Abstract
Objective
To evaluate the feasibility and efficacy of a direct, minimally invasive adenomectomy (MIA) as an alternative to conventional neck exploration (CNE) in patients with primary hyperparathyroidism.
Summary Background Data
Because primary hyperparathyroidism is caused by a solitary adenoma in 85% to 90% of patients, a direct adenomectomy through a mini-incision would theoretically suffice whenever an adenoma is correctly localized on preoperative imaging. If effective, a less invasive method could spare the patient an unnecessary bilateral neck exploration, thus saving time and rendering future surgical procedures in the neck less problematic.
Methods
Between October 1994 and October 1998, 110 consecutive patients with biochemically proven primary hyperparathyroidism who were to undergo surgery were enrolled in this study. Ultrasound and spiral CT were routinely performed as standard preoperative imaging modalities in the first series of 65 patients. In the second series of 45 patients, ultrasound was performed as the sole initial modality; it was supplemented by CT only in case of inconclusive test results. If test results were unequivocal (one adenoma), the patient was offered MIA. CNE was performed if the results were equivocal or if multiglandular disease was suspected.
Results
Overall, 84 patients were selected for MIA and 26 for CNE. In the first series, 2 MIA procedures (2/51) were converted to CNE because of negative perioperative findings. All 65 procedures resulted in normocalcemia. In the second series, all but five (4/33 MIAs, 1/12 CNEs) resulted in normocalcemia. A reexploration (CNE) was performed in three patients, resulting in normocalcemia after resection of a second or third adenoma. Two patients are still awaiting reexploration. In both series together, 78 of the 110 patients were successfully treated with MIA and spared CNE.
Conclusion
MIA is a safe and effective alternative to CNE that may replace CNE in approximately two thirds of all patients.
Conventional treatment for primary hyperparathyroidism (pHPT) consists of systematic bilateral neck exploration, identification of all parathyroid glands, and removal of all pathologically enlarged, hyperfunctioning parathyroid tissue. 1 Because this conventional neck exploration (CNE) is associated with a minimal death rate, a low complication rate, and cure rates of 90% to 97%, it is generally considered an effective and safe procedure, leaving little room for improvement. 1,2
However, various authors have confirmed that pHPT is caused by a solitary adenoma in 85% to 90% of patients. 1–4 In these patients, removal of the affected parathyroid gland alone through a mini-incision would theoretically suffice. Thus, unnecessary exploration of normal parathyroids, making subsequent surgery in the thyroid region unattractive and more prone to complications, might be avoided. Few reports have been published on strategies of less invasive surgery for pHPT, most of which have included unilateral exploration. 5,6 Only the study by Chapuis et al 3 describes a truly minimally invasive strategy, with a mini-incision over the adenoma. Using this procedure, the authors reported they could avoid CNE in approximately 40% of their patients.
Clearly, for such a direct, minimally invasive adenomectomy (MIA) to be successful, optimal preoperative imaging is a prerequisite. This imaging should not only accurately localize pathologically enlarged parathyroid glands, but should also be capable of aiding in the selection of patients for the direct approach by differentiating between solitary and multiglandular disease. Although many imaging techniques have been reported for visualization of hyperfunctioning parathyroids, with varying degrees of success, none has proved sufficiently accurate to revolutionize surgical management in patients with pHPT, with the exception of reoperations. 7,8 Nevertheless, recent work from our group has indicated that preoperative imaging by combined ultrasonography and CT scanning, including the use of cinevision technology, appears to increase sensitivity and specificity rates to acceptable levels. 4 The present study represents a prospective evaluation of MIA in more than 100 patients with pHPT. Initially, combined imaging was used routinely. In the second group of patients, we tried to determine whether ultrasound could be used as the sole initial imaging modality, basing the need for subsequent CT scanning on the ultrasound findings.
METHODS
Patients
Consecutive patients with biochemically proven pHPT who were to undergo surgery were enrolled in this prospective study. Informed consent was obtained. Patients with previous (para)thyroid surgery were excluded. Between October 1994 and October 1998, 110 patients with pHPT were included. The investigated group involved 27 male patients and 83 female patients with a median age of 59.5 years (range 17–84). The median serum calcium level was 2.85 mmol/L (range 2.35–3.80; normal 2.20–2.60) and the median serum parathyroid hormone (PTH) level was 12.8 pmol/L (range 4.9–234; normal <8 in normocalcemic state). All patients underwent preoperative parathyroid imaging.
Protocol
In the first series of 65 patients (group A), both ultrasonography and CT scanning of the neck were routinely performed as standard preoperative imaging modalities. In the second series of 45 patients (group B), ultrasound was done as the sole initial imaging modality. When the ultrasound results were inconclusive, additional CT scanning was performed. The imaging data for each patient were thoroughly discussed by the radiologist and the surgeon. If there was unequivocal radiologic identification of a solitary adenoma, the patient was offered an adenomectomy with MIA. In contrast, when the test results were equivocal, or if multiglandular disease was suspected, the patient underwent a systematic bilateral neck exploration. This protocol has been described elsewhere. 9 An exploration was defined as being successful when a normocalcemic state (or hypocalcemia) was documented on the first postoperative day and confirmed after a minimal follow-up of 1 month. The preoperative findings on imaging were compared with intraoperative observations and postoperative test results (e.g., serum calcium, histology).
Imaging
Ultrasound examinations were performed using a high-end real-time unit (ATL, HDI 900 and 3000, Transducers L5-10 and L7-4; Advanced Technical Laboratories, Bothell, WA) with color Doppler mode. All examinations were performed by the same experienced radiologist (AvD). The patient was positioned in the same way as for surgery: in the supine position with the neck in hyperextension. Modern ultrasound systems, with their discriminatory capacity of structures measuring 3 mm or more, still cannot visualize normal parathyroid glands. However, enlarged, hyperfunctional parathyroids can be discerned not only on the basis of their size and oval shape but also because of their characteristic ultrasound features, including hypoechogenicity, typical intraglandular blood flow, movability, and noncompressibility. 4 All ultrasound findings regarding the location, size, and shape of each parathyroid (adenoma), concomitant thyroid disease, and other abnormalities were recorded and stored in a database.
Spiral CT scanning (Tomoscan 7000, Philips Medical Systems, Eindhoven, The Netherlands) was performed after intravenous administration of a contrast bolus (90 mL Isovist 300 (Schering M, Berlin, Germany); rate 2 mL/sec, delay 25 sec) using a slice thickness of 5 mm and a reconstruction index of 3 mm. Again, parathyroid adenomas were recognized partly by their anatomical position and partly by their vascularity. Essential additional information was obtained from image analysis at the computer workstation (Easy Vision, Philips Medical Systems, Best, The Netherlands). This allows reconstruction of a scanned volume in the cine loop motion. With this dynamic reconstruction, it is possible to go up and down through the scanned volume, making it easier to identify anatomical details that would otherwise be difficult to discern.
Surgery
All explorations were carried out under general anesthesia by the same surgical team. The duration (incision to closure) of each exploration was monitored, together with the exact position of the excised structure.
MIA was performed through a 2-cm-long transverse incision at the medial border of the sternocleidomastoid muscle, precisely over the site where the enlarged gland had been located by preoperative ultrasound. With the sternocleidomastoid muscle and internal jugular vein retracted laterally and the strap muscles medially, the thyroid gland was grasped in Ellis forceps and held in the medioventral direction. This allowed the surgeon to reach the loose areolar tissue of the tracheoesophageal groove (Fig. 1). After identification of the enlarged gland, it was excised in toto and its vascular pedicle ligated. No wound drains were used.
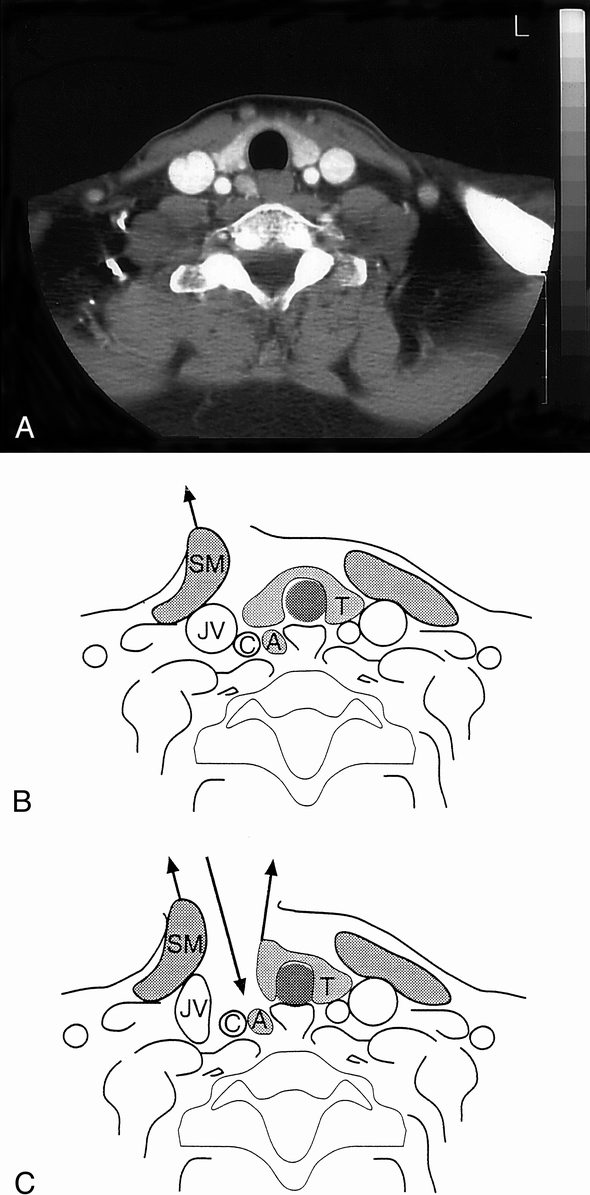
Figure 1. Cross-section images of spiral CT scanning (A) and corresponding schematic drawings (B,C) depicting the route used for the direct approach to a parathyroid adenoma. (B) Lateral retraction of the sternocleidomastoid muscle (SM). (C) Lateral retraction of SM plus the internal jugular vein (JV), along with medioventral retraction of the right lobe of the thyroid gland (T), allows direct approach (downward arrow) to a parathyroid adenoma (A). C, common carotid artery.
CNE was performed through a transverse collar incision. A bilateral, systematic exploration was done identifying as many parathyroid glands as possible before removal of the enlarged, hyperfunctioning gland or glands. Surgical findings, including the size, weight, and location of each gland excised, were noted before preparation for histologic examination.
Evaluation
Immediately after surgery, blood samples were taken at regular intervals. Procedure-related complications were documented and followed up. Patients were discharged as soon as their clinical condition permitted; one criterion for discharge was a serum calcium level of 2.20 mmol/L or more, which was generally achieved on the first or second postoperative day. All patients were seen on follow-up visits after 1 week and 1 month; after that, follow-up was continued by the patient’s physician. At 1 month after surgery, the surgical procedure was determined to be successful or unsuccessful, as defined above. If hypercalcemia persisted, the patient was scheduled to undergo a reexploration.
Recently, we have developed a rapid PTH test for intraoperative assessment. 10 This is useful, because the half-life of this hormone is approximately 5 minutes. The test involves a modification of the computerized immunometric method using chemoluminescence for detection. Although this test was not a subject of the present study, with the use of this test surgery was found to have failed in some of the patients later in this series and they could, therefore, undergo immediate conventional reexploration.
Although all resected parathyroid glands were examined histologically, histologic classification as “adenoma” or “hyperplasia” has been found to correlate poorly with “single” or “multiglandular” disease. 11 In fact, earlier work has shown that the distinction between the two entities may be suppositional. 12,13 Based on these observations, we defined each enlarged, hyperfunctioning parathyroid gland as adenoma for the purpose of this study.
RESULTS
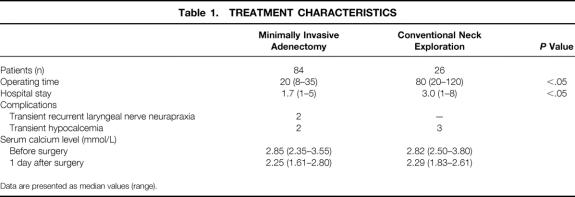
Overall, 84 patients underwent MIA and 26 CNE. Table 1 lists the main characteristics of each procedure. Two patients who underwent MIA had transient neurapraxia of the ipsilateral recurrent laryngeal nerve lasting approximately 2 and 5 months. Both patients had large adenomas in the lower region of the neck. Oral supplementation for transient hypocalcemia was required in five patients (two after CNE, three after MIA). On follow-up at 1 week and 1 month after surgery, serum calcium levels were similar to those on day 1. So far, no late recurrences have been identified after a median follow-up of 23.5 months (range 4–48).
Table 1. TREATMENT CHARACTERISTICS
Data are presented as median values (range).
The excised adenomas varied from 90 to 16,020 mg (median 560; normal <40). All resected adenomas were confirmed histologically. Table 2 shows the number of adenomas retrieved in each treatment group. Ninety percent of the patients in this series had solitary adenomas; 10% had multiple adenomas.
Table 2. NUMBER OF ENLARGED PARATHYROID GLANDS (ADENOMAS) RETRIEVED
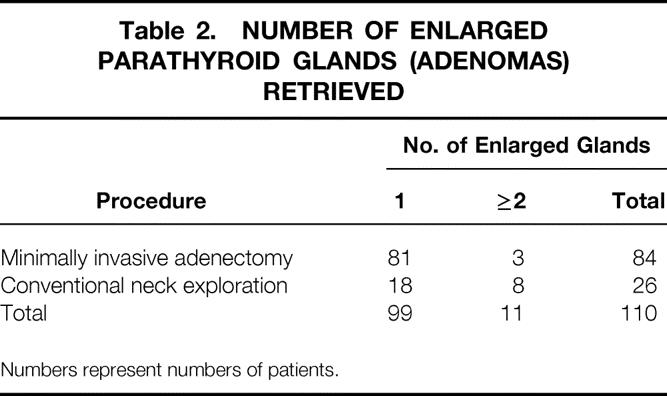
Numbers represent numbers of patients.
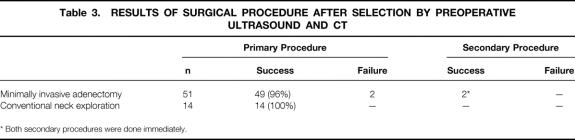
In group A, all 65 patients underwent ultrasound investigation according to protocol. Four patients did not have spiral CT scanning because of pregnancy or claustrophobia. Based on unequivocal localization and identification of a solitary adenoma on imaging, 51 patients were selected for MIA. The remaining 14 patients underwent CNE. Because of negative perioperative findings, two MIA procedures were converted to CNE. In one instance, the causative adenoma was found in a different location than predicted on imaging as a result of significant multinodular enlargement of the thyroid. The adenoma in the second patient was localized correctly but missed during MIA. All 65 procedures resulted in normocalcemia at 1 day, 1 week, and 1 month after surgery (Table 3). Nine patients who were selected for CNE actually had solitary adenomas. Thus, combined imaging with ultrasound and CT (including cinevision) had a sensitivity of 84%, a specificity of 72%, and a positive predictive value of 96% (Table 4).
Table 3. RESULTS OF SURGICAL PROCEDURE AFTER SELECTION BY PREOPERATIVE ULTRASOUND AND CT
* Both secondary procedures were done immediately.
Table 4. PERIOPERATIVE OUTCOME OF PREOPERATIVE IMAGING BY COMBINED ULTRASOUND AND CT
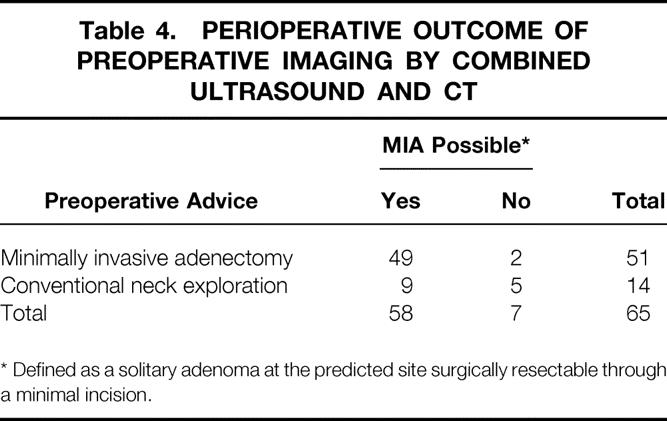
* Defined as a solitary adenoma at the predicted site surgically resectable through a minimal incision.
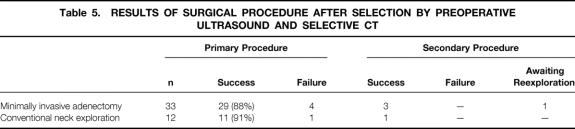
In group B (n = 45), a supplementary spiral CT was performed in 27 patients because of inconclusive test results on ultrasound. In the other 18 patients, a solitary adenoma was diagnosed on ultrasound, and MIA was advised. After CT scanning, another 15 patients were selected to undergo MIA; the remaining 12 underwent CNE (Table 5). In this group, MIA was unsuccessful in four patients, three of whom had undergone ultrasound as the sole preoperative imaging modality. Two of those in whom surgery failed were identified during surgery by rapid PTH testing. These two underwent immediate conventional exploration. In one patient, a second adenoma was ultimately found inside a thyroid cyst; in the other patient, a smaller-than-anticipated adenoma had been removed during MIA, whereas the second, larger adenoma that had been seen on imaging was located more dorsally in the direct vicinity of the first lesion. Both explorations resulted in persistent normocalcemia as well as normal serum PTH levels. The other two cases in which surgery failed involved patients in whom single adenomas fitting the preoperative imaging results were removed during MIA, but hypercalcemia persisted on follow-up. One of these patients underwent successful reexploration, during which a second enlarged gland was retrieved. The other patient is awaiting further surgical exploration. CNE failed in one patient as a result of a missed third enlarged parathyroid gland in the thymus. As shown in Table 6, ultrasound as the sole initial preoperative imaging modality in this series resulted in a sensitivity of 76%, a specificity of 43%, and a positive predictive value of 88%.
Table 5. RESULTS OF SURGICAL PROCEDURE AFTER SELECTION BY PREOPERATIVE ULTRASOUND AND SELECTIVE CT
Table 6. PERIOPERATIVE OUTCOME OF PREOPERATIVE IMAGING BY ULTRASOUND AND SELECTIVE CT
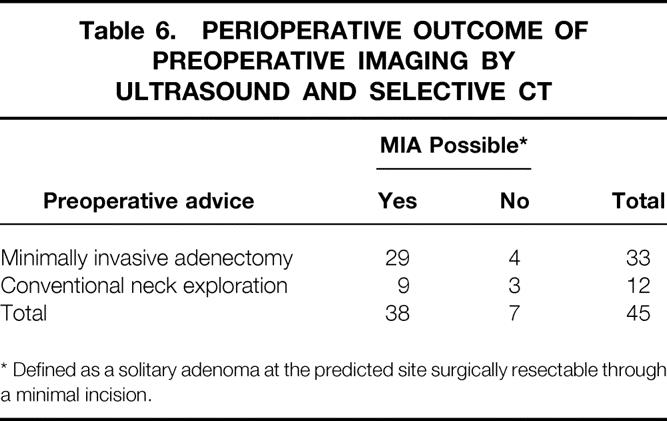
* Defined as a solitary adenoma at the predicted site surgically resectable through a minimal incision.
The overall success rate of MIA was 93% (96% in group A, 88% in group B). The overall success rate for CNE was 96%.
DISCUSSION
Our data confirm, once again, the high frequency (85–90%) of solitary adenomas in patients with pHPT. 1–4 Considering the number of successful MIA procedures as a function of the total number of patients who undergo surgical treatment for pHPT, CNE could be replaced by MIA in 71% of all patients (78/110): 75% of patients in group A (49/65) and 64% of patients in group B (29/45). Clearly, these figures justify the minimally invasive approach described here, which is characterized by a small incision in the skin directly over the affected parathyroid gland, as marked by preoperative ultrasound. The technique uses anatomical planes to reach the adenoma with virtually no unnecessary dissection. This is reflected by the short duration of the procedure. However, experience in this type of surgery is critical, because identification and retrieval of the enlarged gland through a mini-incision are delicate procedures.
The advantages of MIA are clear. The principle of performing minimal dissection when possible and converting to CNE if necessary poses the least possible risk of complications, including minimal risk to the recurrent nerve and the other (normal) parathyroid glands. It also makes subsequent surgery in the (para)thyroid region far less complicated than after CNE. MIA takes significantly less time and is cosmetically attractive, and it could easily be performed in a same-day surgery setting (and eventually under local anesthesia) in selected patients.
Costs must be taken into consideration before any new approach can be definitively evaluated, and the costs, including direct and indirect costs, of MIA versus CNE are the subject of an ongoing study in our department. The additional costs of preoperative ultrasound and CT in every patient undergoing parathyroid surgery may be neutralized by the much shorter operative time and by the shorter hospital stay, with same-day surgery in selected patients. Intraoperative PTH measurement is not specific to this new approach; rather, it should be used in every parathyroid surgical procedure to prevent persistent hyperparathyroidism and the subsequent need for reoperation.
Although the failure rate of MIA is low (4% in this series) if the optimal imaging strategy is routinely followed, one could argue that the procedure might not be as effective as CNE in experienced hands. We believe, however, that MIA in similarly experienced hands does not impose any undue risk for the patient, because the worst outcome would still be a conventional exploration at a later stage, which is currently the standard surgical procedure for pHPT.
The use of rapid PTH testing will undoubtedly make both the patient and the surgeon even more comfortable with MIA, because it allows direct confirmation of success. 14–16 The surgeon can now be immediately aware of a failure, should it occur, and may convert immediately to CNE to attain normocalcemia and euparathyroidism. We have recently developed such a quick PTH test and have included it as a standard perioperative measure. 10 Because this inexpensive test requires approximately 40 minutes, we use the following protocol. Eight minutes after adenomectomy, blood is taken for PTH testing, after which general anesthesia is ended and the patient is brought to the recovery room. Only when the PTH test has confirmed the MIA as successful is the patient allowed to leave the recovery room. If the PTH results are unsatisfactory, the patient can be returned to the next available operating room for subsequent CNE. This possibility is routinely discussed with the patient before surgery. The addition of routine perioperative PTH measurement should result in a nearly 100% immediate success rate.
Our data show that the success rate of MIA depends on the imaging performed before surgery. The sensitivity and specificity rates of combined ultrasound and CT (84% and 72%, respectively) are higher than those reported thus far for these modalities. 7,8,17–19 This may be explained in part by the fact that we adhered strictly to a combined imaging protocol in a prospective fashion, as opposed to the retrospective nature of most other investigations. The thorough discussion of each patient by all members of the multidisciplinary team and the preoperative ultrasound-guided marking of the skin over the suspected adenoma also contributed to this discrepancy.
In addition, the technologic features of the ultrasound and CT devices played a significant role. The use of CT imaging in cinevision mode (i.e., repeatedly going up and down through the field of interest at the CT workstation) is a major advance in parathyroid imaging and a magnificent aid in preoperative planning.
In this series, initial imaging with ultrasound alone, followed by CT only if dictated by the ultrasound findings, produced inferior accuracy compared with the combined imaging strategy. This finding was contrary to our expectations but was in concordance with other published data. 3,8 Specifically, three of the four failures occurred in patients who had undergone only ultrasound, suggesting that selection for CT by ultrasound is less reliable. Most importantly, the presence of multinodular thyroid enlargement is a major obstacle for ultrasonographic examination. 4 Based on our results, we do not support the concept of routine ultrasound alone, followed by CT only in selected patients. In fact, once the data from series B became available and turned out to be so much inferior to those of series A, we returned to the standard preoperative regimen of both CT and ultrasound.
Recent advances in radionuclide scintigraphy, including the use of 99mTechnetium-labeled sestamibi and other cationic complexes, have led to a promising increase in preoperative localization of parathyroid adenomas, with reported sensitivity rates of more than 85%. 20,21 Probe-guided surgery has, therefore, been suggested to facilitate parathyroid exploration. This was recently shown to be particularly true in the reoperative neck. 22 Although the same group advocated radioguided surgery for primary parathyroid procedures as well, 23 this was contradicted in a recent report by Bonjer et al. 24
Regardless of the imaging modalities advocated, several other investigators have proposed less invasive strategies as alternatives to CNE. Most have focused on unilateral neck exploration. 5,14 Because this still requires the dissection of normal parathyroids, such an approach will undoubtedly lack many of the advantages of MIA. Chapuis et al 3 have described a truly minimally invasive technique, performing ultrasound-guided adenomectomy under local anesthesia. Although this was feasible in only approximately 40% of their patients, we believe that in selected patients direct adenomectomy as described in our study could eventually be carried out under local anesthesia as well. This was recently confirmed by Norman and Chheda, 23 who used a direct approach under local anesthesia by reducing the dissection field using nuclear scintigraphy and perioperative localization with a hand-held probe. Alternatively, some surgeons have advocated endoscopy to perform a minimally invasive adenomectomy in selected patients. 25 Although conceptually elegant, this technique appears difficult to learn and certainly requires more operating time than the direct approach described here.
In conclusion, this study has shown that MIA is a safe and effective technique that may be anticipated to replace CNE in approximately 75% of patients undergoing surgical treatment of pHPT. Preoperative imaging should include a combination of ultrasound and CT scanning, including the possibility of cinevision. Perioperative measurement of PTH levels by rapid test methods will improve the surgical management of missed adenomas, permitting prompt CNE if MIA fails.
Footnotes
Correspondence: Theo J.M.V. van Vroonhoven, MD, PhD, Dept. of Surgery, UMC Utrecht, P.O. Box 85500, 3508 GA Utrecht, The Netherlands.
Accepted for publication September 28, 1999.
References
- 1.Van Heerden JA, Grant CS. Surgical treatment of primary hyperparathyroidism: an institutional perspective. World J Surg 1991; 15:688–692. [DOI] [PubMed] [Google Scholar]
- 2.Ryan JA, Lee F. Effectiveness and safety of 100 consecutive parathyroidectomies. Am J Surg 1997; 173:441–444. [DOI] [PubMed] [Google Scholar]
- 3.Chapuis Y, Icard P, Fulla Y, et al. Parathyroid adenomectomy under local anesthesia with intraoperative monitoring of UcAMP and/or 1-84 PTH. World J Surg 1992; 16:570–575. [DOI] [PubMed] [Google Scholar]
- 4.Van Vroonhoven TJMV, van Dalen A. Successful minimally invasive surgery in primary hyperparathyroidism after combined preoperative ultrasound and computed tomography imaging. J Int Med 1998; 243:581–587. [PubMed] [Google Scholar]
- 5.Lucas RJ, Welsh RJ, Glover JL. Unilateral neck exploration for primary hyperparathyroidism. Arch Surg 1990; 125:982–985. [DOI] [PubMed] [Google Scholar]
- 6.Irvin GL, Prudhomme DL, Deriso GT, et al. A new approach to parathyroidectomy. Ann Surg 1994; 219:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller DL. Pre-operative localization and interventional treatment of parathyroid tumors: when and how? World J Surg 1991; 15:706–715. [DOI] [PubMed] [Google Scholar]
- 8.Zmora O, Schachter PP, Heyman Z, et al. Correct preoperative localization: does it permit a change in operative strategy for primary hyperparathyroidism? Surgery 1995; 118:932–935. [DOI] [PubMed] [Google Scholar]
- 9.Smit PC, van Dalen A, van Vroonhoven TJMV. Strategy in asymptomatic and mildly symptomatic primary hyperparathyroidism, new arguments for the surgical option. Neth J Med 1998; 52:95–99. [DOI] [PubMed] [Google Scholar]
- 10.Smit PC, Thijssen JHH, Borel Rinkes IHM, van Vroonhoven TJMV. Perioperative PTH testing: confirmation of successful surgical treatment of primary hyperparathyroidism. Ned Tijdschr Geneesk 1999; 143:742–746. [PubMed] [Google Scholar]
- 11.Bonjer HJ, Bruining HA, Birkenhager JC, et al. Single and multigland disease in primary hyperparathyroidism: clinical follow-up, histopathology, and flow cytometric DNA analysis. World J Surg 1992; 16:737–743. [DOI] [PubMed] [Google Scholar]
- 12.Juhlin C, Rastad J, Klareskog L, et al. Parathyroid histology and cytology with monoclonal antibodies recognizing a calcium sensor of parathyroid cells. Am J Pathol 1989; 135:321–328. [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan E, Yashiro T, Salti G. Primary hyperparathyroidism in the 1990s: choice of surgical procedures in this disease. Ann Surg 1992; 215:300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irvin GC, Deriso GT. A new practical intraoperative parathyroid assay. Am J Surg 1994; 168:466–468. [DOI] [PubMed] [Google Scholar]
- 15.Michelangeli VP, Heyma P, Colman PG, Ebeling PR. Evaluation of a new, rapid and automated immunochemilluminometric assay for the measurement of serum intact parathyroid hormone. Ann Clin Biochem 1997; 34:97–103. [DOI] [PubMed] [Google Scholar]
- 16.Bergenfelz A, Isakkson A, Lindslom P, et al. Measurements of parathyroid hormone in patients with primary hyperparathyroidism undergoing first and reoperative surgery. Br J Surg 1998; 85:1129–1132. [DOI] [PubMed] [Google Scholar]
- 17.Krubsack AJ, Wilson SD, Lawson TC, et al. Prospective comparison of radionuclide, computed tomographic and sonographic localization of parathyroid tumours. World J Surg 1986; 10:579–585. [PubMed] [Google Scholar]
- 18.Roses DF, Sudarsky LA, Sanger J, et al. The use of preoperative localization of adenomas of the parathyroid glands by thallium-technetium scintigraphy, high-resolution ultrasonography and computed tomography. Surg Gynecol Obstet 1989; 168:99–106. [PubMed] [Google Scholar]
- 19.Hasselgren PO, Fidler JP. Further evidence against the routine use of parathyroid ultrasonography prior to initial neck exploration for hyperparathyroidism. Am J Surg 1992; 164:337–340. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell BK, Merell RC, Kinder BK. Localization studies in patients with hyperparathyroidism. Surg Clin North Am 1995; 75:483–498. [DOI] [PubMed] [Google Scholar]
- 21.Pattou F, Huglo D, Proye C. Radionuclide scanning in parathyroid disease. Br J Surg 1998; 85:1605–1616. [DOI] [PubMed] [Google Scholar]
- 22.Norman J, Denham D. Minimally invasive radioguided parathyroidectomy in the reoperative neck. Surgery 1998; 124:1088–1093. [DOI] [PubMed] [Google Scholar]
- 23.Norman J, Chheda H. Minimally invasive parathyroidectomy facilitated by intraoperative nuclear mapping. Surgery 1997; 122:998–1004. [DOI] [PubMed] [Google Scholar]
- 24.Bonjer HJ, Bruining HA, Pols HA, et al. 2-Methoxyisobutulisonitrile probe during parathyroid surgery: tool or gadget? World J Surg 1998; 22:507–512. [DOI] [PubMed] [Google Scholar]
- 25.Miccoli P, Bendinelli C, Vignali E, et al. Endoscopic parathyroidectomy: report of an initial experience. Surgery 1998; 124:1077–1080. [DOI] [PubMed] [Google Scholar]