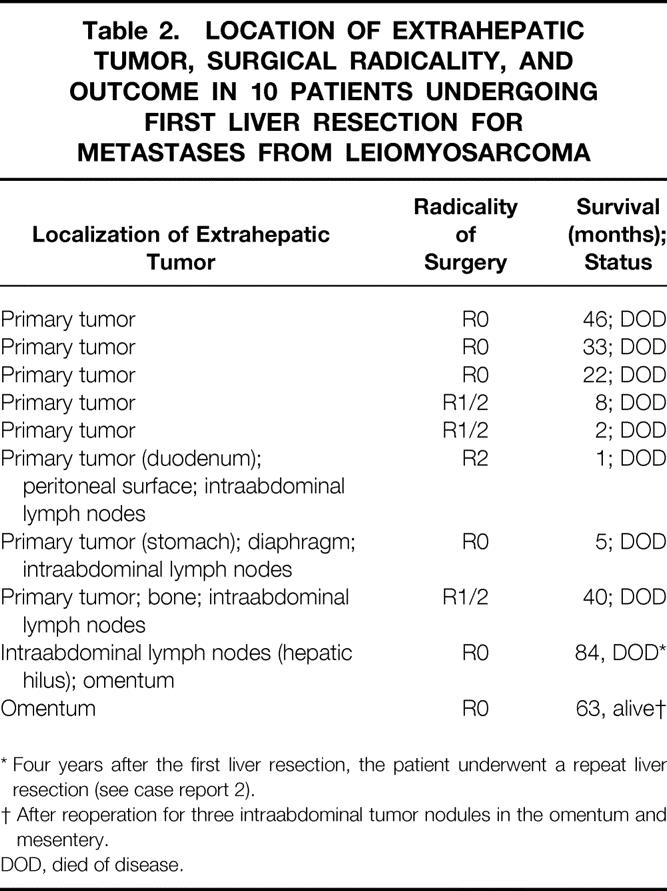
* Four years after the first liver resection, the patient underwent a repeat liver resection (see case report 2).
† After reoperation for three intraabdominal tumor nodules in the omentum and mesentery.
DOD, died of disease.
In the 13 patients with no extrahepatic tumor at the time of liver resection, an R0 resection was possible in 9. In these patients, median survival was 25 months (range 1–63 months); one patient survived more than 5 years (5-year survival rate 11%). After an R1/2 resection in patients with no extrahepatic tumor, the median survival was 37 months (range 33–49 months), with no 5-year survivor.
In the 10 patients with an extrahepatic tumor at the first liver resection, six R0 and four R2 resections were achieved. After R0 resection, the median survival was 39.5 months (range 5–84 months), with a 5-year survival rate of 33%. After R1/2 resection, the median survival was 5 months.
Statistical analysis failed to reveal any significant difference in survival after R0 resection in patients with versus without extrahepatic tumor.
Extracorporeal Liver Resection
In five patients, a total of six extracorporeal hepatic resections were performed. There were three ex situ and three ante situm resections. Complications occurred in four of six (one hemorrhage, two lymph fistulas, and one bile leakage), with no surgical deaths. After ex situ resection, patient survival was 13, 18, and 36 months (case report 1, below); after ante situm resection, it was 32.5 and 84 months (case report 2, below). In all patients the cause of death was tumor recurrence.
Patient 1
In this patient, several hepatic metastases from a leiomyosarcoma of the stomach were removed by an ex situ resection. 4 A few months later, a local recurrence in the small bowel was removed. The patient died of intrahepatic and extrahepatic tumor recurrence 3 years after liver resection.
Patient 2
In this patient, an ante situm resection (presumed R0 resection) was performed for large metastases in the right liver lobe (adjacent to the confluence of the liver veins) and one metastasis in the left lateral segments 10 years after diagnosis of a leiomyosarcoma of the small bowel. In addition, a tumor nodule in the omentum and tumor-involved lymph nodes in the hepatic hilus were removed. Four years later, the patient was found to have multiple bilateral intrahepatic recurrent lesions that almost completely obstructed the vena cava. Despite the presence of extrahepatic tumor, an ante situm resection was again performed. Overall, 11 hepatic metastases were excised, 3 metastases were locally destroyed by fulguration, and extrahepatic tumor nodules in the mesentery and on the surface of the peritoneum were removed. The patient died of diffuse intraabdominal tumor spread 7 years after the first and 3 years after the second antesitum resection.
DISCUSSION
Liver resection is the only potentially curative treatment for hepatic metastases of colorectal cancer. In larger series, 5-year survival rates of up to 39% have been reported. 5 Although an R0 resection has been shown to be the most decisive prognostic factor for liver resection in colorectal metastases, there is still discussion regarding the selection criteria and prognostic factors. In contrast, in neuroendocrine hepatic metastases, even incomplete removal of hepatic tumorous masses may lead to significantly prolonged survival or may at least achieve effective palliation. 6,7 Regarding liver resection for hepatic metastases of noncolorectal, nonneuroendocrine hepatic metastases, little information is available in the surgical literature. In particular, there are only a few reports on resection of liver metastases from leiomyosarcoma, although the liver is a common site of recurrence after curative resection of the primary tumor. 1,8–17
So far, the most common treatment for liver metastases from leiomyosarcoma has been chemotherapy (ifosfamide, mitomycin, and doxorubicin regimens). The reported tumor response rates are poor, with a duration of response of only a few weeks or months. 18,19 Recently, more encouraging results have been obtained by chemoembolization with polyvinyl alcohol sponge particles mixed with cisplatin powder, followed by intrahepatic arterial infusion of vinblastine. With this therapy, Mavligit et al 20 reported a 70% tumor response rate (>50% regression) and a median duration of regression of 12 months.
Our results with liver resection for hepatic metastases from leiomyosarcoma are favorable in comparison with all treatment alternatives so far reported in the literature. Although the results of liver resection cannot be fully compared with the above-mentioned data on chemotherapy or chemoembolization (because patients who are suitable for surgery usually represent a positive selection and often are in better physical condition than patients with unresectable cancer), the median survival of 32 months and the 5-year survival rate of 20% achieved after R0 resection would appear to justify an aggressive surgical approach. In addition, our data indicate that nothing less than complete resection of all tumorous masses must be the aim of aggressive surgery, because the only long-term survivors were patients with an R0 resection. Thus, even extracorporeal surgery to ensure complete removal of intrahepatic tumor is justified. Although our data on this aggressive surgical approach are too few to permit valid conclusions to be drawn, the courses of the reported five patients (with one surviving 7 years) support the use of extracorporeal liver resection in the case of extensive hepatic tumor involvement.
In our series, median survival after R1/2 resection was 20.5 months. This is considerably longer than that reported for chemoembolization or systemic chemotherapy. However, a detailed analysis shows that the results of R1/2 resection are biased by the short survival after such resection in patients with additional extrahepatic tumor (median survival 5 months). In contrast, statistical analysis revealed no difference in survival after R0 and R1/2 resection in patients without extrahepatic tumor. Thus, the indication for an operation for hepatic metastases from leiomyosarcoma should be based on an exact preoperative diagnostic workup aimed at determining whether an R0 resection (especially in the presence of an additional extrahepatic tumor) appears possible. The comparatively high rate of noncurative resections of 35% in our series is due to the fact that in view of the poor results of nonsurgical treatment modalities, a surgical approach was favored, even in doubtful circumstances.
This aggressive approach might be one reason for the slightly higher rate of death and complications in our series than in recent reports in the literature. Another explanation for the higher complication rate is the large number of extended hepatectomies and even extracorporeal liver resections in our series, although the two fatal complications were associated with only small liver resections (segmentectomies).
Because of the small number of patients and the differing tumor characteristics (extrahepatic tumor, synchronous or metachronous metastases, extent of resection), there is no point in performing further statistical analysis. However, our data do not suggest that metachronous metastases are associated with a better prognosis than a synchronous tumor. This is in contrast to Ng et al, 3 who reported significantly better survival when there was an interval of at least 18 months between removal of the primary tumor and the diagnosis of metastatic spread.
Further, our data show that evidence of an extrahepatic tumor at the time of liver resection has no influence on survival if complete removal of both liver metastases and extrahepatic tumor is achieved. Thus, the presence of extrahepatic tumor growth should be regarded as a contraindication for liver resection only if an R0 resection does not appear possible. In our series, extrahepatic tumor was located exclusively in the abdominal cavity in all but one patient. This is important because intraabdominal metastases of leiomyosarcoma are associated with a much better prognosis than extraabdominal tumor spread. According to Ng et al, 3 extraabdominal metastases occur less frequently than intraabdominal metastases and signal a grave prognosis. Thus, our conclusion to consider liver resection even in the presence of extrahepatic tumor can be valid only in patients with metastases in the abdominal cavity.
So far, few case reports on disease-free long-term survivals after hepatic resection for metastases from leiomyosarcoma have been published. 8,12 The largest series of liver resections in this tumor entity reported so far contained 18 patients, with only a single patient surviving for more than 5 years. 11 Similarly, Foster and Lundy 10 reported a 2-year survival rate of 36% and a 5-year survival rate of 9% in 11 patients. Jaques et al 1 found a median survival of 30 months after resection of hepatic metastases from intestinal sarcomas in 14 patients (in 10 patients the primary tumor was a leiomyosarcoma) and a 100% recurrence rate, predominantly in the liver. Similarly, Ng et al 3 described a median survival of 33 months in five patients after curative liver resection, compared with 18 months after R1/2 resection and only 14 months in unresectable tumors. These data are consistent with the median survival of 32 months and the 5-year survival rate of 20% after R0 resection in the present series. In addition, the median survival of 31 months after a second liver resection in our series appears to suggest that patients with intrahepatic tumor recurrence may also benefit from repeat liver resection. It is obvious that a repeat hepatic resection is feasible only in highly selected patients, and thus the good results of surgery are least to some extent due to favorable patient selection. One of these selection criteria might be the fact that in our series, in patients undergoing repeat liver resection all but one of the tumors were classified as low-grade leiomyosarcomas. Despite these encouraging results, there was not a single 5-year survivor after repeat liver resection, and all of these patients died of disseminated tumor recurrence, irrespective of whether R0 or R1/2 resection had been achieved. This indicates that there is hardly any chance of cure in repeat liver resection, because such patients usually do not have isolated intrahepatic lesions, but rather systemic tumor spread.
As reported in the literature, experience with liver transplantation for hepatic cancer secondary to a gastrointestinal leiomyosarcoma is limited. Olthoff et al 21 reported on one patient who had no evidence of recurrence 70 months after a liver transplant for a gastrointestinal leiomyosarcoma. These data have not been confirmed by others. The Pittsburgh group reported on six patients undergoing cluster transplantation for metastatic stromal tumors; a tumor recurrence rate of 83% was noted. At the time of reporting, four of the six patients had died, and the median survival was approximately 31 months (938 days). Two patients were still alive at 19 months (with recurrence) and 49 months after transplantation. 22,23 Although the median survival of these patients is no different from that reported after liver resection, total hepatectomy and liver transplantation, the most aggressive surgical approach to liver malignancies, has not gained acceptance as treatment for hepatic metastases originating from leiomyosarcoma.
In conclusion, our results, as well as the data reported in the literature, support the notion that hepatic resection for metastases from leiomyosarcoma appears to be valuable in carefully selected patients. Although tumor recurrence is frequent, the aim of hepatic resection must be an R0 resection to offer the chance of cure. Extrahepatic tumor should not be considered a contraindication for resection if complete removal of both hepatic metastases and nonhepatic cancer appears possible. In view of the unfavorable results of chemotherapy and chemoembolization, aggressive surgical therapy appears justified, with the aim of improving the otherwise poor prognosis of patients with hepatic metastases from leiomyosarcoma.
Footnotes
Correspondence: Hauke Lang, MD, Klinik und Poliklinik für Allg. und Transplantationschirurgie, Universitätsklinikum Essen, Hufelandstr. 55, 45122 Essen, Germany.
E-mail: hauke.lang@uni-essen.de
Accepted for publication November 10, 1999.
References
- 1.Jaques DP, Coit DG, Casper ES, Brennan MF. Hepatic metastases from soft-tissue sarcoma. Ann Surg 1995; 221:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffe BM, Donegan WL, Watson F, Spratt JS. Factors influencing survival in patients with untreated hepatic metastases. Surg Gynecol Obstet 1968; 127:1–11. [PubMed] [Google Scholar]
- 3.Ng EH, Pollock RE, Romsdahl MM. Prognostic implications of patterns of failure for gastrointestinal leiomyosarcoma. Cancer 1992; 69:1334–1341. [DOI] [PubMed] [Google Scholar]
- 4.Pichlmayr R, Bretschneider H, Kirchner E, et al. Ex situ-Operation an der Leber. Eine neue Möglichkeit der Leberchirurgie. Langenbecks Arch Chir 1988; 373:122–126. [DOI] [PubMed] [Google Scholar]
- 5.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19:59. [DOI] [PubMed] [Google Scholar]
- 6.McEntee, Nagorney DM, Kvols LK et al. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery 1990; 108:1091–1096. [PubMed] [Google Scholar]
- 7.Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg 1995; 169:36. [DOI] [PubMed] [Google Scholar]
- 8.Berney T, Mentha G, Roth AD, Morel P. Results of surgical resection of liver metastases from non-colorectal primaries. Br J Surg 1998; 85:1423–1427. [DOI] [PubMed] [Google Scholar]
- 9.Coburn CS, Makowka L, Langer B, et al. Examination of patient selection and outcome for hepatic resection for metastatic disease. Surg Gynecol Obstet 1987; 165:239–246. [PubMed] [Google Scholar]
- 10.Foster JH, Lundy J. Liver metastases. Curr Probl Surg 1981; 18:157–202. [DOI] [PubMed] [Google Scholar]
- 11.Harrison LE, Brennan MF, Newman E, et al. Hepatic resection for non-colorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery 1997; 121:625–632. [DOI] [PubMed] [Google Scholar]
- 12.Morrow CE, Grage TB, Sutherland DER, Najarian JS. Hepatic resection for secondary neoplasms. Surgery 1982; 92:610–614. [PubMed] [Google Scholar]
- 13.Riesener KP, Winkeltau G, Klemm M, Schumpelick V. Chirurgische Therapie von Lebermetastasen. Therapieverfahren, Ergebnisse und Prognosefaktoren. Langenbecks Arch Chir 1994; 379:321. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz SI. Hepatic resection for noncolorectal non-neuroendocrine metastases. World J Surg 1995; 19:72–75. [DOI] [PubMed] [Google Scholar]
- 15.Seifert JK, Junginger T. Leberresektionen bei Metastasen nicht-colorectaler Primärtumoren. Chirurgie 1996; 67:161–168. [PubMed] [Google Scholar]
- 16.Stehlin JS, De Ipolyi PD, Greeff PJ, et al. Treatment of cancer of the liver: twenty years’ experience with infusion and resection in 414 patients. Ann Surg 1988; 208:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf RE, Goodnight JE, Krag DE, Schneider PD. Results of resection and proposed guidelines for patient selection in instances of noncolorectal hepatic metastases. Surg Gynecol Obstet 1991; 173:454–460. [PubMed] [Google Scholar]
- 18.Edmondson JH, Ryan LM, Blum RH, et al. Randomized comparison of doxorubicin alone versus ifosphamide or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993; 11:1269–1275.8315424 [Google Scholar]
- 19.Casper ES, Christman KL, Schwartz GK, et al. Edatrexate in patients with soft tissue sarcoma: activity in malignant fibrous histiocytoma. Cancer 1993; 72:766–770. [DOI] [PubMed] [Google Scholar]
- 20.Mavligit GM, Zukwiski AA, Ellis LM, et al. Gastrointestinal leiomyosarcoma metastatic to the liver: durable tumor regression by hepatic chemoembolization infusion with cisplatin and vinblastine. Cancer 1995; 95:2083–2088. [DOI] [PubMed] [Google Scholar]
- 21.Olthoff KM, Millis M, Rosove MH, et al. Is liver transplantation justified for the treatment of hepatic malignancies? Arch Surg 1990; 125:1261–1268. [DOI] [PubMed] [Google Scholar]
- 22.Alessiani M, Tzakis A, Todo S, et al. Assessment of five-year experience with abdominal organ cluster transplantation. J Am Coll Surg 1995; 180:1–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Iwatsuki S, Tzakis A, Todo S, et al. Liver transplantation for metastatic hepatic malignancies. Hepatology 1993; 18:723. [Google Scholar]