Abstract
Objective
To evaluate the influence of the width and histologic involvement of the resection margin on postoperative recurrence after resection of hepatocellular carcinoma (HCC).
Summary Background Data
The significance of the resection margin in hepatectomy for HCC remains controversial. A precise evaluation of the effects of the width and histologic involvement of the resection margin on postoperative recurrence is required to clarify the issue.
Methods
Two hundred eighty-eight patients with macroscopically complete resection of HCC were divided into groups with narrow (<1 cm) or wide (≥1 cm) resection margins. The two groups were compared for postoperative recurrence rate and pattern of recurrence. A further analysis was performed to investigate the effects of histologic involvement of the resection margin on postoperative recurrence.
Results
Recurrence rates were similar between 150 patients with a narrow margin and 138 patients with a wide margin; the groups were comparable in other clinicopathologic variables. Most recurrent tumors occurred in the liver remnant at a segment distant from the resection margin or at multiple segments. Thirty-four patients had margin involved histologically by microscopic invasion from the main tumor (n = 13), venous tumor thrombi (n = 13), or microsatellites separate from the main tumor (n = 8). These patients had significantly higher recurrence rates than those with a histologically clear margin. However, a positive histologic margin was not a significant risk factor for recurrence by multivariate analysis. Tumor stage and perioperative transfusion were the only independent risk factors.
Conclusions
The width of the resection margin did not influence the postoperative recurrence rates after hepatectomy for HCC. A positive histologic margin was associated with a higher incidence of postoperative recurrence, but in most patients this was related to the underlying venous invasion or microsatellites. Most intrahepatic recurrences were considered to arise from intrahepatic metastasis by means of venous dissemination, which a wide resection margin could not prevent.
Hepatectomy for hepatocellular carcinoma (HCC) has become a safe operation, with a low death rate, as a result of advances in surgical techniques and perioperative management. 1 The long-term survival, however, is still unsatisfactory because of the high incidence of intrahepatic recurrence. 2 Resection margin is a surgical factor that has been evaluated for its influence on the long-term outcome after resection of HCC, but its significance remains controversial.
A few studies have shown that a resection margin of less than 1 cm was an adverse prognostic factor of long-term survival, 3–8 but others found no correlation between the width of the resection margin and the long-term prognosis. 9–15 This has resulted in a discrepancy among hepatic surgeons in the definition of “curative” resection for HCC. Some consider a margin of at least 1 cm necessary for cure, 3,4,6 whereas others define it as grossly complete tumor removal. 9,11 The incidence of actual histologic involvement of the resection margin was reported in only a few studies, 16–20 and its prognostic significance has not been clarified.
The main concern of a narrow or positive resection margin is postoperative recurrence, in particular recurrence in the liver remnant. In most previous studies, the significance of the resection margin was assessed only as one of the possible factors affecting survival. With few exceptions, 5,6,9,12 these studies did not examine in detail the effects of the resection margin on the incidence and pattern of recurrence. To clarify the significance of the resection margin in hepatectomy for HCC, a precise evaluation of the relation between the resection margin and postoperative recurrence is required. Based on a prospectively collected database, we conducted a detailed analysis of the effects of the width and histologic involvement of the resection margin on the incidence and pattern of recurrence after resection of HCC.
PATIENTS AND METHODS
Patients and Follow-Up
Between January 1989 and December 1997, 309 patients underwent hepatectomy for HCC with macroscopically complete resection of tumor in the Department of Surgery at the University of Hong Kong at Queen Mary Hospital. Twenty-one patients who died in the hospital were excluded, and the remaining 288 patients were the subjects of this study.
All patients were regularly followed up at our outpatient clinic and were prospectively monitored for recurrence by serum alpha-fetoprotein level assessment monthly and an ultrasound or contrast CT scan, together with chest x-ray, every 2 to 4 months. Suspected intrahepatic recurrence was confirmed by hepatic angiography, postlipiodol CT scan, and if necessary percutaneous needle biopsy. A computerized database has been established since 1989 for prospective collection of clinicopathologic data of all patients, including the macroscopic width and histologic involvement of the resection margin as assessed by pathologists. Any postoperative recurrence was entered into the database immediately on diagnosis.
By the time of analysis, all patients had been followed up for at least 1 year. Postoperative recurrence developed in 178 patients during a median follow-up period of 27 months (150 with intrahepatic recurrence and 28 with extrahepatic recurrence).
Resection Margin Width
Patients were classified according to the width of the resection margin, defined as the shortest macroscopic distance from the edge of tumor to the line of transection, into a narrow margin or a wide margin group. The narrow margin group consisted of patients with a margin width less than 1 cm, the wide margin group of patients with a margin width of 1 cm or more. These two groups were compared for postoperative recurrence rates and the pattern of recurrence in terms of type of recurrence (intrahepatic or extrahepatic), time of recurrence (≤1 or >1 year), and site of intrahepatic recurrence (Fig. 1). A further analysis of the effects of margin width on postoperative recurrence was performed in subgroups of patients stratified according to tumor size (≤5 or >5 cm), underlying liver histology (cirrhotic or noncirrhotic liver), and extent of resection (major or minor).
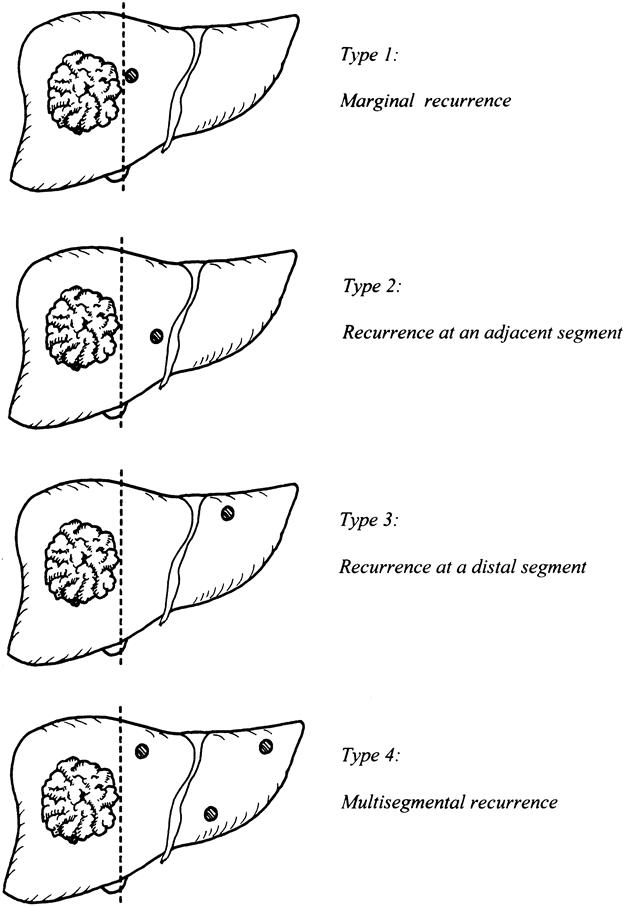
Figure 1. Classification of intrahepatic recurrence according to site of recurrence in the liver remnant (type 2 included recurrence in the same segment after subsegmentectomy).
Histologic Margin Involvement
A separate analysis was carried out to evaluate the influence of histologic margin involvement on the incidence and pattern of recurrence. Patients were classified into those with a positive or a negative microscopic margin. A positive margin was defined as the presence of tumor cells at the line of transection detected by histologic examination and was further subdivided into three patterns: microscopic involvement by the main tumor, involvement by venous permeation, and involvement by discrete microscopic satellite nodules. The effect of a positive margin on postoperative recurrence was evaluated by univariate analysis followed by multivariate analysis, taking into account other host, tumor, and surgical factors that could influence the risk of recurrence.
Transarterial lipiodolized chemotherapy (TAC) using cisplatin was given at 3 to 4 weeks after surgery in some patients with a positive margin; others did not receive chemotherapy. The two groups were compared for long-term survival results and recurrence rates.
Statistical Analysis
Comparisons between groups were performed using the chi-square test with Yates’ correction (or the Fisher test where appropriate) for nominal variables, and the unpaired t test was used for continuous variables. Cumulative survival and recurrence rates were evaluated by the Kaplan-Meier method and compared by the log-rank test. The Cox stepwise regression model was used for multivariate analysis. All statistical analyses were performed using statistical software (SPSS, Chicago, IL). P < .05 was considered statistically significant.
RESULTS
Effects of Resection Margin Width on Postoperative Recurrence
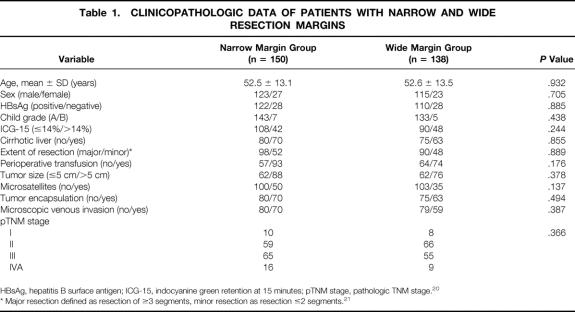
Among 288 patients with macroscopically complete resection of HCC, 150 had a narrow resection margin (mean 0.5 cm, SD 0.2 cm), and 138 had a wide resection margin (mean 2.4 cm, SD 1.0 cm). Table 1 shows the clinicopathologic data of these two groups of patients. There were no significant differences in any of the host, tumor, or surgical factors.
Table 1. CLINICOPATHOLOGIC DATA OF PATIENTS WITH NARROW AND WIDE RESECTION MARGINS
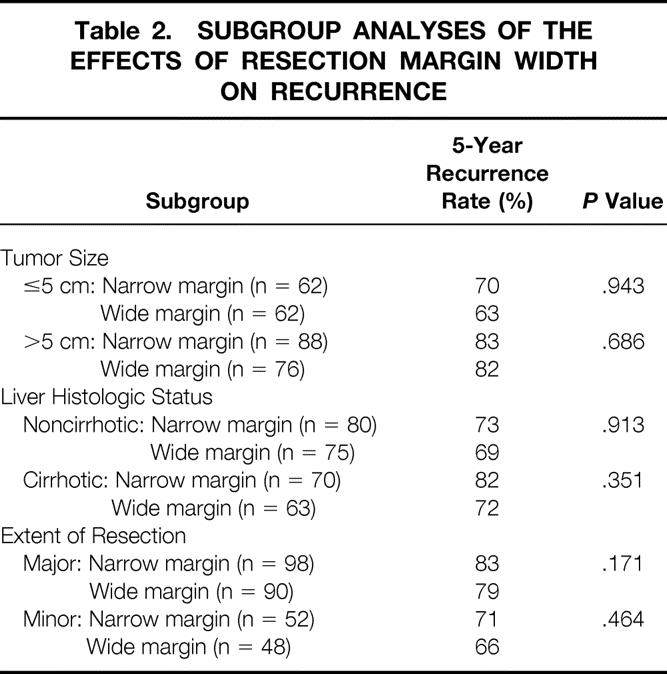
Long-term outcomes in terms of overall survival and postoperative recurrence rates were similar between the two groups. The 1-, 3-, and 5-year survival rates were, respectively, 54%, 34%, and 22% in the narrow margin group and 55%, 35%, and 25% in the wide margin group (median survival 14.6 vs. 16.0 months, P = .495). The 1-, 3-, and 5-year cumulative recurrence rates were, respectively, 47%, 66%, and 78% in the former group and 45%, 65%, and 75% in the latter group (P = .943) (Fig. 2). There were no significant differences in the survival (P = .742) or recurrence rates (P = .652) when patients in the wide margin group were divided into subgroups (margin width of 1–2 cm vs. >2 cm). Analyses after stratification of patients according to tumor size, liver cirrhotic status, and extent of resection revealed no significant correlation between the width of the resection margin and postoperative recurrence in any patient subgroup (Table 2).
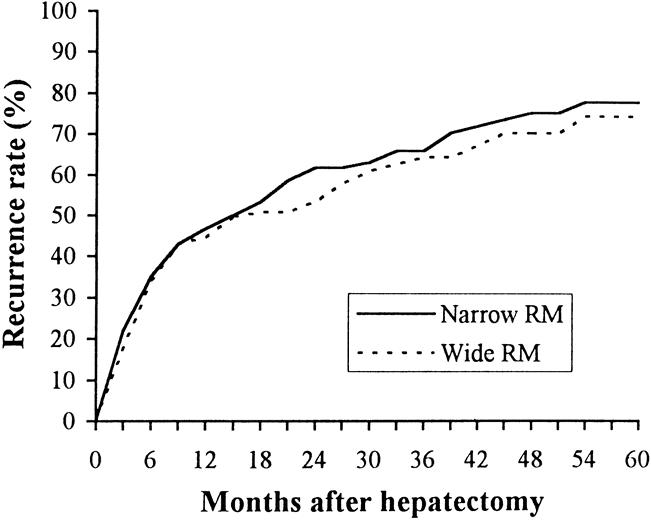
Figure 2. Cumulative postoperative recurrence rate in patients with narrow or wide resection margin (RM).
Table 2. SUBGROUP ANALYSES OF THE EFFECTS OF RESECTION MARGIN WIDTH ON RECURRENCE
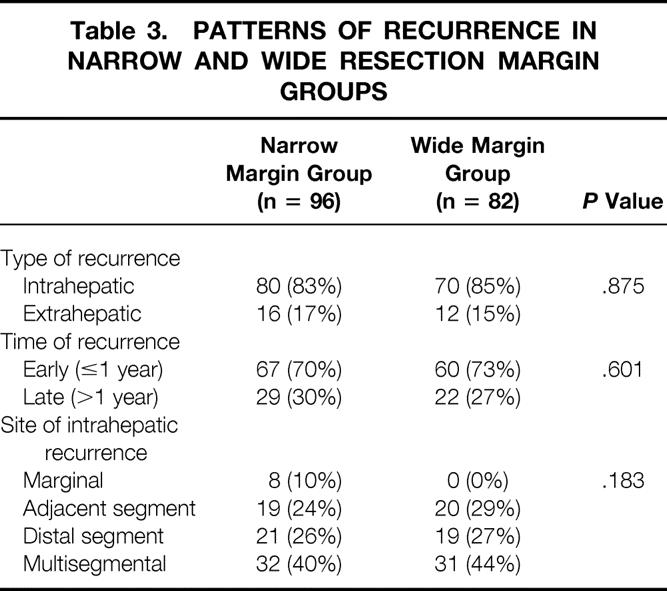
During the follow-up period, recurrence developed in 96 patients (64%) in the narrow margin group and 82 (59%) in the wide margin group. There were no significant differences between the two groups in terms of type of recurrence, time of recurrence, and site of intrahepatic recurrence (Table 3). The only remarkable difference was that all marginal recurrences were observed in the narrow margin group; however, this constituted only a small proportion of the intrahepatic recurrences even in this group. Most of the recurrent tumors developed at a distal segment or multiple segments in both groups.
Table 3. PATTERNS OF RECURRENCE IN NARROW AND WIDE RESECTION MARGIN GROUPS
Effects of Histologic Margin Involvement on Postoperative Recurrence
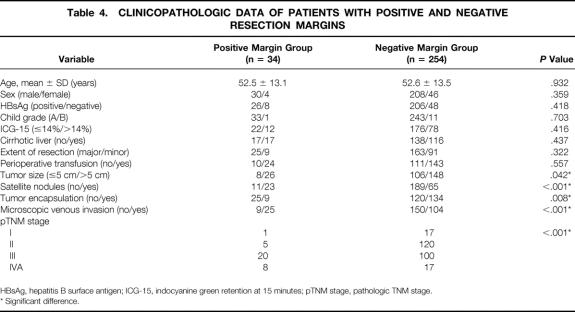
Thirty-four patients (12%) had a positive microscopic margin. Twenty-six of these patients had a narrow resection margin; the other eight had a wide margin. Table 4 shows a comparison of the host, tumor, and surgical factors between the groups with positive and negative margins. The positive margin group had a significantly higher frequency of adverse tumor factors, including tumor size more than 5 cm, absence of tumor capsule, presence of microsatellites, venous invasion, and advanced pTNM stage.
Table 4. CLINICOPATHOLOGIC DATA OF PATIENTS WITH POSITIVE AND NEGATIVE RESECTION MARGINS
HBsAg, hepatitis B surface antigen; ICG-15, indocyanine green retention at 15 minutes; pTNM stage, pathologic TNM stage.
* Significant difference.
The long-term survival rates in the positive margin group were 73%, 33%, and 29% at 1, 3, and 5 years, respectively; median survival was 17 months. These rates were significantly worse than in the negative margin group (83%, 61%, and 50%; median survival 54 months) (P = .004). The cumulative recurrence rates at 1, 3, and 5 years in the positive margin group were 62%, 83%, and 83%, significantly higher than in the negative margin group (44%, 63%, and 73%) (P = .004, Fig. 3).
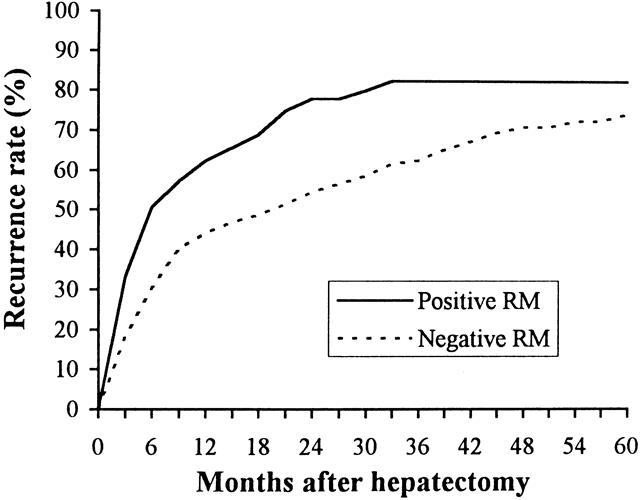
Figure 3. Cumulative postoperative recurrence rate in patients with histologically positive or negative resection margin (RM).
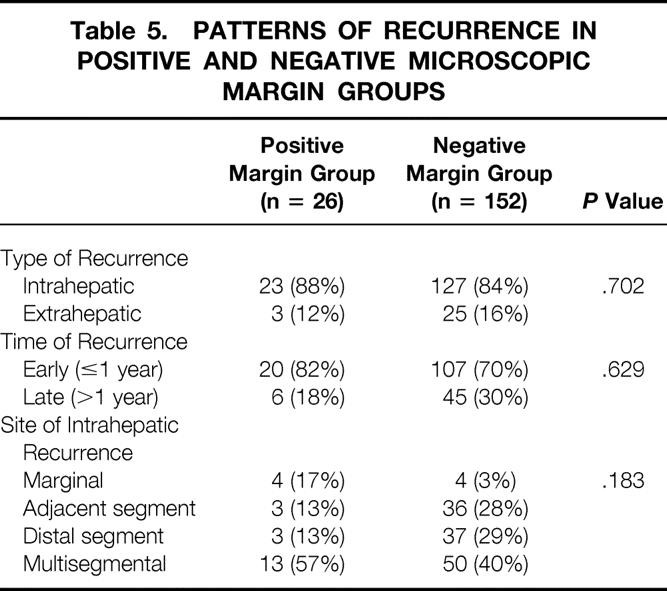
During the follow-up period, recurrence developed in 26 patients (76%) with a positive margin and 152 patients (60%) with a negative margin. There were no significant differences between the two groups in type of recurrence, time of recurrence, and site of intrahepatic recurrence (Table 5).
Table 5. PATTERNS OF RECURRENCE IN POSITIVE AND NEGATIVE MICROSCOPIC MARGIN GROUPS
The prognostic significance of a positive margin on postoperative recurrence was further evaluated by multivariate analysis that included all the host, tumor, and surgical factors listed in Table 4. In addition to positive microscopic margin (P = .004), five other factors were found to be significant risk factors for postoperative recurrence by univariate analysis: tumor size more than 5 cm (P = .006), presence of microsatellites (P = .010), venous invasion (P < .001), pTNM stage III/IV (P < .001), and perioperative transfusion (P < .001). After multivariate analysis, only pTNM stage (risk ratio 1.9798, 95% confidence interval 1.5968–2.4548, P < .001) and perioperative blood transfusion (risk ratio 1.1934, 95% confidence interval 1.0131–2.1654, P = .027) were independent risk factors for recurrence. Histologic involvement of the resection margin did not have an independent prognostic significance in relation to postoperative recurrence by multivariate analysis (P = .332).
A closer look at the pattern of histologic margin involvement revealed microscopic involvement by the main tumor in 13 patients, venous permeation at the margin in 13 patients, and involvement by discrete microscopic satellite nodules in 8 patients. The long-term survival of the first group (median survival 34 months) was significantly better than that of the latter two groups (median survival 11 and 16 months respectively, P = .005). All patients with microscopic involvement by the main tumor had a narrow macroscopic margin. In contrast, only eight patients with involvement by venous permeation and five patients with involvement by microsatellites had a narrow margin.
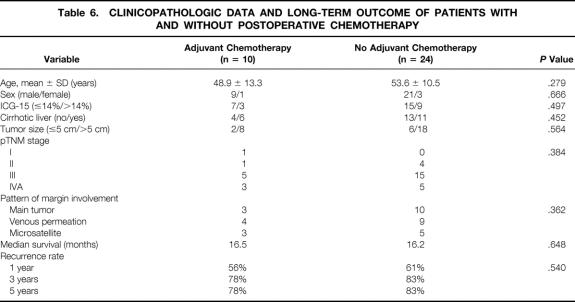
Postoperative TAC was given to 10 patients with a positive margin; the other 24 patients did not receive chemotherapy. These two groups of patients were comparable in terms of preoperative liver function, underlying liver histology, tumor size, pTNM stage, and pattern of histologic margin involvement (Table 6). There were no significant differences in the median survival and disease recurrence rate.
Table 6. CLINICOPATHOLOGIC DATA AND LONG-TERM OUTCOME OF PATIENTS WITH AND WITHOUT POSTOPERATIVE CHEMOTHERAPY
DISCUSSION
The significance of the resection margin in hepatectomy for HCC is an important but unresolved issue. In general, wide excision of a malignant tumor with an adequate margin is considered important to ensure disease eradication and prevent recurrence. However, such a concept may not be applicable to HCC, which is characterized by two unique pathologic features. First, intrahepatic spread occurs mainly by means of portal venous invasion, 22–25 which is entirely different from the way other tumors invade into surrounding tissue. Second, multicentric recurrence is common and could occur anywhere in the liver remnant. 24,25
In this study, the narrow and wide margin groups both had a high recurrence rate (78% and 75%, respectively, at 5 years), and most recurrences occurred in the liver remnant. A 5-year intrahepatic recurrence rate of 75% to 100% after resection of HCC has been reported in other studies. 2,25,26 Venous invasion and the presence of satellite nodules have been found to be the main risk factors for intrahepatic recurrence, 2,18,24,26 indicating intrahepatic metastasis as a major mechanism. In a previous pathologic study from our institution, serial sections and histologic examination of 23 resected liver specimens with HCC revealed the presence of either microsatellites or histologic venous permeation beyond 1 cm from the resection margin in 20 specimens, and it was concluded that no distance could ensure disease clearance. 27 This contention was supported by the finding in the present study that a wide resection margin was not associated with reduced postoperative recurrence rates. The propensity of HCC to disseminate by means of the portal venous system means that intrahepatic metastasis is likely to be present beyond 1 or 2 cm in most patients. Intrahepatic recurrence could also arise from multicentric carcinogenesis in the liver remnant, which also cannot be prevented by a wide resection margin.
An analysis of the relation between the resection margin and the pattern of recurrence showed that in both the narrow and wide margin groups, most recurrences occurred in the liver remnant at a distal segment or multiple segments, indicating an origin from either intrahepatic metastasis or multicentric carcinogenesis. Most of the recurrences occurred within 1 year after hepatectomy in both groups, suggesting that most recurrences were probably due to intrahepatic metastasis. 28 The only effect of a wide resection margin appeared to be the prevention of marginal recurrence, which may be regarded as a true local recurrence related to inadequate margin. However, marginal recurrence constituted only 4% of all postoperative recurrences; thus, a wide resection margin is considered to have limited value.
Tumor size, underlying cirrhosis, and the extent of resection are the main determinants of the feasible resection margin during hepatectomy. Hence, subgroup analyses were performed by stratifying patients according to these three factors. No significant effect of the margin width on postoperative recurrence could be demonstrated in either large or small tumors. The 5-year recurrence rate was substantial even in patients with small tumors, regardless of the margin width. Other authors have observed a 5-year intrahepatic recurrence rate of approximately 70% after resection of HCC less than 2 or 3 cm, suggesting a high incidence of intrahepatic metastasis or multicentric occurrence even in small HCCs. 29,30 The margin width did not have prognostic significance in relation to the underlying liver cirrhotic status or the extent of resection, either. This has an important implication for resection of HCC associated with cirrhosis. Cirrhotic liver has a limited capacity for regeneration, and limited resection is an important technique to avoid postoperative liver failure and death. 31,32 Preservation of liver function reserve may also enhance the long-term prognosis by allowing effective treatment options to be used should recurrence develop. 28,33 However, an extensive resection of nontumorous liver is often necessary to obtain a wide margin, especially when the tumor is close to a major vessel. Our findings strongly suggest that functional liver parenchyma should not be sacrificed for the sake of obtaining a wide margin, especially in patients with limited liver function reserve.
An adverse effect of histologic involvement of the resection margin on long-term survival after resection of HCC was reported in a retrospective study of patients who underwent surgery in the 1970s and 1980s in our department. 16 In that study, the relation between a positive margin and the pattern of recurrence was not investigated, and a detailed analysis of the pattern of histologic margin involvement was unavailable. A prospective database established since 1989 has permitted a more precise examination of the relation between histologic margin involvement and recurrence, providing insights into the significance of histologic margin involvement after resection of HCC.
A positive margin was a significant factor for recurrence by univariate analysis but not multivariate analysis. Perioperative transfusion and pTNM stage were the only independent risk factors. Two previous studies have reported an adverse prognostic effect of histologic margin involvement. 17,19 In this study, we examined for the first time the pattern of recurrence in patients with a positive margin. We also analyzed in detail the different patterns of histologic involvement; to our knowledge, these have not been studied before. Most of the intrahepatic recurrences occurred at a distal segment or at multiple segments rather than at the resection line, even in patients with a positive margin. Margin involvement by microscopic satellite nodules or venous tumor thrombi accounted for the majority of recurrences. Both are factors linked to intrahepatic metastasis, 2,24,26 and thus these two patterns of histologic involvement could be regarded as a marker for disseminated intrahepatic disease. This could explain the worse prognosis in these patients compared with patients with microscopic involvement by the main tumor. Only the latter could be regarded as having true residual disease in the usual sense. A 1-cm margin was effective in preventing microscopic involvement by the main tumor, but it could not prevent margin involvement by microsatellites or microscopic venous thrombi. The intriguing relation between histologic margin involvement and the presence of venous invasion or microsatellites explained why a positive margin was not found to be an independent risk factor for recurrence. Both venous invasion and satellite nodules have been incorporated into the pTNM staging, which was by far the most significant independent risk factor for postoperative recurrence. Our findings were echoed by the results of another study showing that a positive margin was an adverse prognostic factor by univariate analysis, but venous invasion was the single most important predictor of long-term outcome. 18 Perioperative transfusion was another independent risk factor of recurrence in our study, and it probably enhanced intrahepatic metastasis by suppressing the antitumor immune mechanism. 34
The role of postoperative therapy for patients with a positive margin has not been addressed before in the literature, and hence we did not have a definite policy in these patients. TAC was given to 10 patients at the discretion of the operating surgeon, taking into account each patient’s wishes. A previous study found a possible value of postoperative TAC after resection of HCC. 35 Our data failed to show a benefit of TAC in patients with a positive margin, but a prospective trial would be needed to clarify its role. Adjuvant therapy has so far proved disappointing in preventing recurrence after resection of HCC, and aggressive management of recurrence appears to be the best way of improving the long-term outcome. 28,36
In conclusion, this study showed that a wide resection margin during hepatectomy for HCC is not an effective strategy to reduce the risk of postoperative recurrence. A 1-cm resection margin may be desirable to ensure microscopic clearance from the main tumor and avoid marginal recurrence. However, most of the recurrences were related to intrahepatic metastasis or multicentric occurrence and hence could not be prevented by a wide margin. Inability to obtain a resection margin of 1 cm should not be regarded as a contraindication to resection of HCC. In patients with limited liver function reserve, preservation of liver parenchyma should take priority over a wide resection margin. Histologic involvement of the resection margin was associated with an increased risk of postoperative recurrence, but this was related to the underlying vascular invasion and intrahepatic metastasis in most patients. With the high incidence of recurrence in the liver remnant from intrahepatic metastasis or multicentric occurrence, resection of HCC could not be considered “curative” in a strict sense, irrespective of the resection margin. Regular postoperative surveillance for recurrence is mandatory for all patients, and effective management of recurrence is currently the most practical strategy to prolong survival after hepatectomy for HCC.
Footnotes
Correspondence: Ronnie Tung-Ping Poon, MS, Dept. of Surgery, Queen Mary Hospital, 102 Pokfulam Rd., Hong Kong, China.
Accepted for publication June 21, 1999.
References
- 1.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999; 229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494. [DOI] [PubMed] [Google Scholar]
- 3.Lee CS, Sung JL, Hwang LY, et al. Surgical treatment of 109 patients with symptomatic and asymptomatic hepatocellular carcinoma. Surgery 1986; 99:481–490. [PubMed] [Google Scholar]
- 4.The Liver Cancer Study Group of Japan. Predictive factors for long-term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer 1994; 74:2772–2780. [DOI] [PubMed] [Google Scholar]
- 5.Chen MF, Hwang TL, Jeng LB, et al. Postoperative recurrence of hepatocellular carcinoma: two hundred five consecutive patients who underwent hepatic resection in 15 years. Arch Surg 1994; 129:738–742. [DOI] [PubMed] [Google Scholar]
- 6.Chau GY, Lui WY, Tsay SH, et al. Prognostic significance of surgical margin in hepatocellular carcinoma resection: an analysis of 165 Child A patients. J Surg Oncol 1997; 66:122–126. [DOI] [PubMed] [Google Scholar]
- 7.Nonami T, Harada A, Kurokawa T, Nakao A, Takagi H. Hepatic resection for hepatocellular carcinoma. Am J Surg 1997; 173:288–291. [DOI] [PubMed] [Google Scholar]
- 8.Lise M, Bacchetti S, Da Pian P, et al. Prognostic factors affecting long-term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer 1998; 82:1028–1036. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida Y, Kanematsu T, Matsumata T, Takenaka K, Sugimachi K. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis. Further evaluation of limited hepatic resection. Ann Surg 1989; 209:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka N, Okamoto E, Toyosaka A, et al. Prognostic factors after hepatectomy for hepatocellular carcinoma. A univariate and multivariate analysis. Cancer 1990; 65:1104–1110. [DOI] [PubMed] [Google Scholar]
- 11.Jwo SC, Chiu JH, Chau GY, Loong CC, Lui WY. Risk factors linked to tumor recurrence of human hepatocellular carcinoma after hepatic resection. Hepatology 1992; 16:1367–1371. [DOI] [PubMed] [Google Scholar]
- 12.Ouchi K, Matsubara S, Fukuhara K, Tominaga T, Matsuno S. Recurrence of hepatocellular carcinoma in the liver remnant after hepatic resection. Am J Surg 1993; 166:270–273. [DOI] [PubMed] [Google Scholar]
- 13.Kosuge T, Makuuchi M, Takayama T, et al. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepato-Gastroenterology 1993; 40:328–332. [PubMed] [Google Scholar]
- 14.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994; 106:720–727. [DOI] [PubMed] [Google Scholar]
- 15.Fuster J, Garcia-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg 1996; 223:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai ECS, Ng IOL, You KT, et al. Hepatectomy for large hepatocellular carcinoma: the optimal resection margin. World J Surg 1991; 15:141–145. [DOI] [PubMed] [Google Scholar]
- 17.Savage AP, Malt RA. Survival after hepatic resection for malignant tumours. Br J Surg 1992; 79:1095–1101. [DOI] [PubMed] [Google Scholar]
- 18.Vauthey JN, Klimstra D, Franceschi D, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg 1995; 169:28–35. [DOI] [PubMed] [Google Scholar]
- 19.Paquet KJ, Gad HA, Lazar A, et al. Analysis of factors affecting outcome after hepatectomy of patients with liver cirrhosis and small hepatocellular carcinoma. Eur J Surg 1998; 164:513–519. [DOI] [PubMed] [Google Scholar]
- 20.Hermanek P, Sobin LH. TNM Classification of Malignant Tumours. Berlin: Springer-Verlag; 1987: 52–55.
- 21.Couinaud C. Le Foie: Etudes Anatomiques et Chirugicales. Paris: Masson & Cie; 1957: 283–289.
- 22.Matsumata T, Kanematsu T, Takenaka K, et al. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology 1989; 9:457–460. [DOI] [PubMed] [Google Scholar]
- 23.Shirabe K, Kanematsu T, Matsumata T, et al. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology 1991; 14:802–805. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto J, Kosuge T, Takayama T, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg 1996; 83:1219–1222. [PubMed] [Google Scholar]
- 25.Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg 1991; 214:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada S, Shimada K, Yamamoto J, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology 1994; 106:1618–1624. [DOI] [PubMed] [Google Scholar]
- 27.Lai ECS, You KT, Ng IOL, Shek TWH. The pathological basis of resection margin for hepatocellular carcinoma. World J Surg 1993; 17:786–791. [DOI] [PubMed] [Google Scholar]
- 28.Poon RTP, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999; 299:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi E, Maeda T, Matsumata T, et al. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology 1995; 108:768–775. [DOI] [PubMed] [Google Scholar]
- 30.Kumada T, Nakano S, Takeda I, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology 1997; 25:87–92. [DOI] [PubMed] [Google Scholar]
- 31.Kanematsu T, Takenaka K, Matsumata T, et al. Limited hepatic resection is effective for selected cirrhotic patients with primary liver cancer. Ann Surg 1984; 199:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bismuth H, Houssin D, Ornowski J, Meriggi F. Liver resections in cirrhotic patients: a Western experience. World J Surg 1986; 10:311–317. [DOI] [PubMed] [Google Scholar]
- 33.Yasui M, Harada A, Torii A, et al. Impaired liver function and long-term prognosis after hepatectomy for hepatocellular carcinoma. World J Surg 1995; 19:439–443. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery 1994; 115:303–309. [PubMed] [Google Scholar]
- 35.Takenaka K, Yoshida K, Nishizaki T, et al. Postoperative prophylactic lipiodolization reduces the intrahepatic recurrence of hepatocellular carcinoma. Am J Surg 1995; 169:400–405. [DOI] [PubMed] [Google Scholar]
- 36.Farges O, Regimbeau JM, Belghiti J. Aggressive management of recurrence following surgical resection of hepatocellular carcinoma. Hepato-Gastroenterology 1998; 45:1275–1280. [PubMed] [Google Scholar]