Abstract
Objective
To identify factors influencing the occurrence of cancer in the rectal remnant in patients with familial adenomatous polyposis (FAP) after colectomy and ileorectal anastomosis (IRA).
Summary Background Data
The risk for rectal cancer in patients with FAP after colectomy and IRA remains a major concern.
Methods
Between 1955 and 1997, 371 patients (206 men, 165 women) from the Registry of Hereditary Colorectal Tumors underwent colectomy and IRA as a primary surgical procedure. Survival was estimated using the Kaplan-Meier method. Cox proportional hazard models were fitted to assess the relative excess risk of rectal cancer and to control for confounding factors. A multivariate analysis was performed to assess the relation between cancer risk in the rectum and sex, age, number of rectal polyps, colon cancer, and APC germline mutation.
Results
Median follow-up was 81 months. Eighty-nine patients (24%) had colon cancer at the time of surgery. The APC mutation was found in 200 patients. In 27 patients, cancer developed in the retained rectum 1 to 26 years after surgery. The incidence of rectal carcinoma appears to increase with time: at 10, 15, and 20 years after surgery, the cumulative risk was 7.7%, 13.1%, and 23.0%, respectively. Multivariate analysis identified as independent predictors the presence of colon cancer at IRA and a mutation occurring between codons 1250 and 1464; both factors increased the risk nine times.
Conclusions
The presence of cancer at IRA and APC mutation type are the most important risk factors for the future development of cancer in the rectal remnant in patients with FAP.
Familial adenomatous polyposis (FAP), an autosomal dominantly inherited disorder with a penetrance of nearly 100%, is characterized by the progressive development of hundreds of adenomatous colorectal polyps, some of which inevitably progress to cancer. The clinical features of this syndrome and its variants have been known for many years. Diagnosis still relies largely on the detection of numerous colorectal polyps during the second or third decade of life, as well as extracolonic lesions.
Mutations of the APC gene, identified in 1991, are responsible for the disease in most FAP families. Several reports have shown a correlation among mutations occurring within specific regions of APC, the severity of polyposis, and the presence of extracolonic manifestations. 1–6
Treatment for FAP is the prophylactic surgical resection of affected colon to prevent malignant degeneration of adenomas. Colectomy with ileorectal anastomosis (IRA) is the most common surgical procedure in many institutions. This procedure carries low rates of complications and death and produces a good functional outcome. However, the rectal remnant should be carefully observed for the possible occurrence of cancer. Beginning in the 1980s, several reports have indicated that the risk of rectal cancer gradually increases with time, amounting to 10% to 55% after 20 years of follow-up. 5,7–15 However, some have reported spontaneous regression of the remaining polyps in the retained rectum, 16,17 for a reduced proliferation of the rectal mucosa. 18–20
The aims of this study were to examine the risk of rectal cancer in patients with FAP after IRA and to identify clinical or genetic factors that can predict the development of cancer in the retained rectum.
METHODS
In 1980, the Hereditary Colorectal Tumor Registry of families with hereditary nonpolyposis colorectal cancer and FAP was founded at the National Cancer Institute in Milan. By the end of 1997, 569 FAP families were enrolled throughout the country. Data concerning patient demographics, patient features (sex, age at diagnosis of FAP, number of polyps at first examination, distribution of polyps, age at diagnosis of colorectal cancer, and cancer location), and surgical details were obtained at inclusion in the registry and from medical records.
Adenomas were classified according to their histopathologic characteristics, size, and location. The preoperative number of adenomas of the rectum was determined by reviewing endoscopic examinations and was divided into three groups (<10, 10–30, and >30 polyps). Histologic examination and polyp fulguration or removal were undertaken at every follow-up when appropriate.
The presence and type of APC mutations were assessed using different methods, including single-strand conformation polymorphism assay, blue/white assay, and protein truncation test, followed by sequence analysis, as described elsewhere. 21–23 When a mutation was identified in the family proband, its occurrence was verified in all affected relatives included in the analysis. Subjects were subdivided into three groups: mutation between codons 1250 and 1464 (correlated with a profuse phenotype) 1, mutation before codon 1250 and after codon 1464, or mutation not found.
The date of surgery was set at the index date for time calculation. “Last date” was defined as the date of diagnosis of rectal cancer or the date of last contact or death.
Survival was estimated using the Kaplan-Meier product limit method. Differences among groups were tested using the log-rank test. To assess the relative excess risk of rectal cancer and to control for confounding factors, proportional hazard models (including sex, age at IRA, and all available prognostic factors) were fitted, computing hazard ratios (HR) and the corresponding 95% confidence intervals (95% CI). The proportional assumption was examined with log-log survival plots or by adding time-dependent interaction terms to the model. Statistical analysis was performed using the Statistical Analysis System version 6.12 (SAS, Cary, NC).
RESULTS
Between 1988 and 1997, the registry collected 569 FAP pedigrees comprising 879 patients. Of these, 431 who underwent surgical procedures other than IRA and 77 with rectal cancer at surgery were excluded from the analysis. The study group consisted of 371 patients (206 men, 166 women) from 97 kindreds. None of the patients was participating in any chemoprevention trials.
Mean age at time of surgery was 32 years (33 for men, 31 for women). Median duration of follow-up after surgical treatment was 81 months (range 1–515).
Colon cancer was present in the colectomy specimen of 89 patients (24.0%); 50 were early stage (Dukes’ A or B) and 39 were advanced stage. Thirty-one patients had multiple locations in the colon. The mean age of patients with colon cancer at initial surgery was significantly higher than in cancer-free patients (45.1 vs. 28.6, Wilcoxon rank-sum test, P = .0001).
Seventy-seven patients had more than 30 rectal polyps at the time of initial colectomy. The presence of synchronous cancer increased with the polyp count (chi-square for trend, 4.67;P = .03).
The APC mutation was sought in 297 patients and was found in 200 of them (67.3%). Thirty-six patients (12.1%) had a mutation between codons 1250 and 1464. The mean number of rectal polyps found was 42.5 for mutations detected between codons 1250 and 1464 and 22.0 for mutations before codon 1250 or after codon 1464 (Wilcoxon rank-sum test, P = .001). In contrast, the presence of mutations between codons 1250 and 1464 was not associated with colon cancer at surgery (Fisher exact test, P = .6).
Follow-up revealed development of cancer in the retained rectum in 27 patients (15 men, 12 women), with a median follow-up of 102 months (range 1–26 years). Of the patients in whom rectal cancer developed, 50% had colon cancer and 58.3% had more than 30 polyps in the rectum at initial colectomy.
The rectal cancer was diagnosed at early stage (Dukes’ A or B) in 22 patients (81.5%). Eighteen were still alive and disease-free at last follow-up, six died of metastatic diffusion, and three died of extracolonic malignancy.
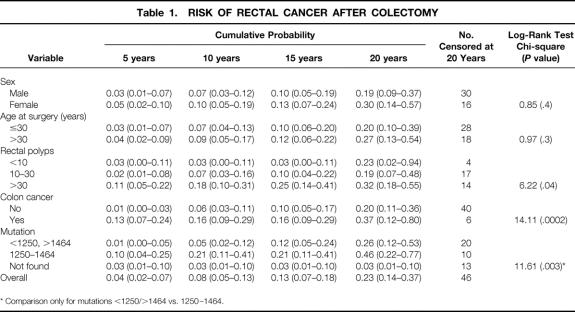
Figure 1 shows the cumulative risk of developing rectal cancer per years of follow-up after surgery. At 10, 15, and 20 years after surgery, the cumulative risk was 7.7%, 13.1%, and 23.0%, respectively. Table 1 shows the cumulative risk of rectal cancer for several variables at 5, 10, 15, and 20 years after surgery.
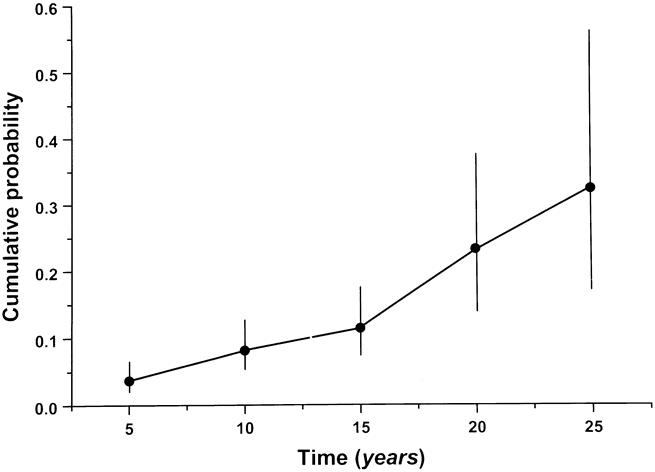
Figure 1. Cumulative probability of developing rectal cancer after colectomy and ileorectal anastomosis during 25 years of follow-up.
Table 1. RISK OF RECTAL CANCER AFTER COLECTOMY
* Comparison only for mutations <1250/>1464 vs. 1250–1464.
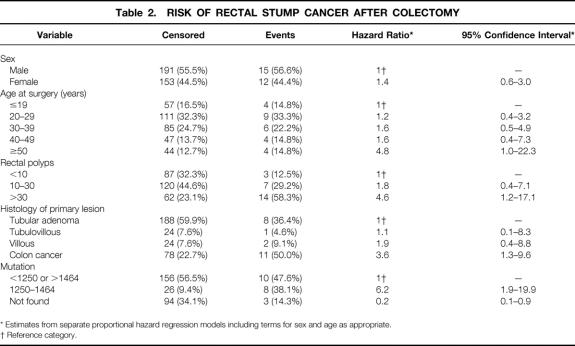
Univariate analysis (Table 2) showed that the risk of developing rectal cancer was significantly higher in patients with colon cancer at the initial operation (HR = 3.6, 95% CI 1.3–9.6), in patients with more than 30 polyps in the rectum at the initial surgery (HR = 4.6, 95% CI 1.2–17.9), and with mutations between codons 1250 and 1464 (HR = 6.2, 95% CI 1.9–19.9).
Table 2. RISK OF RECTAL STUMP CANCER AFTER COLECTOMY
* Estimates from separate proportional hazard regression models including terms for sex and age as appropriate.
† Reference category.
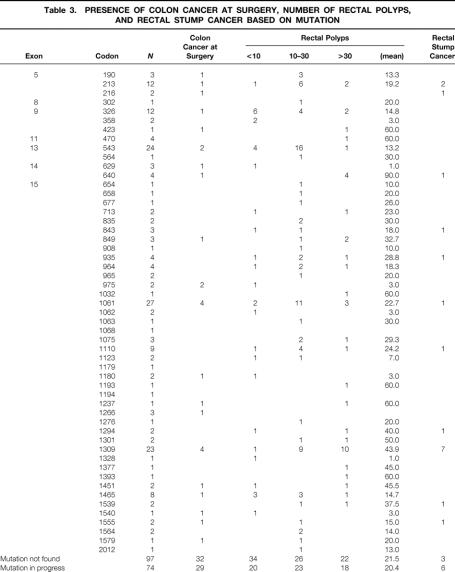
Multivariate analysis identified as independent predictors of rectal cancer the presence of cancer in the resected colon (HR = 3.2, 95% CI 1.1–9.8) and mutations between codons 1250 and 1464 (HR = 4.4, 95% CI 1.3–15.0) (Table 3) . The coexistence of cancer in the resected colon and a mutation between codons 1250 and 1464 increased ninefold the risk of rectal cancer (HR = 8.7, 95% CI 3.1–24.7).
Table 3. PRESENCE OF COLON CANCER AT SURGERY, NUMBER OF RECTAL POLYPS, AND RECTAL STUMP CANCER BASED ON MUTATION
DISCUSSION
The risk of rectal cancer in patients with FAP remains a major concern at the time of prophylactic surgery. The surgeon and patient often must choose between surgical procedures with distinctive issues: total colectomy with IRA is considered safer in terms of complications and quality of life, but restorative proctocolectomy eliminates the cancer risk.
Studies of patients with FAP after IRA have found an incidence of rectal cancer of 7.1% to 32.1%. 5,7–14 A large-scale study from the Scandinavian countries involving 297 patients found a rectal cancer risk of 9% after 20 years and 13% after 25 years of follow-up. 13 Feinberg et al 24 found a 15% risk of rectal cancer after 15 years. A Japanese study of 1,050 patients with FAP showed a much higher risk of developing rectal cancer in the retained rectum: 13% after 10 years and 37% after 20 years. 14 Several investigations conducted by the St. Mark’s Polyposis Registry reported a high incidence of postoperative rectal cancer. 8,25 Nugent and Phillips 26 reviewed the risk of rectal cancer in 224 patients and found a 20% risk of developing cancer in the retained rectum after 10 years. Differences between studies may be due to different patient characteristics, different therapeutic strategies, the paucity of long-term results in large series of patients, variable follow-up policies, or the wide confidence intervals of estimates related to the few series with follow-up of more than 20 years.
Age at colectomy, sex, number of polyps, presence of cancer in the colectomy specimen, and length of the remnant rectum have been implicated as independent risk factors. 13,27 After the identification of APC mutations, genotype has been investigated as a risk factor. Several reports have found a correlation between specific mutations and the density of adenomas, the severity of the FAP phenotype, and the presence of extracolonic disease, 1–4,6 but one report has correlated the presence of specific mutations with the risk of subsequent rectal cancer, supporting a possible role of genotyping in the management of patients with FAP. 5 These authors found that patients with APC mutations beyond codon 1275 had a high risk of developing rectal cancer in the retained rectum. However, this result was obtained without taking into consideration the already confirmed clinical risk factors (e.g., number of rectal adenomas, presence of colon cancer at surgery), and therefore the real weight of the genotype could not be clearly evaluated. Wu et al 6 proposed that patients with a mutation at codon 1309 should undergo proctocolectomy because they have a more extended rectal disease.
Our findings suggest that the presence of a specific mutation associated with a severe phenotype, the presence of colon cancer at surgery, and a large number of rectal adenomas are strong predictors of cancer in the rectal stump after IRA. The estimated risk increased from 4 to 10 times for single or combined factors, and it was comparable to data published in the literature.
Our results confirm that important factors can be identified to predict the fate of the rectal stump in patients with FAP. The decision to undergo prophylactic surgery is a complex one, because many determinants must be considered to balance life expectancy and quality of life. Restorative proctocolectomy should be proposed to patients based on clinical features (presence of cancer, number of rectal adenomas) and also the genotype. In the future, when the functional aspects of each APC gene mutation are better understood, the controversy over rectum sparing will probably be settled.
APPENDIX
The Hereditary Colorectal Tumors Registry
M.P. Baldacci, Milano, F. Barberani, Terni, T. Bruni, Mantova, L. Bucci, Napoli, G. Carassale, Firenze, M. Cataldi, Como, S. Civitelli, Siena, L. Dall’Oglio, Roma, G.P. Ferulano, Napoli, P. Fracasso, Roma, R. Gentilini, Trento, M. Grazia, Verona, L. Grossano, Milano, A. Heouaine, Genova, A. Lorenzotti, Roma, L. Maculotti, Milano, M. Malerba, Roma, C. Marchese, Torino, C. Massazza, Varese, M. Messina, Siena, M. Moretti, Perugia, A. Paterlini, Brescia, M. Pescatori, Roma, S. Piffer, Trento, M. Pinna Pintor, Torino, M. Pisani, Piacenza, M. Ponz De Leon, Modena, A. Renda, Napoli, G. Ribotta, Roma, G. Roggia, Trento, G. Sansonetti, Milano, E. Scarcello, Pisa, P. Setti Carraro, Milano, P. Tansini, Piacenza, A. Tempesta, Napoli, G. Toti, Milano, F. Tricarico, Foggia, S. Valabrega, Roma.
Footnotes
Correspondence: Lucio Bertario, MD, Istituto Nazionale Tumori, Via Venezian, 1, 20133 Milan, Italy.
Supported by the Italian Association for Cancer Research.
Accepted for publication August 6, 1999.
References
- 1.Nagase H, Miyoshi Y, Horii A, et al. Correlation between the location of germ-line mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res 1992; 52:4055–4057. [PubMed] [Google Scholar]
- 2.Phillip P, Letteboer T, Gelbert L, et al. Identical APC exon 15 mutations result in a variable phenotype in familial adenomatous polyposis. Hum Molec Genet 1993; 2:925–931. [DOI] [PubMed] [Google Scholar]
- 3.Gayther SA, Wells D, Chapman P, et al. Regionally clustered APC mutations are associated with a severe phenotype and occur at high frequency in new mutation cases of adenomatous polyposis coli. Hum Molec Genet 1994; 3 (1):53–56. [DOI] [PubMed] [Google Scholar]
- 4.Nugent KP, Phillips RK, Hodgson SV, et al. Phenotypic expression in familial adenomatous polyposis: partial prediction by mutation analysis. Gut 1994; 35:1622–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasen HF, van der Luijt RB, Slors JF, et al. Molecular genetic tests as a guide to surgical management of familial adenomatous polyposis. Lancet 1996; 348:433–435. [DOI] [PubMed] [Google Scholar]
- 6.Wu JS, Paul P, McGannon EA, Church JM. APC genotype, polyp number, and surgical options in familial adenomatous polyposis. Ann Surg 1998; 227:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bess MA, Adson MA, Elveback LR, Moertel CG. Rectal cancer following colectomy for polyposis. Arch Surg 1980; 115:460–467. [DOI] [PubMed] [Google Scholar]
- 8.Bussey HJ, Eyers AA, Ritchie SM. The rectum in adenomatous polyposis: the St. Mark’s policy. Br J Surg 1985; 72:S29–31. [DOI] [PubMed] [Google Scholar]
- 9.Heimann TM, Bolnick K, Aufses AH Jr. Results of surgical treatment for familial polyposis coli. Am J Surg 1986; 152:276–278. [DOI] [PubMed] [Google Scholar]
- 10.Sarre RG, Jagelman DG, Beck GJ, et al. Colectomy with ileorectal anastomosis for familial adenomatous polyposis: the risk of rectal cancer. Surgery 1987; 101:20–26. [PubMed] [Google Scholar]
- 11.Slors JF, den Hartog Jager FC, Trum JW, et al. Long-term follow-up after colectomy and ileorectal anastomosis in familial adenomatous polyposis coli. Is there still a place for the procedure? Hepato-Gastroenterology 1989; 36 (2):109–112. [PubMed] [Google Scholar]
- 12.Skinner MA, Tyler D, Branum GD, et al. Subtotal colectomy for familial polyposis. A clinical series and review of the literature. Arch Surg 1990; 125:621–624. [DOI] [PubMed] [Google Scholar]
- 13.De Cosse JJ, Bulow S, Neale K, et al. Rectal cancer risk in patients treated for familial adenomatous polyposis. The Leeds Castle Polyposis Group. Br J Surg 1992; 79:1372–1375. [DOI] [PubMed] [Google Scholar]
- 14.Iwama T and Mishima Y. Factors affecting the risk of rectal cancer following rectum-preserving surgery in patients with familial adenomatous polyposis. Dis Colon Rectum 1994; 37:1024–1026. [DOI] [PubMed] [Google Scholar]
- 15.Heiskanen I, Jarvinen HJ. Fate of the rectal stump after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Intl J Colorectal Dis 1997; 12 (1):9–13. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard TB. Familial polyposis of the colon: the fate of the retained rectum after colectomy in children. Am Surg 1957; 23:577–585. [PubMed] [Google Scholar]
- 17.Nicholls RJ, Springall RG, Gallagher P. Regression of rectal adenomas after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Br Med J (Clin Res Ed) 1988; 296:1707–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farmer KC, Phillips RK. Colectomy with ileorectal anastomosis lowers rectal mucosal cell proliferation in familial adenomatous polyposis. Dis Colon Rectum 1993; 36 (2):167–171. [DOI] [PubMed] [Google Scholar]
- 19.Cats A, Kleibeuker JH, Kuipers F, et al. Changes in rectal epithelial cell proliferation and intestinal bile acids after subtotal colectomy in familial adenomatous polyposis. Cancer Res 1992; 52:3552–3557. [PubMed] [Google Scholar]
- 20.Spagnesi MT, Tonelli F, Dolara P, et al. Rectal proliferation and polyp occurrence in patients with familial adenomatous polyposis after sulindac treatment. Gastroenterology 1994; 106:362–366. [DOI] [PubMed] [Google Scholar]
- 21.Gismondi V, Bafico A, Biticchi R, et al. Characterization of 19 novel and six recurring APC mutations in Italian adenomatous polyposis patients, using two different mutation detection techniques. Human Mutation 1997; 9:370–373. [DOI] [PubMed] [Google Scholar]
- 22.Giarola M, Stagi L, Bertario L, et al. Screening for mutations of the APC gene in 66 Italian Familial Adenomatous Polyposis patients: evidence for phenotypic differences in cases with and without identified mutation. Human Mutat 1999; 13:116–123. [DOI] [PubMed] [Google Scholar]
- 23.Bulow S. The risk of developing rectal cancer after colectomy and ileorectal anastomosis in Danish patients with polyposis coli. Dis Colon Rectum 1984; 27:726–729. [DOI] [PubMed] [Google Scholar]
- 24.Feinberg SM, Jagelman DG, Sarre RG, et al. Spontaneous resolution of rectal polyps in patients with familial polyposis following abdominal colectomy and ileorectal anastomosis. Dis Colon Rectum 1988; 31:169–175. [DOI] [PubMed] [Google Scholar]
- 25.Thomson JP. Familial adenomatous polyposis: the large bowel. Ann R Coll Surg Engl 1990; 72:177–180. [PMC free article] [PubMed] [Google Scholar]
- 26.Nugent KP, Phillips RK. Rectal cancer risk in older patients with familial adenomatous polyposis and ileorectal anastomosis: a cause for concern. Br J Surg 1992; 79:1204–1206. [DOI] [PubMed] [Google Scholar]
- 27.Jang YS, Steinhagen RM, Heimann TM. Colorectal cancer in familial adenomatous polyposis. Dis Colon Rectum 1997; 40:312–316. [DOI] [PubMed] [Google Scholar]