Abstract
Objective
To investigate the role of angiotensin II as a mediator of burn- and sepsis-induced gut ischemia and reperfusion injury and to determine whether treatment with the angiotensin II inhibitor DuP753 can attenuate mucosal injury and bacterial translocation in a burn/endotoxemia porcine model.
Summary Background Data
Thermal injuries and endotoxemia have been shown to induce ischemia and reperfusion injury to the intestine, leading to increased mucosal permeability and bacterial translocation. Angiotensin II, the production of which has been reported to increase after burn, is thought to be one of the primary mediators of postburn mesenteric vasoconstriction.
Methods
An ultrasonic flow probe was inserted into the superior mesenteric artery and a catheter into the superior mesenteric vein in 21 female pigs. After 5 days, all animals were anesthetized, and 14 received 40% total body surface area third-degree burn. DuP753 was administered intravenously at 1 μg/kg to seven pigs immediately after burn. Eighteen hours after burn, 100 μg/kg Escherichia coli lipopolysaccharide (LPS) was intravenously administered. Systemic and splanchnic hemodynamics were measured and blood samples were drawn for blood gas analysis. Plasma conjugated dienes (PCDs), an index of lipid peroxidation, were measured every 6 hours. Intestinal permeability was assessed every 6 hours by measuring the lactulose/mannitol excretion ratio. At the end of the study (42 hours), tissue samples were harvested for bacteriologic cultures.
Results
Burn caused a significant decrease in mesenteric blood flow, to approximately 58% of baseline. Postburn endotoxemia significantly reduced the blood flow in the superior mesenteric artery to 53% of baseline. Treatment with DuP753 prevented postburn vasoconstriction and subsequently abrogated the impact of postburn endotoxemia on blood flow in the superior mesenteric artery. Mesenteric oxygen supply was significantly reduced after burn and endotoxin to 60% and 51% of baseline levels, respectively. DuP753 administration significantly improved mesenteric oxygen supply after both insults. Burn- and LPS-induced mesenteric hypoxia, as indicated by decreased mesenteric oxygen consumption, was also ameliorated by DuP753 treatment. PCD levels were significantly elevated 8 hours after burn. LPS caused a higher and prolonged increase in PCD levels. Treatment with DuP753 significantly reduced PCD levels after burn and after LPS. Intestinal permeability, as assessed by the lactulose/mannitol ratio, showed 6-fold and 12-fold increases after thermal injury and LPS, respectively. In contrast, the lactulose/mannitol ratio was only doubled in DuP753-treated animals. Bacterial translocation was significantly increased after burn and endotoxin. The incidence of bacterial translocation in the DuP753-treated animals was similar to that in the sham group.
Conclusions
Angiotensin II appears to play a pivotal role in the burn- and endotoxin-induced intestinal ischemia and reperfusion injury, with subsequent increases in permeability and bacterial translocation. Postburn administration of the angiotensin II receptor antagonist DuP753 significantly reduces the extent of these events.
After major thermal injuries, elevated serum endotoxin levels have been documented in humans. 1,2 Endotoxin levels were found to increase within 24 hours after injury, 2 peaking on days 3 and 4 after burn. 1
Previous studies have investigated the impact of thermal injuries or endotoxin on intestinal mucosal integrity. The association between thermal injuries and the dysfunction of the intestinal mucosal barrier has been documented. A significant increase in intestinal permeability in thermally injured patients was reported within 24 hours and throughout the first 2 weeks after injury. 3,4 This alteration in the mucosal barrier may contribute to the translocation of indigenous bacteria and endotoxins into the circulation. 5 In turn, endotoxin has been shown to impair gut barrier function in humans: a brief exposure to circulating endotoxin was reported to increase the permeability of the normal gut. 6
The cause and the clinical significance of the altered intestinal mucosal integrity under such conditions remain unclear. Based on experimental studies, intestinal ischemia and reperfusion injury appears to be the most likely mechanism underlying this phenomenon. 7 Mesenteric vasoconstriction may be the result of a plethora of vasomediators, one of which is angiotensin II. The production of angiotensin II has been reported to increase significantly after thermal injuries. 8 Therefore, we postulated that angiotensin II may be one of the primary mediators of postburn mesenteric vasoconstriction. We investigated in this study whether treatment with the angiotensin II antagonist DuP753 can attenuate burn- and sepsis-induced gut ischemia and reperfusion injury and abrogate the detrimental impact on mucosal barrier function in a chronic porcine burn/sepsis model.
METHODS
Experimental protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch (ACUC #90-09-103).
Surgical Preparation
Studies were performed in 21 female minipigs (weight 20–25 kg). After an overnight fast, the pigs were sedated with intramuscular ketamine (10 mg/kg) and mechanically ventilated with 2% to 2.5% halothane after endotracheal intubation. A bilateral subcostal incision was performed. A transit time ultrasonic flow probe (6–8 mm, Transonic Systems Inc., Ithaca, NY) was placed on the superior mesenteric artery. A 6.5F catheter was positioned in the superior mesenteric vein. A Witzel jejunostomy was also performed using a 12F Foley catheter.
After surgery, the animals were kept in recovery slings for 24 hours, then placed in runs for 5 days with free access to food and water. On the day of the experiment, the animals were anesthetized. Through a neck incision, a catheter was placed through the right common carotid artery into the abdominal aorta, and a Swan-Ganz thermal dilution catheter (Model 93 A-131-5F, American Edwards Laboratories, Anasco, PR) was positioned in the pulmonary artery through the right jugular vein. A 12F Foley catheter was inserted in the urinary bladder.
Experimental Design
The animals were kept in special slings for monitoring. Throughout the study, all animals received enteral feeding at 25 mL/hr and nothing orally. Baseline data were collected after complete recovery from anesthesia.
Pigs were randomized into three groups:
1. The burn/lipopolysaccharide (LPS) group (n = 7) had a 40% total body surface area third-degree flame burn under general anesthesia, as described above. The pigs were resuscitated according to the Parkland formula and received lactated Ringer’s solution (4 mL/kg/percentage of total body surface area burned) starting immediately after the burn; half was given in the first 8 hours after burn and the remainder in the next 16 hours. Eighteen hours after burn, 100 μg/kg Escherichia coli LPS (0111:B4, Difco, Detroit, MI) was administered intravenously. During the second day of the experiment, burned animals received lactated Ringer’s solution at 3.5 mL/m2 burned area and 2 mL/kg/hr for daily maintenance.
2. The sham group (n = 7) had a sham burn under anesthesia. Eighteen hours later, the animals received the diluent (0.9% NaCl) used for the endotoxin. Lactated Ringer’s solution was administered at 2 mL/kg/hr for daily maintenance.
3. The treatment group (n = 7) underwent the same procedure as the burn/LPS group, except for the administration of DuP753 (DuPont Merck, Wilmington, DE) intravenously in a dose of 1 μg/kg immediately after burn.
Mean arterial and central venous pressures were measured using transducers (P231D, Statham Gould, Oxnard, CA) connected to an Electronic Medicine Honeywell Recorder (Honeywell Inc., Pleasantville, NY) for electronic calculation of mean pressures. Cardiac output was determined by the thermal dilution technique using a Swan-Ganz catheter and a cardiac output computer (Model 9520, American Edwards Laboratories, Irvine, CA).
Mesenteric arterial blood flow was measured with a transit time ultrasonic flow probe connected to a T101 ultrasonic meter (Transonic Systems Inc., Ithaca, NY).
Systemic and splanchnic hemodynamics were measured and blood samples were drawn for determinations of arterial, mixed venous, and portal blood gases at baseline and 14 consecutive time points, starting 1 hour after burn.
The systemic vascular resistance index (SVRI) and mesenteric vascular resistance (MVR) were calculated as follows:
Cardiac index (L/min/m2) = cardiac output (L/min)/body surface area
SVRI (dyne · sec · cm−5 · m2) = ([mean arterial pressure − central venous pressure] × 80)/cardiac index
MVR (dyne · sec · cm−5) = ([mean arterial pressure − central venous pressure] × 80]/mesenteric arterial blood flow
Systemic oxygen delivery, systemic oxygen consumption, mesenteric oxygen delivery, and mesenteric oxygen consumption were calculated as follows:
Systemic oxygen delivery = cardiac index × arterial oxygen content × 10 (mL/min/m2)
Systemic oxygen consumption = cardiac index × (arterial oxygen content − mixed venous oxygen content) × 10 (mL/min/m2)
Mesenteric oxygen delivery = mesenteric arterial blood flow × arterial oxygen content/100 (mL/min)
Mesenteric oxygen consumption = mesenteric arterial blood flow × (arterial oxygen content − mesenteric oxygen content)/100 (mL/min)
Arterial oxygen content (mL/dL) equals (Hb × 1.34) SaO2 + (PaO2 × 0.0031); mixed venous oxygen content (mL/dL) equals (Hb × 1.34) SvO2 + (PvO2 × 0.0031); mesenteric oxygen content (mL/dL) equals (Hb × 1.34) SmO2 + (PmO2 × 0.0031).
Blood samples for plasma conjugated diene (PCD) assays were taken from the arterial line catheter at baseline and then every 6 hours, beginning 1 hour after burn.
Permeability Assessment
After the animals had recovered from the surgical instrumentation, a solution of 10 g lactulose and 5 g mannitol, diluted in 60 mL distilled water (1,160 mOsm/kg), was given through the jejunostomy tube, and urine was collected for a 6-hour period to obtain baseline measurements. Lactulose/mannitol (L/M) assessments were repeated every 6 hours. At the completion of collection, the urine was divided into aliquots and frozen at 20°C until assayed. Urinary L/M concentrations were simultaneously determined by the technique described by Fleming et al, 9 using high-pressure liquid chromatography coupled with pulsed amperometric detection. Urine was diluted 2- to 20-fold with deionized water, depending on the collection volume. One milliliter diluted urine was mixed with internal saccharide standards, desalted, vortex-mixed, centrifuged, and filtered. Fifty milliliters of the filtrate was injected onto a 250 × 40-mm anion exchange column (Dionex Carbopak, PAI, Houston, TX) and eluted with 0.15 mol/L NaOH, 1 mL/min, at 20°C. Detection was by pulsed amperometric detection with a working gold electrode and silver/silver chloride reference electrode, with a detection potential of +0.05, oxidation potential of +0.06, and reduction potential of −0.95V. Quantification was by peak height analysis and peak height ratios, with internal standardization. This method offers excellent separation of the carbohydrates and precise detection at low concentrations (lactulose 0.3 mg/L). The amount of each sugar excreted in the urine during 6 hours was then converted to a percentage of the amount of the given sugar, reflecting the excretion fraction of each sugar. By dividing the lactulose and mannitol excretion fractions, a permeability index—the L/M ratio—was calculated.
Conjugated Diene Assay
Conjugated dienes were measured according to the method of Till et al. 10 PCDs were extracted from the plasma by using a 2:1 (vol/vol) mixture of chloroform and methanol. Seven milliliters of the chloroform/methanol mixture, preheated to 45°C, was added to 0.5 mL plasma. The mixture was vigorously agitated for 2 minutes and centrifuged for 5 minutes at 3,000 rpm for 5 minutes at 4°C. The lower layer was aspirated and pipetted into a test tube and dried under a direct flow of nitrogen gas. The residue was reconstituted with 1.5 mL heptane and read on a spectrophotometer at 233 nanometers (Spectronic 1001, Milton Roy Co., Houston, TX).
Testing for Bacterial Translocation
At the end of the 42 hours, animals were anesthetized with 10 mg/kg intravenous ketamine and killed with 5 mL intravenous saturated KCl. Using aseptic technique, through a midline laparotomy incision, peritoneal fluid and tissue samples from the proximal and distal mesenteric lymph nodes, spleen, liver, kidney, lung, jejunum, ileum, cecum, and colon were taken for bacteriologic cultures. Collected tissue samples were weighed, and 0.5 g of each was homogenized in a tissue grinder with 4.5 mL nonbacteriostatic saline to create a 1:10 dilution of the original sample; 0.1 mL and 0.01 mL (of the 1:10 dilution) were inoculated onto a MacConkey agar plate and a Columbia Nutrient Agar plate for isolation of gram-negative and gram-positive organisms, respectively. Therefore, one colony would represent 1 × 102 and 1 × 103 colony-forming units per gram of tissue, respectively, for each inoculum size. Limits of detection were 100 organisms per gram of tissue. Inoculated plates were incubated at 37°C for 24 and 48 hours and read with a Darkfield Quebec Colony Counter (Model 3330, American Optical Co., Buffalo, NY). Cultures were considered positive when more than 100 colonies per gram of tissue were found. All bacterial isolates were identified by biotype using a Microscan 4 bacterial analyzer (Baxter, Sacramento, CA).
Statistical Analysis
Data are presented as mean ± SEM. Within-group analysis was performed by analysis of variance for repeated measurements with the Dunnett post hoc test. Between-groups analysis was performed by analysis of variance for factorial analysis with the Bonferroni post hoc test. Bacteriologic tissue culture results were analyzed by the Fisher exact test. P < .05 was considered statistically significant.
RESULTS
Systemic Hemodynamics
Baseline hemodynamic measurements were similar in all groups. All animals survived the study period (Figs. 1 and 2).
Figure 1. Systemic hemodynamic parameters: mean arterial pressure (MAP) and central venous pressure (CVP) after 40% total body surface area third-degree burn (0 hour) and endotoxin administration (18 hours after burn). The angiotensin II inhibitor DuP753 was administered intravenously immediately after burn. Differences were not statistically significant:P < .05, * vs. baseline, # vs. sham, ¥ vs. DuP753.
Figure 2. Systemic hemodynamic parameters: cardiac output (CO) and systemic vascular resistance index (SVRI) after burn (0 hour) and endotoxin (18 hours). The hemodynamic alteration was more pronounced after LPS infusion. DuP753 treatment had a positive impact on CO and SVRI. P < .05, * vs. baseline, # vs. sham, ¥ vs. DuP753.
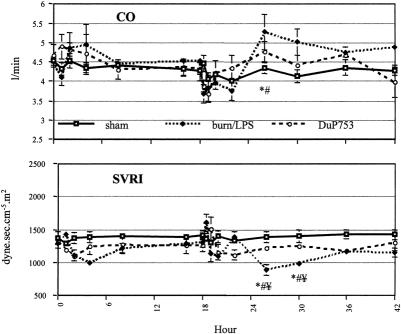
After thermal injury, no significant differences were observed between groups in mean arterial or central venous pressure. Cardiac output showed a slight increase during the first 6 hours, returning to baseline 8 hours after burn. This increase was associated with a concomitant fall in SVRI to 78% of baseline.
After LPS administration, a typical biphasic response was observed. The hemodynamic alteration was more pronounced during the second phase: after a marked drop in cardiac output to 77% of baseline, a hyperdynamic period began 8 hours after endotoxin administration. At this time point, SVRI dropped to 69% of baseline. Mean arterial pressure showed a 14% decrease immediately after LPS infusion in both burn/LPS groups. DuP753 treatment ameliorated the alteration in systemic circulation occurring after burn and LPS administration.
Mesenteric Hemodynamics
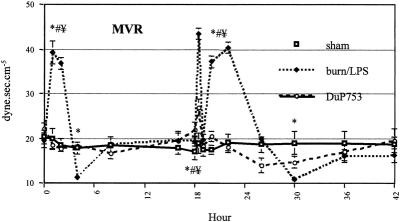
Although cardiac output did not fall after thermal injury, mesenteric arterial blood flow decreased significantly to approximately 58% of baseline during the first 4 hours after burn (Fig. 3). In contrast to the previously observed postburn reduction in systemic vascular resistance, mesenteric vascular resistance showed a significant increase (201% of baseline) during the early postburn phase (Fig. 4). During the late postburn phase, starting 4 hours after insult, a mesenteric reperfusion phase became manifested: mesenteric arterial blood flow increased by 44% over baseline. Compared with burned animals not receiving the angiotensin II antagonist, DuP753-treated animals showed no reduction in mesenteric arterial blood flow after burn; in fact, it increased by 33% over baseline during the early postburn phase. Animals in the DuP753 treatment group maintained, unlike the nontreated burned animals, a stable mean vascular resistance near baseline during this early mesenteric vasoconstrictive phase.

Figure 3. Burn (0 hour) and endotoxin administration (18 hours after burn) significantly reduced mesenteric blood flow (Qm). Treatment with DuP753 prevented the impact of burn/sepsis on Qm. P < .05, * vs. baseline, # vs. sham, ¥ vs. DuP753.
Figure 4. Changes in mesenteric vascular resistance (MVR) after burn (0 hour) and endotoxin administration (18 hours). P < .05, * vs. baseline, # vs. sham, ¥ vs. DuP753.
At 18 hours after burn, mesenteric hemodynamic measurements were comparable to baseline levels in all groups.
Administration of LPS to burned animals resulted in a biphasic response of mesenteric ischemia and reperfusion. The second insult yielded a significant mesenteric vasoconstriction, with an increase of mean vascular resistance to 222% of baseline during the first 8 hours after LPS infusion.
Similarly, mesenteric arterial blood flow decreased significantly to 53% of baseline during the same time of peak mesenteric vascular resistance. After an initial moderate drop in mesenteric arterial blood flow by 13% of baseline, burned animals treated with DuP753 showed no signs of mesenteric vasoconstriction after the second insult (LPS): no marked increase was noticed in mean vascular resistance. Compared with nontreated animals, DuP753 treatment resulted in a significant improvement of mesenteric arterial blood flow after the second insult (LPS); 4 hours after LPS infusion, mesenteric arterial blood flow reached 112% of baseline in the DuP753 group, whereas the flow was still significantly reduced to 57% of baseline in the burn/LPS group.
Systemic Oxygen Delivery and Consumption
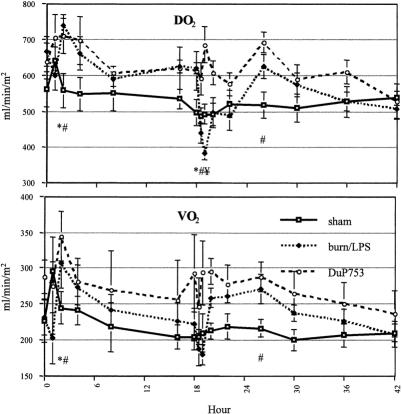
After an initial reduction, systemic oxygen delivery and consumption showed a marked increase during the first 4 hours after burn (Fig. 5). Administration of LPS yielded a significant drop in oxygen delivery during the first hour; oxygen consumption remained unchanged at this time point. Animals treated with DuP753 remained at baseline levels after LPS infusion. During the post-LPS hyperdynamic phase, both oxygen delivery and consumption increased.
Figure 5. Effects of burn (0 hour) and endotoxin administration (18 hours) on systemic oxygen delivery (DO2) and oxygen consumption (VO2). P < .05, * vs. baseline, # vs. sham, ¥ vs. DuP753.
Mesenteric Oxygen Delivery and Consumption
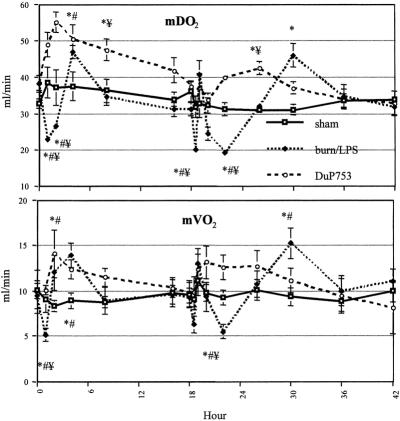
During the first 2 hours after burn, mesenteric oxygen delivery and consumption showed a significant decrease to 60% and 53% of baseline, respectively (Fig. 6). In contrast, animals treated with DuP753 showed an improvement in postburn mesenteric oxygen delivery and consumption, to 128% and 112% of baseline, respectively.
Figure 6. Mesenteric oxygen supply (mDO2) and oxygen consumption (mVO2) were significantly reduced after burn (0 hour) and endotoxin administration (18 hours after burn). These detrimental effects were ameliorated by DuP753 treatment. P < .05, * vs. baseline, # vs. sham, ¥ vs. DuP753.
The second insult (LPS) yielded a dramatic reduction in mesenteric oxygen delivery, to 51% of baseline. Postburn treatment with DuP753 prevented this impact of LPS, and mesenteric oxygen delivery remained at baseline. Similarly, after LPS infusion, reduced mesenteric oxygen consumption was improved in animals receiving DuP753 (58% vs. 127% of baseline).
Conjugated Diene Assay
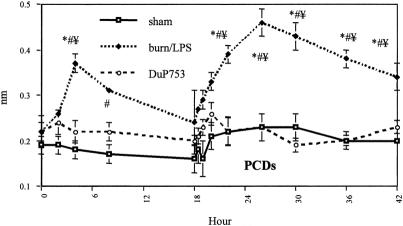
Levels of PCDs were significantly increased in the burn/LPS group at 4 and 8 hours after burn (Fig. 7). Postburn PCDs reached 168% of baseline at 4 hours, and the differences compared with sham-burned animals were significant. Eight hours after burn, PCDs showed a 182% increase compared with control animals. DuP753 treatment resulted in a significant decrease of postburn PCD levels at 4 hours. LPS infusion to burn animals yielded a higher and prolonged elevation of PCDs, showing an increase of 178% of baseline at 4 hours and 210% of baseline at 8 hours after LPS infusion. PCD levels remained significantly elevated in the burn/LPS group until the end of the study period. In contrast, animals treated with DuP753 showed no elevation in PCD levels after LPS administration.
Figure 7. Plasma conjugated diene (PCD) levels, an index of ischemia and reperfusion injury-induced lipid peroxidation, were significantly elevated after burn and endotoxin administration. Postburn treatment with DuP753 yielded a significant reduction in PCD levels after burn and endotoxin challenges. P < .05, * vs. baseline, # vs. sham, ¥ vs. DuP753.
Lactulose and Mannitol Assay
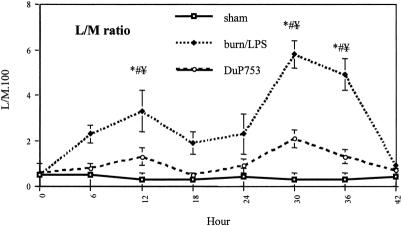
There were no differences between groups in baseline levels of the L/M ratio (Fig. 8). The L/M ratio for burned animals was moderately increased at 6 hours after burn. Intestinal permeability, as indicated by the L/M ratio, showed a sixfold increase at 12 hours after burn in the burn/LPS group but only a twofold increase in the treatment group. The differences between groups were significant at this time point. The changes in mucosal permeability, as measured by the L/M ratio, were more pronounced after LPS administration to burned animals. The L/M excretion ratio was 12- and 10-fold elevated in the burn/LPS group at 12 and 18 hours after burn, respectively. In contrast, treated animals showed only a threefold and twofold increase at these time points. Differences between groups were significant.
Figure 8. Changes in intestinal permeability, as assessed by the lactulose/mannitol (L/M) excretion ratio, after burn (0 hour) and endotoxin administration (18 hours after -burn). DuP753 was administered intravenously immediately after burn. P < .05, * vs. baseline, # vs. sham, ¥ vs. DuP753.
Quantitative Bacteriologic Culture of Tissue Samples
The rates of positive tissue cultures were significantly higher in animals receiving burn and LPS compared with the sham group (6/7 vs. 1/7, P < .05). Animals treated with DuP753 showed rates of positive tissue cultures comparable to those of the control animals (Fig. 9). Specific tissue isolates and their origin from animals in the three groups are shown in Table 1. Only tissue cultures that were positive with enteric bacteria of the same biotype as that found in the intestine of the corresponding animal were interpreted as evidence of bacterial translocation. In the burn/LPS group, 86% of the animals showed bacterial translocation to the mesenteric lymph nodes and lung, 71.4% to the spleen and liver, and 43% to the kidney. In contrast, only 14% of the treated animals showed bacterial translocation to the mesenteric lymph nodes and lung (P < .05), 28.5% to the liver, 14% to the spleen (P = .053), and none to the kidney.
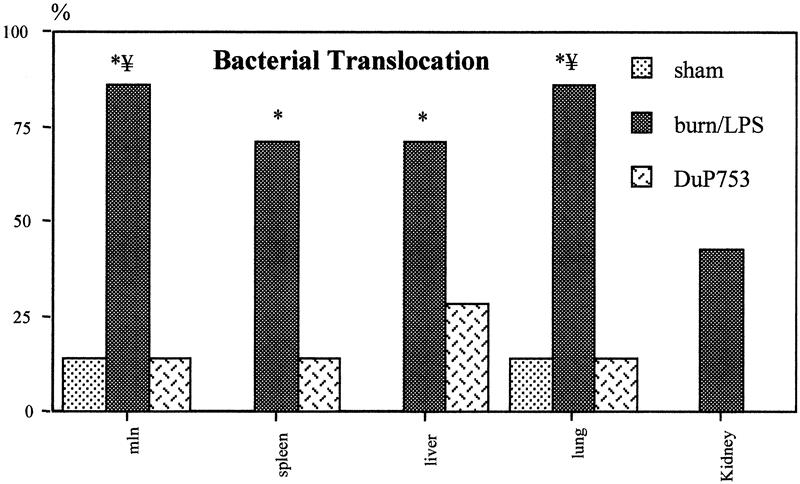
Figure 9. Burn and endotoxin resulted in a significant increase in the rates of positive tissue cultures with enteric bacteria. The incidence of bacterial translocation to remote organs was significantly reduced in animals treated with DuP753. Data are presented as percentages of harvested tissue samples. P < .05, * vs. baseline, # vs. sham, ¥ vs. DuP753.
Table 1. SPECIFIC TISSUE ISOLATES FROM THE THREE STUDY GROUPS

c, cecum; i, ileum; j, jejunum; MLN, mesenteric lymph nodes.
DISCUSSION
There is accumulating evidence that failure of the gut barrier function may play an important role in the initiation or progression of the hypermetabolic response and multiple organ dysfunction syndrome that frequently occur after major trauma. 11,12 Although the causal relation between the gut as a reservoir of enteric bacteria and endotoxins and the development of sepsis and/or multiple organ dysfunction syndrome remains to be defined, bacterial translocation has been associated with various pathologic conditions, including thermal injury 7 and endotoxemia. 13 Intestinal ischemia with a resultant reperfusion injury appears the most likely mechanism in the pathophysiology of decreased intestinal mucosal integrity, occurring shortly after thermal injury. 7 Later in the postburn period, altered intestinal permeability seems to be related to the episodes of endotoxemia to which burned patients are frequently exposed. 1,2 In such situations, intestinal ischemia and sepsis can become self-sustaining because endotoxin, gut- or tissue-derived, has been documented to decrease intestinal blood flow and promote bacterial translocation. 14–16 In addition to the elucidation of the molecular mechanisms underlying the injury of sepsis-induced intestinal ischemia, the identification of these mediators may facilitate the development of interventions to modulate the mesenteric blood flow and prevent subsequent pathologic events.
Our data confirm those of previous studies reporting the alteration of mesenteric hemodynamics secondary to thermal injury. 17,18 Despite indicators of adequate resuscitation (i.e., minor changes in cardiac output, mean arterial pressure, and central venous pressure), a significant reduction in mesenteric blood flow was observed in this study during the early phase after burn. Thus, altered systemic hemodynamics could not be solely responsible for the observed postburn mesenteric vasoconstrictive phase. The significant increase in mesenteric vascular resistance during the first 4 hours after burn, despite the marked decrease in systemic vascular resistance, suggests the involvement of certain mediators selectively acting on the mesenteric vasculature.
The renin-angiotensin axis appears to play a critical role in the pathophysiology of thermal injury. Hilton and Marullo 8 reported a fourfold increase in angiotensin II levels 6 hours after burn. Angiotensin II is a potent vasoconstrictor that exhibits important mesenteric selectivity, thought to be caused by an increased affinity of the angiotensin II receptors on the splanchnic vascular smooth muscle. 19 In a pig model of cardiogenic shock, a disproportionate mesenteric ischemia resulting from selective splanchnic vasospasm was observed. 20 Ablation of the renin-angiotensin axis was shown to abolish this response. Moreover, the observed hemodynamic changes were found to correlate with serum angiotensin II concentrations and could be reproduced in the absence of shock by the infusion of angiotensin II. 21
Our data clearly show that postburn treatment with DuP753, a specific angiotensin II receptor antagonist, 22 can prevent postburn vasoconstriction and subsequently abrogate the impact of postburn endotoxemia on superior mesenteric artery blood flow. In contrast to an approximately 50% decrease in mesenteric arterial blood flow, occurring as early as the first hour after burn, an initial increase of 11% in mesenteric arterial blood flow was observed after DuP753 treatment. Postburn administration of DuP753 was found to prevent burn-induced mesenteric vasoconstriction: mesenteric vascular resistance remained near baseline in treated animals, compared with an almost 100% increase in nontreated animals.
Systemic oxygen delivery and consumption showed a pattern of increase similar to that seen in cardiac output after burn. In contrast, mesenteric oxygen supply was significantly reduced during this early postburn phase. This observation also confirms the independence of the postburn altered mesenteric circulation and oxygenation, with respect to the systemic circulation. 18,23,24 At the same time, mesenteric oxygen demand showed a reduction that was dependent on the mesenteric oxygen supply. This postburn pathologic supply-dependent gut oxygen consumption was observed before in experimental models. 18,25 This early decreased mesenteric oxygen consumption indicates the inability of the gut to compensate for inadequate oxygen delivery by increasing oxygen extraction, resulting in tissue hypoxia. Early hypoxia in the splanchnic region is suggested as a plausible mechanism behind the development of secondary organ failure, especially in sepsis. 26 Animals receiving DuP753 showed no decrease in mesenteric oxygen delivery after burn. Administration of DuP753 after burn resulted in a significant improvement in mesenteric oxygen delivery (2 hours after burn, 49.14 mL/min [DuP753] vs. 23.03 [burn], P < .05). Postburn mesenteric hypoxia, as indicated by a decreased mesenteric oxygen consumption, was not observed in animals in the treatment group. The action of DuP753 appears to be selective. The enhancement in oxygen supply to meet the increased oxygen demand was noticed only in the mesenteric circulation; no differences were found between treated and untreated burned animals with respect to systemic oxygen delivery or consumption.
Although no important changes were noticed in systemic oxygen delivery or consumption in both burn/LPS and DuP753 groups after endotoxin infusion, the impact of this second insult on the mesenteric oxygenation was dramatic. A typical biphasic response was observed, with a more pronounced flow-dependent hypoxic period. The first 8 hours after LPS administration in burned animals were marked by a significant decrease in mesenteric oxygen delivery. Mesenteric oxygen consumption showed a pathologic dependence on oxygen delivery, leading to an oxygen debt that limits metabolism. LPS alone has been shown to cause such an alteration in mesenteric oxygenation. 24 These results could be explained by a defect in microvascular regulation of blood flow that interfered with the optimal distribution of a limited supply of oxygen, in accordance with tissue oxygen needs. 27 Our data demonstrate that these negative effects of LPS on mesenteric oxygenation can be prevented by DuP753 treatment. After LPS challenge, burned animals in the treatment group showed a significant improvement in their mesenteric oxygenation status compared with nontreated animals (consumption/delivery 4 hours after LPS infusion: 12.57/40.08 mL/min [DuP753] vs. 5.47/19.37 [burn], P < .05).
We measured intestinal permeability by assessment of the L/M ratio. The L/M ratio was increased sixfold 12 hours after burn. These results are in agreement with those of previous reports. Deitch 3 documented a threefold increase in intestinal permeability in burned patients during the first 24 hours after injury with use of the L/M ratio. Postburn administration of DuP753 resulted in a significant reduction of L/M ratios in burned animals. This treatment effect on postburn intestinal permeability may be beneficial in reducing the susceptibility to infection in burned patients, because a relation between increased permeability and postburn infections has been reported in humans. 4,28 Intestinal permeability was studied in burned patients during the first 2 weeks by measuring the L/M ratio. 4 There was a clear correlation between the increased L/M ratio on postburn day 2 and the occurrence of significant clinical infections during the first 2 postburn weeks. Ziegler et al 28 studied patients 2 weeks after injury by the L/M ratio and noted that only infected burned patients had altered permeability. However, their patients were studied in the late postburn period, during which other factors, such as endotoxemia, could have contributed to the reported altered permeability. Endotoxin alone has been reported to increase intestinal permeability in humans. 6 Thus, it appears that burned patients are exposed to two episodes of increased permeability: first, the initial burn trauma, and second, the insult of endotoxemia. In our model of combined burn/LPS, the impact of endotoxin, administered 18 hours after burn, on intestinal permeability was dramatic. Intestinal permeability, as indicated by the L/M ratio, showed a significant and prolonged increase in burned animals receiving LPS (12- and 10-fold increase at 12 and 18 hours after LPS infusion, respectively). The postburn blocking of angiotensin II significantly reduced these detrimental effects of postburn endotoxemia on intestinal permeability.
Because the permeability was increased in the burn/LPS group, the incidence of positive tissue cultures with enteric bacteria was also significantly elevated. Eighty-six percent of the animals with combined burn and LPS insults showed bacterial translocation. The observed correlation between increased permeability and bacterial translocation in these animals suggests a causal relation. Another finding in our study that may confirm this relation is the observation of a significant decrease in bacterial translocation rates in the treatment group. The bacterial translocation rates of animals receiving burn and LPS and treated with DuP753 and those of the sham animals did not differ significantly (14–28% vs. 14%). The involvement of angiotensin in the occurrence of bacterial translocation after burn and bacterial challenge has recently been reported. 29 Mice pretreated with angiotensin-converting enzyme inhibitor showed a lower incidence of bacterial translocation and a higher survival rate after burn and bacterial challenge compared with control animals. In the current study, a more target-specific angiotensin-blocking agent was given immediately after the first insult (burn).
The exact mechanisms by which major burns and endotoxemia affect intestinal permeability and bacterial translocation remain to be elucidated. However, as previously suggested, ischemia and reperfusion injury appears to play a pivotal role in this complex process. The resultant oxygen-derived free radicals have been implicated in the subsequent tissue damage that results in intestinal mucosal injury. 16,30,31 Although oxidant-induced damage may involve many cell components, peroxidative decomposition of membrane lipids has been considered as the basis of cell injury. 32 According to Tribble et al, 32 lipid peroxidation is important in oxidative injury because it increases the number of free radical chain reactions, compromises detoxification systems, and causes direct deleterious effects, because lipid peroxidation products themselves are considered toxic. Lipid peroxidation, initiated by hydroxyl radicals, results in the formation of lipid-derived free radicals such as conjugated dienes, lipid hydroperoxide radicals, and lipid hydroperoxides. 30 Measurement of conjugated dienes is frequently used as an index of lipid peroxidation. In the current study, PCD concentrations showed a 68% increase at 4 hours after burn and a return to baseline at 18 hours after burn. After LPS infusion, the elevation of PCDs was augmented and prolonged, beginning as early as 2 hours after LPS infusion, peaking (210% of baseline) at 8 hours after LPS infusion, and remaining elevated until the end of the study period. Administration of DuP753 directly after burn yielded a significant reduction in the postburn reperfusion injury, as indicated by the PCD levels. Treated animals showed no significant increase in their PCD levels compared with baseline and sham animal levels. From these data, we cannot determine whether the PCDs are solely the product of intestinal ischemia and reperfusion injury. These radicals could have their origins from other organs, such as the liver, burned skin, and lung. 33,34 However, the observation that systemic hemodynamic parameters (cardiac output, mean arterial pressure, and central venous pressure) showed no significant changes because of adequate resuscitation and the finding that mesenteric arterial blood flow was significantly reduced under the same conditions argue for the concept that the gut is, at least in part, a major contributor to these lipid-derived free radicals. The beneficial effect of DuP753 on mesenteric arterial blood flow and mesenteric oxygenation after burn and endotoxin challenges may explain the action of DuP753 treatment in attenuating burn and endotoxemia reperfusion injury, as assessed by PCD measurements.
In conclusion, major burn appears to cause ischemia and reperfusion injury, with a subsequent increase in intestinal permeability and bacterial translocation, all of which are augmented by a second insult of endotoxemia. Our data indicate that angiotensin II plays a central role in this process. Blocking angiotensin II receptor by DuP753 can attenuate these detrimental effects of burn and endotoxin.
Footnotes
Correspondence: Tamer Tadros, MD, Dept. of Surgery, University Hospital Rotterdam, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands.
DuP753 was provided by DuPont Merck Pharmaceutical Co., Wilmington, Delaware.
Accepted for publication October 27, 1999.
References
- 1.Winchurch RA, Thupari JN, Munster AM. Endotoxemia in burn patients: levels of circulating endotoxins are related to burn size. Surgery 1987; 102:808–812. [PubMed] [Google Scholar]
- 2.Dobke MK, Simoni J, Ninnemann JL, Garrett J, Harnar TJ. Endotoxemia after burn injury: effect of early excision on circulating endotoxin levels. J Burn Care Rehabil 1989; 10:107–111. [DOI] [PubMed] [Google Scholar]
- 3.Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery 1990; 107:411–416. [PubMed] [Google Scholar]
- 4.LeVoyer T, Ciofii WG, Pratt L, et al. Alteration in intestinal permeability after thermal injury. Arch Surg 1992; 127:26–30. [DOI] [PubMed] [Google Scholar]
- 5.Deitch EA. The role of intestinal barrier failure and bacterial translocation, in the development of systemic infection and multiple organ failure. Arch Surg 1990; 125:403–404. [DOI] [PubMed] [Google Scholar]
- 6.O’Dwyer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg 1988; 123:1459–1464. [DOI] [PubMed] [Google Scholar]
- 7.Herndon DN, Ziegler ST. Bacterial translocation after thermal injury. Crit Care Med 1993; 21:S50–S54. [DOI] [PubMed] [Google Scholar]
- 8.Hilton JG, Marullo DS. Trauma-induced increases in plasma vasopressin and angiotensin II. Life Sci 1987; 41:2195–2200. [DOI] [PubMed] [Google Scholar]
- 9.Fleming SC, Kapembwa MS, Laker MF, Levin GE, Griffin GE. Rapid and simultaneous determination of lactulose and mannitol in urine, by HPLC with pulsed amperometric detection, for use in studies of intestinal permeability. Clin Chem 1990; 36:797–799. [PubMed] [Google Scholar]
- 10.Till GO, Hatherill JR, Tourtellotte WW, Lutz MJ, Ward PA. Lipid peroxidation and acute lung injury after thermal trauma to skin. Evidence of a role for hydroxyl radical. Am J Pathol 1985; 119:376–384. [PMC free article] [PubMed] [Google Scholar]
- 11.Baue AE. Multiple Organ Failure; Patient Care and Prevention. St Louis: Mosby Year Book; 1990.
- 12.Deitch EA. Multiple organ failure. Ann Surg 1992; 216:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deitch EA, Berg R, Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch Surg 1987; 122:185–190. [DOI] [PubMed] [Google Scholar]
- 14.Xu D, Qi L, Guillory D, Cruz N, Berg R, Deitch EA. Mechanisms of endotoxin-induced intestinal injury in a hyperdynamic model of sepsis. J Trauma 1993; 34:676–682. [DOI] [PubMed] [Google Scholar]
- 15.Navaratnam RL, Morris SE, Traber DL, et al. Endotoxin (LPS) increases mesenteric vascular resistance (MVR) and bacterial translocation (BT). J Trauma 1990; 30:1104–1113. [DOI] [PubMed] [Google Scholar]
- 16.Deitch EA, Specian RD, Berg RD. Endotoxin-induced bacterial translocation and mucosal permeability: role of xanthine oxidase, complement activation, and macrophage products. Crit Care Med 1991; 19:785–791. [DOI] [PubMed] [Google Scholar]
- 17.Tokyay R, Loick HM, Traber DL, Heggers JP, Herndon DN. Effects of thromboxane synthetase inhibition on postburn mesenteric vascular resistance and the rate of bacterial translocation in a chronic porcine model. Surg Gynecol Obstet 1992; 174:125–132. [PubMed] [Google Scholar]
- 18.Tokyay R, Zeigler ST, Kramer GC, et al. Effects of hypertonic saline dextran resuscitation on oxygen delivery, oxygen consumption, and lipid peroxidation after burn injury. J Trauma 1992; 32:704–712. [DOI] [PubMed] [Google Scholar]
- 19.Gunther S, Gimbrone MA Jr, Alexander RW. Identification and characterization of the high-affinity vascular angiotensin II receptor in rat mesenteric artery. Circ Res 1980; 47:278. [DOI] [PubMed] [Google Scholar]
- 20.Reilly PM, MacGowan S, Miyachi M, Schiller HJ, Vickers S, Bulkley GB. Mesenteric vasoconstriction in cardiogenic shock in pigs. Gastroenterology 1992; 102:1968–1979. [DOI] [PubMed] [Google Scholar]
- 21.Reilly PM, Bulkley GB. Vasoactive mediators and splanchnic perfusion. Crit Care Med 1993; 21:S55–S68. [DOI] [PubMed] [Google Scholar]
- 22.Timmermans PB, Wong PC, Chiu AT, Herblin WF. Nonpeptide angiotensin II receptor antagonists. Trends Pharmacol Sci 1991; 12:55–62. [DOI] [PubMed] [Google Scholar]
- 23.Dahn MS, Lange P, Lobdell K, Hans B, Jacobs LA, Mitchell RA. Splanchnic and total body oxygen consumption differences in septic and injured patients. Surgery 1987; 101:69–80. [PubMed] [Google Scholar]
- 24.Fink MP. Adequacy of gut oxygenation in endotoxemia and sepsis. Crit Care Med 1993; 21:S4–S8. [DOI] [PubMed] [Google Scholar]
- 25.Demling RH, Knox J, Youn YK, LaLonde C. Oxygen consumption early postburn becomes oxygen delivery-dependent with the addition of smoke inhalation injury. J Trauma 1992; 32:593–598. [DOI] [PubMed] [Google Scholar]
- 26.Arvidsson D, Rasmussen I, Almqvist P, Niklasson F, Haglund U. Splanchnic oxygen consumption in septic and hemorrhagic shock. Surgery 1991; 109:190–197. [PubMed] [Google Scholar]
- 27.Nelson DP, Samsel RW, Wood LD, Schumacker PT. Pathological supply dependence of systemic and intestinal O2 uptake during endotoxemia. J Appl Physiol 1988; 64:2410–2419. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler TR, Smith RJ, ODwyer ST, Demling RH, Wilmore DW. Increased intestinal permeability associated with infection in burn patients. Arch Surg 1988; 123:1313–1319. [DOI] [PubMed] [Google Scholar]
- 29.Gennari R, Alexander JW, Boyce ST, Lilly N, Babcock GF, Cornaggia M. Effects of the angiotensin-converting enzyme inhibitor enalapril on bacterial translocation after thermal injury and bacterial challenge. Shock 1996; 6:95–100. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83. [DOI] [PubMed] [Google Scholar]
- 31.Ma L, Ma JW, Deitch EA, Specian RD, Berg RD. Genetic susceptibility to mucosal damage leads to bacterial translocation in a murine burn model. J Trauma 1989; 29:1245–1251. [DOI] [PubMed] [Google Scholar]
- 32.Tribble DL, Aw TY, Jones DP. The pathophysiological significance of lipid peroxidation in oxidative cell injury. Hepatology 1987; 7:377–383. [DOI] [PubMed] [Google Scholar]
- 33.Demling RH, LaLonde C. Systemic lipid peroxidation and inflammation induced by thermal injury persists into the post-resuscitation period. J Trauma 1990; 30:69–74. [DOI] [PubMed] [Google Scholar]
- 34.Daryani R, LaLonde C, Zhu D, Weidner M, Knox J, Demling RH. Effect of endotoxin and a burn injury on lung and liver lipid peroxidation and catalase activity. J Trauma 1990; 30:1330–1334. [DOI] [PubMed] [Google Scholar]