Abstract
Objective
To evaluate the clinical significance of preoperative serum levels of interleukin-10 (IL-10) and interleukin-6 (IL-6) in patients with resectable hepatocellular carcinoma (HCC).
Summary Background Data
IL-10 is an immunosuppressive factor and IL-6 is a multifunctional cytokine that plays a role in host defense mechanisms. Both have been reported to be related to the disease prognosis in some human solid tumors. Their role in human HCC has not been investigated.
Methods
Preoperative serum samples of 67 patients with HCC who underwent potentially curative resection and 27 normal healthy donors were assayed. Levels of IL-10 and IL-6 were determined by enzyme-linked immunosorbent assay. The clinical significance of serum IL-10 and IL-6 was evaluated and compared with conventional clinicopathologic factors.
Results
Levels of IL-10 and IL-6 were significantly higher in patients with HCC than in healthy subjects. There was no correlation between IL-10 and IL-6 levels. Tumor resection resulted in a decrease in IL-10 and IL-6 levels. On univariate analysis, patients with high IL-10 levels had a worse disease-free survival, but IL-6 levels had no correlation with the disease-free survival. Multivariate analysis identified IL-10 levels as a predictor of postresectional outcome, in addition to the well-established clinical risk factors.
Conclusions
In patients with HCC, the preoperative serum IL-10 level is related to the clinical outcome. IL-10 may play an important role in the progression of HCC.
Hepatocellular carcinoma (HCC) is a common malignancy that often occurs in patients with chronic liver disease, usually resulting from type B or C virus infection. Although surgical resection is an effective treatment for the disease, the rate of tumor recurrence after resection is high. 1 Occult microscopic intrahepatic or extrahepatic metastasis and background factors of the diseased liver, including active inflammation, 2,3 had been regarded as significant risk factors for tumor recurrence.
Interleukin-10 (IL-10) is a pleiotropic cytokine produced by macrophages, T-helper 2 cells, and B lymphocytes (CD5 subset) and can both stimulate and suppress the immune response. 4 IL-10 has been shown to inhibit various immune functions, such as antigen presentation, cytokine production, macrophage activation, and antigen-specific T-cell proliferation. By interfering with the costimulatory function of antigen-presenting cells (e.g., downregulation of class II MHC expression of monocytes and costimulatory molecule expression of macrophages), IL-10 reduces antigen-specific T-cell proliferation. Recently, it has been proposed that IL-10 plays a key role in the oncogenetic and metastatic ability of neoplasms, 5,6 and increased levels have been found in the plasma of patients with different histotypes of solid and hematopoietic tumors. 7–9
Interleukin-6 (IL-6), a multifunctional cytokine produced by a range of cells, plays a central role in host defense mechanisms. 10 Recently, it was shown that IL-6 is produced by human epidermal cells and epidermoid carcinoma cell lines, 11 as well as other epithelial tumors, including bladder carcinoma, 12 renal cell carcinoma, 13 and ovarian cancer. 14 Increased serum IL-6 levels have been found in patients with multiple myeloma, 15 renal cell carcinoma, 16 bladder carcinoma, 17 head and neck cancer, 18 ovarian cancer, 19 and cholangiocarcinoma. 20
Serum IL-10 levels have been observed to be significantly elevated in patients with type C chronic liver disease, and IL-10 may be related to the development of HCC. 21 High serum IL-6 levels had been observed in patients with advanced HCC. 22 However, it is not known whether serum levels of IL-10 and IL-6 are related to the prognosis of patients with relatively early and resectable disease. The present study was designed to investigate the serum level of IL-10 and IL-6 in patients with resectable HCC, to determine whether human hepatoma cell lines can produce IL-10 and IL-6 in vitro, and to elucidate the possible role of IL-10 and IL-6 in tumor biology and their prognostic impact.
PATIENTS AND METHODS
Patients
Preoperative serum samples of 67 patients (age 63.4 ± 1.5 years; male:female ratio, 8.6:1) with histologically confirmed HCC who underwent hepatic resection for HCC at Veterans General Hospital—Taipei were assayed. No patient had obvious infection (fever, leukocytosis) at the time of blood withdrawal, which was performed immediately before surgery. Serum samples were also obtained from a control population of 27 normal healthy donors with comparable sex and age distribution characteristics. Normal volunteers had no history of gastrointestinal complaints.
Tumor Cell Lines
Human hepatoma cell lines Hep3B, HepG2, 23 and HA22T/VGH 24 were cultured in DMEM (Gibco/BRL, Grand Island, NY) containing 10% fetal bovine serum, 0.01 mg/mL gentamicin, and 0.1 mmol/L nonessential amino acid. Cells were grown in a CO2 incubator at 37°C, with 5% CO2 and 95% filtered air. From each of three human hepatoma cell lines, 2 × 106 cells were seeded in 25-cm2 culture flasks in 5 mL culture medium. IL-6 and IL-10 proteins were determined in spent media collected 24, 48, 72, and 96 hours after seeding.
Measurement of IL-10 and IL-6 Levels
Serum samples were kept at −80°C and thawed immediately before the determination of the cytokine levels. IL-10 and IL-6 levels were determined using enzyme-linked immunosorbent assay kits (Quantikine, R&D Systems, Inc., Minneapolis, MN). All samples were measured in duplicate. Sensitivity was 1.5 and 0.79 pg/mL for IL-10 and IL-6, respectively.
Surgical Procedures and Follow-Up
The surgical procedures have been described previously. 25–27 After surgery, the resected specimens were examined meticulously by both the surgeon and the pathologist. The specimens were cut into slices approximately 10 mm thick. The macroscopic features of the tumor, including size, number, capsular formation, and tumor venous invasion, were recorded. DNA ploidy, analyzed with fresh tissue samples, was measured with a flow cytometer (Epics Profiles, Coulter Electronics, Hialeah, FL), as previously described. 28,29 After surgery, patients were followed up every 2 to 3 months with measurement of serum α-fetoprotein (AFP), ultrasonography, CT, or MRI. If recurrence was suspected, angiography was performed. Intrahepatic tumor recurrence was diagnosed when the results of at least two of those five modalities were positive.
Statistical Analysis
Values are expressed as median (ranges) or as mean ± SEM when appropriate. IL-10 and IL-6 levels for patients and for healthy subjects were compared using the Wilcoxon rank-sum test. Statistical comparisons of other variables were made using the Student t test, the chi-square test, or the Fisher test, as appropriate. Survival rates were calculated by the Kaplan-Meier method, 30 and survival curves were compared using the log-rank test. For IL-10 and IL-6, Kaplan-Meier estimates were performed defining the best cutoff values for discrimination between poor and good survival. Univariate and multivariate analyses of prognosis for the patient’s background factors were performed using the Cox proportional hazards model. 31 Statistical difference was defined as P < .05.
RESULTS
IL-10 and IL-6 Levels
Serum IL-10 levels (median 9.8 pg/mL, range 2.3–50.7) were significantly higher in the 67 patients with HCC than in the 27 healthy subjects (median 6.3 pg/mL, range 3.6–13.3) (P = .001) (Fig. 1). Taking 12 pg/mL as the cutoff value, 24 patients with HCC (36%) had an IL-10 level of more than 12 pg/mL.
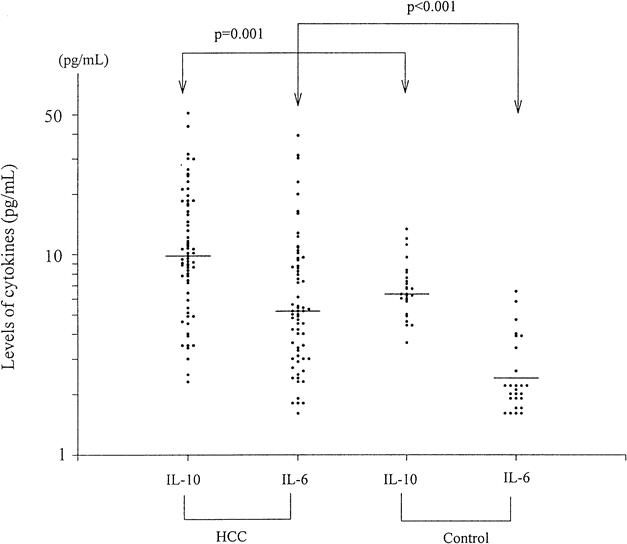
Figure 1. Serum levels of interleukin-10 and interleukin-6 on a logarithmic scale in patients with hepatocellular carcinoma and healthy subjects. Horizontal lines indicate the median values.
Serum IL-6 levels (median 5.2 pg/mL, range 1.6–39.1) were also significantly higher in the 67 patients with HCC than in the 27 healthy subjects (median 2.4 pg/mL, range 1.6–6.5) (P < .001). Taking 6.0 pg/mL as the cutoff value, 27 patients with HCC (40%) had an IL-6 level of more than 6.0 pg/mL.
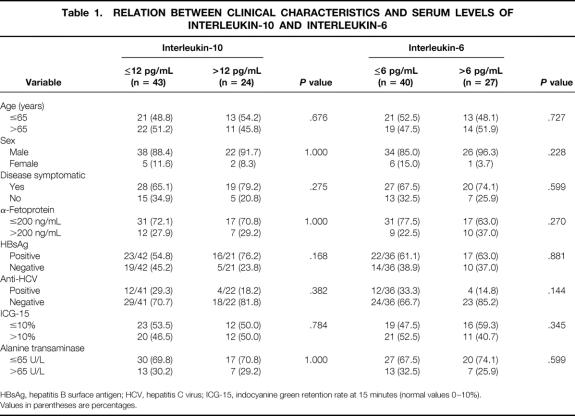
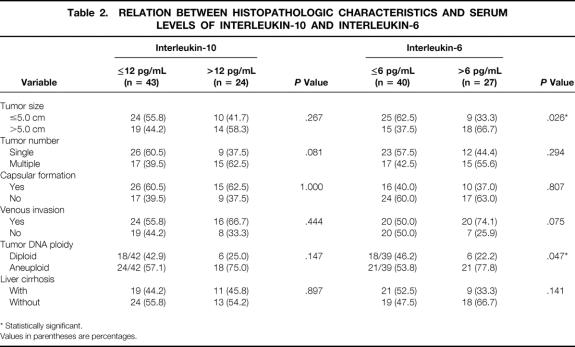
The clinicopathologic features of the patients stratified according to the IL-10 and IL-6 levels are shown in Tables 1 and 2. No factor was found to be related significantly to the serum IL-10 level, but tumor size and DNA ploidy status correlated significantly with the serum IL-6 level. In patients with HCC, the serum IL-10 levels did not correlate with serum IL-6 levels (r = 0.0704, P = .571) (Fig. 2).
Table 1. RELATION BETWEEN CLINICAL CHARACTERISTICS AND SERUM LEVELS OF INTERLEUKIN-10 AND INTERLEUKIN-6
HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; ICG-15, indocyanine green retention rate at 15 minutes (normal values 0–10%).
Values in parentheses are percentages.
Table 2. RELATION BETWEEN HISTOPATHOLOGIC CHARACTERISTICS AND SERUM LEVELS OF INTERLEUKIN-10 AND INTERLEUKIN-6
* Statistically significant.
Values in parentheses are percentages.
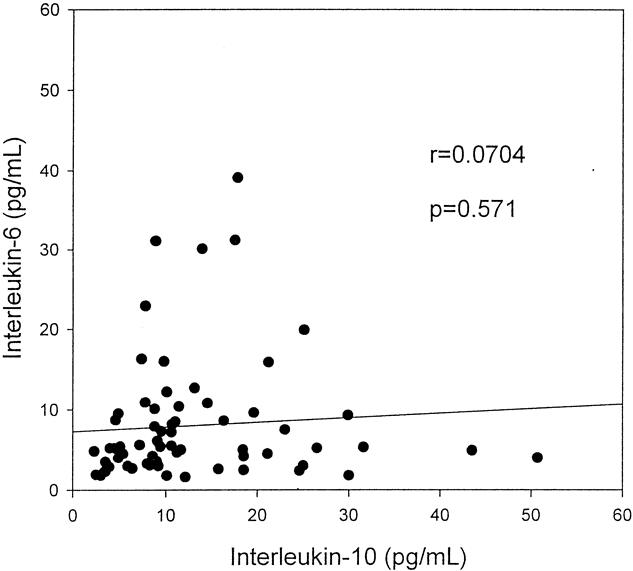
Figure 2. Scattergram of serum levels of interleukin-10 and interleukin-6 in patients with hepatocellular carcinoma. No significant correlations were noted.
Serial IL-10 and IL-6 Levels
Serial serum IL-10 levels were measured in 10 patients with HCC who had elevated levels before surgery and were disease-free at the time postoperative levels were assessed. Eight of these patients also had elevated IL-6 levels. Decreased IL-10 levels were observed during a period of 6 to 13 months in all of these patients (Fig. 3). Decreased IL-6 levels were observed in nine of these patients (Fig. 4).

Figure 3. Serial serum levels of interleukin-10 in 10 patients with hepatocellular carcinoma.
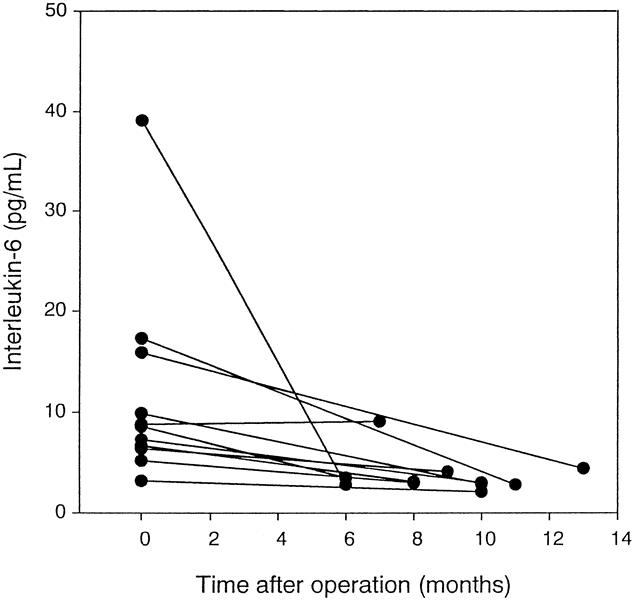
Figure 4. Serial serum levels of interleukin-6 in 10 patients with hepatocellular carcinoma.
Effect of IL-10 Levels on Survival
Patients with high IL-10 levels had a significantly worse disease-free survival (P = .007, log-rank test) (Fig. 5). No significant difference in survival was noted between patients with high and low IL-6 levels (P = .647, log-rank test) (Fig. 6). Univariate and multivariate analyses using the Cox proportional hazards model showed that IL-10 level was an independent prognostic factor (risk ratio = 4.0202, P = .0016) (Table 3).
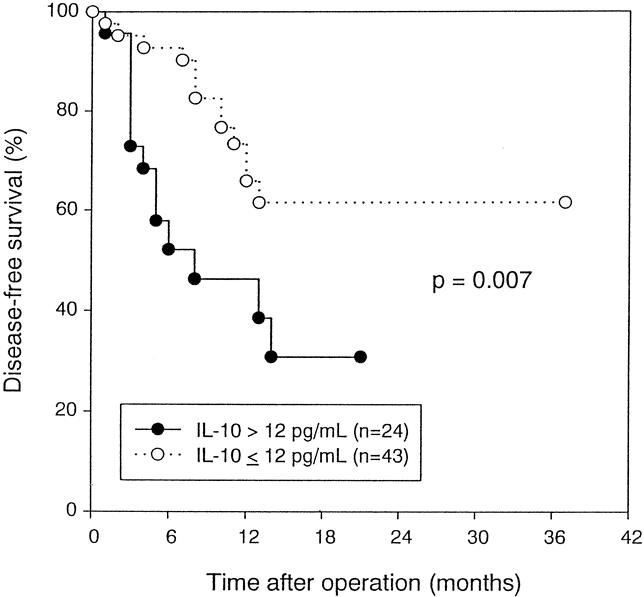
Figure 5. Disease-free survival of patients with hepatocellular carcinoma stratified by serum interleukin-10 level before resection (P = .007, log-rank test).
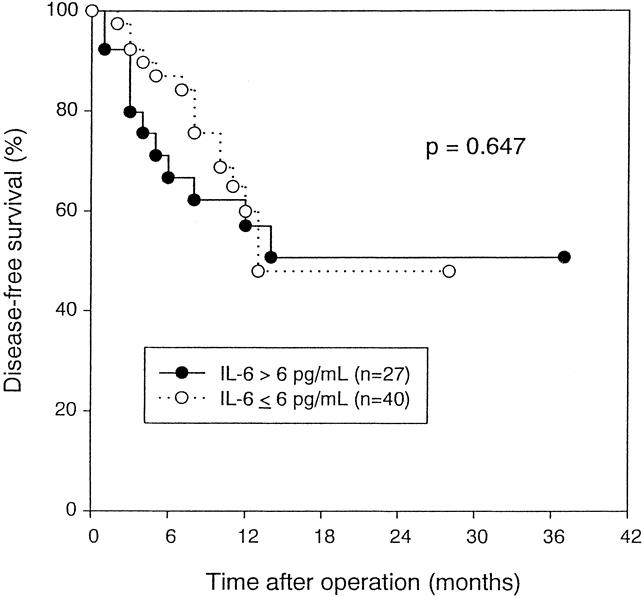
Figure 6. Disease-free survival of patients with hepatocellular carcinoma stratified by serum interleukin-6 level before resection (P = .647, log-rank test).
Table 3. UNIVARIATE AND MULTIVARIATE ANALYSES OF POSSIBLE PROGNOSTIC FACTORS
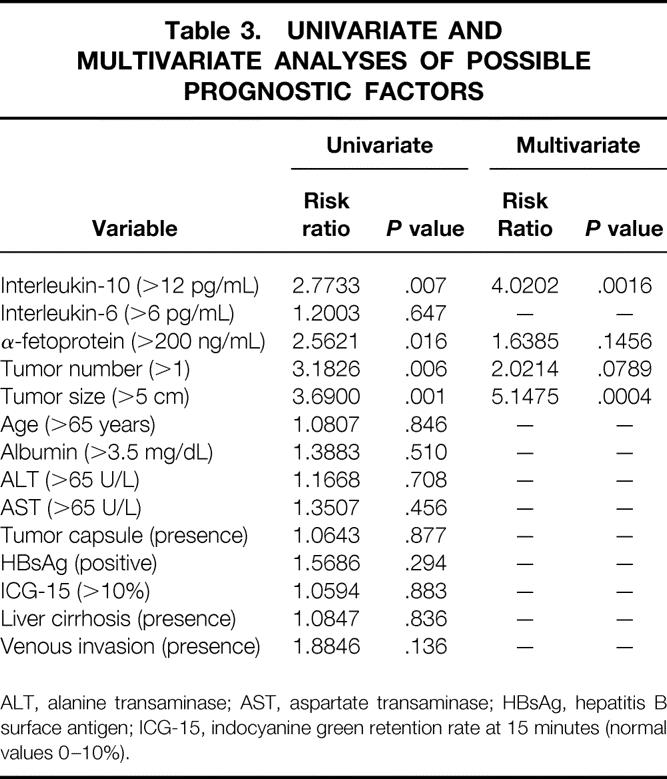
ALT, alanine transaminase; AST, aspartate transaminase; HBsAg, hepatitis B surface antigen; ICG-15, indocyanine green retention rate at 15 minutes (normal values 0–10%).
Production of IL-10 and IL-6 by Human Hepatoma Cell Lines
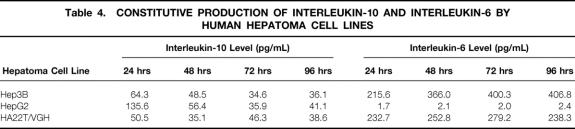
IL-10 was detected in spent media from three human hepatoma cell lines, with levels from 34.6 to 135.6 pg/mL (Table 4). In contrast, Hep3B and HA22T cells secreted high levels of IL-6, but negligible levels was found in spent media of HepG2 cells.
Table 4. CONSTITUTIVE PRODUCTION OF INTERLEUKIN-10 AND INTERLEUKIN-6 BY HUMAN HEPATOMA CELL LINES
DISCUSSION
We found that serum IL-10 and IL-6 levels were increased significantly in patients with resectable HCC compared with healthy subjects. Tumor resection resulted in a decrease of IL-10 and IL-6 levels. Multivariate analysis found serum IL-10 levels to be an independent prognostic factor for disease-free survival after tumor resection.
Elevated serum IL-10 levels have been observed in patients with chronic liver disease, including chronic hepatitis and cirrhosis, 21,32 and the levels are closely associated with disease progression. Kakumu et al 21 found that IL-10 levels correlated with serum alanine aminotransferase values, suggesting that this cytokine may also reflect the degree of inflammation in the liver. This increment in serum IL-10 during the course of chronic disease of the liver may be ascribed to increased IL-10 production by several cell types, including CD4+ T cells, monocytes/macrophages, and B cells. IL-10 may be produced in response to the intrahepatic inflammatory changes as an antiinflammatory cytokine. The antiinflammatory characteristics of IL-10 downregulate the host immune response 33 and provide a mechanism for modulating the deleterious overreaction by the host immune response during the progression of chronic viral infection. 21 A high IL-10 level was suggested to contribute to a relative state of immunosuppression and, in patients with HCC, may help the tumor cell to escape host immune surveillance and potentiate tumor cells to metastasize.
In addition to being produced at the site of inflammatory changes with activated infiltrating mononuclear cells in the liver, the high serum IL-10 levels in patients with HCC presumably also resulted from secretion of IL-10 by tumor cells. Secretion of IL-10 by human hepatocellular tumors had been observed previously. 34 This assumption is also supported by our observations that hepatoma cancer cell lines secreted IL-10 and that serum IL-10 levels decreased after tumor-bearing tissues were removed. However, the IL-10 levels after surgery were still higher than the average for healthy subjects (see Figs. 1 and 3). One of the key functional parameters of an immunologic response to tumors is the local production of cytokine at the tumor site. The observation that certain human epithelial cancer cell lines elaborate significant quantities of IL-10 suggests a potential role for this molecule in solid tumor development and in host–tumor interactions, and autocrine production of IL-10 by tumor cells may have a role in HCC development and antitumor immunity. 35
We found that IL-10 concentrations were not related to those of IL-6. This finding may indicate that an independent response occurs between IL-10 and IL-6 in HCC-related immunity.
In contrast to IL-10, serum IL-6 levels were not elevated in patients with chronic liver disease. 36 In patients with advanced HCC, elevated levels of IL-6 have been found, correlating with the tumor burden. 22 Consistent with this finding, our study showed that serum IL-6 levels were also elevated in patients with relatively early and resectable disease and were also correlated with tumor size (see Table 2). In a study of 14 patients with advanced HCC, Goydos et al 20 found that IL-6 levels were higher than in healthy subjects, but with no correlation between IL-6 and AFP levels. Similarly, IL-6 levels did not correlate with AFP levels in our study (see Table 1). In addition, there was no significant survival difference between patients with high or low levels of serum IL-6.
In our study, elevated IL-10 levels had no correlation with other adverse prognostic factors, including tumor size, tumor number, and venous invasion (see Table 2). Serum IL-10 was found to be an independent prognostic factor on multivariate analysis, indicating the important biologic significance of this cytokine in the HCC disease process. The immunosuppressive effects of IL-10 may play a major role in the development of neoplastic processes by suppressing macrophage activation and interferon-γ production, thereby crippling two potential mediators of an antitumor response. The functional consequences of IL-10 binding to its receptors on tumor cells could be the prevention of programmed cell death and the promotion of proliferation. 37 In addition, IL-10 may contribute to the development of an environment favorable to neoplastic cells and to the enhancement of their metastatic potential. IL-10 may influence the expression or activity of as-yet-untested or unknown adhesion molecules and may affect the migratory abilities of immune effector cells or the metastatic abilities of tumor cells. 5 Production of IL-10 by tumors or other cells is mostly likely to be beneficial to the tumor but detrimental to the patient. The possible biologic mechanisms responsible for the prognostic value of serum IL-10 levels in patients with HCC are under investigation in our laboratory.
We speculate that chronic liver disease provokes both inflammatory and antiinflammatory responses, with the latter responsible for the high serum levels of IL-10 found in certain patients. The tumor may benefit accidentally from the antiinflammatory effect from the host. In addition, IL-10 can be produced vigorously by some tumor cells. High circulating and local levels of IL-10 result, allowing tumor cells to elude host defense mechanisms and affecting the tumor’s metastatic potential. However, an active inflammatory status in the liver may result in further tumor appearance. 3,38 The result is potentiation of recurrence after resection. If this hypothesis is true, the inhibition of IL-10 production and the administration of anti–IL-10 should be able to disrupt this cascade. The inflammatory response should also be suppressed. In HCC, IL-10 may be a promising cytokine for future cancer immunotherapy or gene therapy protocols to restore host cellular antitumor response by antagonizing the IL-10–mediated immunosuppressive events.
Footnotes
Correspondence: Chin-Wen Chi, PhD, Dept. of Medical Research and Education, Veterans General Hospital—Taipei, Shih-pai, Taipei, Taiwan, 11217.
Supported by grants from the National Science Council of the Republic of China (NSC 87-2314-B-075-053) and Veterans General Hospital, Taipei, Taiwan.
Accepted for publication November 8, 1999.
References
- 1.Okada S, Shimada K, Yamamoto J, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology 1994; 106:1618–1624. [DOI] [PubMed] [Google Scholar]
- 2.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994; 106:720–727. [DOI] [PubMed] [Google Scholar]
- 3.Ko S, Nakajima Y, Kanehiro H, et al. Significant influence of accompanying chronic hepatitis status on recurrence of hepatocellular carcinoma after hepatectomy. Ann Surg 1996; 224:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard M, O’Garra A, Ishida H, et al. Biologic properties of interleukin-10. J Clin Immunol 1992; 12:239–247. [DOI] [PubMed] [Google Scholar]
- 5.Holland G, Zlotnik A. Interleukin-10 and cancer. Cancer Invest 1993; 11:751–758. [DOI] [PubMed] [Google Scholar]
- 6.Nabioullin R, Sone S, Mizuno K, et al. Interleukin-10 is a potent inhibitor of tumor cytotoxicity by human monocytes and alveolar macrophages. J Leukocyte Biol 1994; 55:437–442. [DOI] [PubMed] [Google Scholar]
- 7.Fortis C, Foppoli M, Gianotti L, et al. Increased interleukin-10 serum levels in patients with solid tumors. Cancer Lett 1996; 104:1–5. [DOI] [PubMed] [Google Scholar]
- 8.Merville P, Rousset F, Banchereau J, et al. Serum interleukin-10 in early stage multiple myeloma. Lancet 1992; 340:1544–1545. [DOI] [PubMed] [Google Scholar]
- 9.Blay JY, Burdin N, Rousset F, et al. Serum interleukin-10 in non-Hodgkin’s lymphoma: a prognostic factor. Blood 1993; 82:2169–2174. [PubMed] [Google Scholar]
- 10.Hirano T, Akira S, Taga T. Biological and clinical aspects of interleukin-6. Immunol Today 1990; 11:443–449. [DOI] [PubMed] [Google Scholar]
- 11.Kirnbauer R, Kock A, Schwarz T, et al. IFN-b2, B cell differentiation factor 2, or hybridoma growth factor (IL-6) is expressed and released by human epidermal cells and epidermoid carcinoma cells. J Immunol 1989; 16:1017–1019. [PubMed] [Google Scholar]
- 12.Rawle FC, Shields J, Smith SH, et al. B cell growth and differentiation induced by supernatants of transformed epithelial cell lines. Eur J Immunol 1986; 16:1017–1019. [DOI] [PubMed] [Google Scholar]
- 13.Miki S, Iwano M, Miki Y, et al. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett 1989; 250:607–610. [DOI] [PubMed] [Google Scholar]
- 14.Watson JM, Sensintaffar JL, Berek JS, Martinez-Maza O. Constitutive production of IL-6 by ovarian cancer cell lines and by primary ovarian tumor cultures. Cancer Res 1990; 50:6959–6965. [PubMed] [Google Scholar]
- 15.Bataille R, Jourdan M, Zhang XG, Klein B. Serum levels of interleukin 6, a potent myeloma growth factor, as a reflection of disease severity in plasma cell dyscrasia. J Clin Invest 1989; 84:2008–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsukamoto T, Kumanoto Y, Miyao N, et al. Interleukin-6 in renal cell carcinoma. J Urol 1992; 148:1778–1782. [DOI] [PubMed] [Google Scholar]
- 17.Seguchi T, Yokokawa K, Sugao H, et al. Interleukin-6 activity in urine and serum in patients with bladder carcinoma. J Urol 1992; 148:791–794. [DOI] [PubMed] [Google Scholar]
- 18.Gallo O, Gori AM, Attanasio M, et al. Acute-phase proteins and interleukin-6 serum level in head and neck cancer. Arch Otolaryngol 1992; 118:1336–1337. [DOI] [PubMed] [Google Scholar]
- 19.Berek JS, Chung C, Kaldi K, et al. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol 1991; 164:1038–1043. [DOI] [PubMed] [Google Scholar]
- 20.Goydos JS, Brumfield AM, Frezza E, et al. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma. Validation of utility as a clinical marker. Ann Surg 1998; 227:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakumu S, Okumura A, Ishikawa T, et al. Serum levels of IL-10, IL-15 and soluble tumor necrosis factor-alpha (TNF-α) receptors in type C chronic liver disease. Clin Exp Immunol 1997; 109:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaguarnera M, Fazio I Di, Laurino A, et al. Role de l’interleukine 6 dans le carcinoma hepatocellulaire. Bull Cancer 1996; 83:379–384. [PubMed] [Google Scholar]
- 23.Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 1980; 209:497–499. [DOI] [PubMed] [Google Scholar]
- 24.Chang C, Hu CP, Tang T, et al. The differentiation properties of human hepatoma cell lines. In: Chan EH, Lin KJ, Huang PC, eds. Molecular Biology of Neoplasia. Taipei: Academica Sinica; 1985: 262–269.
- 25.Lui WY, Chau GY, Loong CC, et al. Hepatic segmentectomy for curative resection of primary hepatocellular carcinoma. Arch Surg 1995; 130:1090–1097. [DOI] [PubMed] [Google Scholar]
- 26.Chau GY, Lui WY, Tsay SH, et al. Prognostic significance of surgical margin in the resection of hepatocellular carcinoma: an analysis of 165 Child A patients. J Surg Oncol 1997; 66:122–126. [DOI] [PubMed] [Google Scholar]
- 27.Lee NH, Chau GY, Lui WY, et al. Surgical treatment and outcome in patients with a hepatocellular carcinoma greater than 10 cm in diameter. Br J Surg 1998; 85:1654–1657. [DOI] [PubMed] [Google Scholar]
- 28.Chiu JH, Kao HL, Wu LW, et al. Prediction of relapse or survival after resection in human hepatomas by DNA flow cytometry. J Clin Invest 1992; 89:539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jwo SC, Chiu JH, Chau GY, et al. Risk factors linked to tumor recurrence of human hepatocellular carcinoma after hepatic resection. Hepatology 1992; 16:1367–1371. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–481. [Google Scholar]
- 31.Cox DR. Regression models and life-tables. J R Stat Soc B 1972; 34:187–220. [Google Scholar]
- 32.Cacciarelli TV, Martinez OM, Gish RG, et al. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and posttreatment with interferon alfa. Hepatology 1996; 24:6–9. [DOI] [PubMed] [Google Scholar]
- 33.Moore KW, O’Garra A, de Waal Malefyt R, et al. Interleukin 10. Annu Rev Immunol 1994; 11:165–190. [DOI] [PubMed] [Google Scholar]
- 34.Matsuguchi I, Okamura S, Kawasaki C, Niho Y. Production of interleukin-6 from human liver cell lines: production of interleukin-6 is not concurrent with the production of α-fetoprotein. Cancer Res 1990; 50:7457–7459. [PubMed] [Google Scholar]
- 35.Gastl GA, Abrams JS, Nanus DM, et al. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer 1993; 55:96–101. [DOI] [PubMed] [Google Scholar]
- 36.Wu CW, Wang SR, Chao MF, et al. Serum interleukin-6 reflects disease status of gastric cancer. Am J Gastroenterol 1996; 91:1417–1422. [PubMed] [Google Scholar]
- 37.Baiocchi RA, Ross ME, Tan JC, et al. Lymphomagenesis in the SCID-hu mouse involves abundant production of human interleukin-10. Blood 1995; 85:1063–1074. [PubMed] [Google Scholar]
- 38.Chiu JH, Wu LH, Kao HL, et al. Can determination of the proliferative capacity of the non-tumor part predict the risk of the tumor recurrence in the liver remnant after resection of the human hepatocellular carcinoma? Hepatology 1993; 18:96–102. [PubMed] [Google Scholar]