Abstract
Objective
To compare the healing response of sequential topically applied cytokines to that of each cytokine alone and to a placebo in pressure ulcers, and to evaluate the molecular and cellular responses.
Summary Background Data
Because of a deficiency of cytokine growth factors in chronic wounds and the reversal of impaired healing in animal models, pressure ulcer trials have been performed with several exogenously applied growth factors. Because single-factor therapy has not been uniformly successful, combination or sequential cytokine therapy has been proposed. Laboratory data have suggested that sequential treatment with granulocyte-macrophage/colony-stimulating factor (GM-CSF)/basic fibroblast growth factor (bFGF) might augment the previously reported effect of bFGF alone.
Methods
A masked, randomized pressure ulcer trial was performed comparing sequential GM-CSF/bFGF therapy with that of each cytokine alone and with placebo during a 35-day period. The primary measure was wound volume decrease over time. Cytokine wound levels and mRNA levels were serially determined. Fibroblast-populated collagen lattices (FPCLs) were constructed from serial fibroblast biopsies. Cellular ultrastructure was evaluated by electron microscopy. Changes in ease of surgical closure and its relative cost were determined.
Results
Ulcers treated with cytokines had greater closure than those in placebo-treated patients. Patients treated with bFGF alone did the best, followed by the GM-CSF/bFGF group. Patients treated with GM-CSF or bFGF had higher levels of their respective cytokine after treatment. Patients with the greatest amount of healing showed higher levels of platelet-derived growth factor (PDGF) on day 10 and transforming growth factor beta (TGFβ1) on day 36. Message for the bFGF gene was upregulated after treatment with exogenous bFGF, suggesting autoinduction of the cytokine. FPCLs did not mimic the wound responses. Ultrastructure of wound biopsies showed response to bFGF. Treatment with any of the cytokines improved the wound by allowing easier wound closure. This was most marked for the bFGF-alone treatment, with a cost savings of $9,000 to $9,200.
Conclusions
Treatment with bFGF resulted in significantly greater healing than the other treatments in this trial. The clinical response appeared to be related to upregulation of the bFGF message and to increased levels of PDGF-AB, bFGF, and TGFβ1 in the wounds and changes in ultrastructure. The resultant improvements could be correlated with cost savings.
The normal response to tissue injury is a timely and orderly reparative process that results in sustained restoration of anatomical and functional integrity. 1 In chronic wounds, the healing process is prolonged and incomplete, proceeding in an uncoordinated manner and resulting in a poor anatomical and functional outcome. 2 Lack of cellular and molecular signals required for normal wound repair processes such as resolution of inflammation, angiogenesis, deposition of extracellular matrix, contraction, epithelialization, and remodeling may be a major contributing factor to poor healing of chronic wounds such as pressure ulcers. Cytokines, especially the subclass of growth factors, provide many of the cellular and molecular signals necessary for normal healing. 3,4
Mast and Schultz 5 and Tarnuzzer et al 6 have postulated that in chronic wounds, repeated trauma, ischemia, and infection increase the level of proinflammatory cytokines, increase the level of matrix metalloproteinases, decrease the presence of tissue inhibitors of metalloproteinases, and lower the level of growth factors. Cooper et al, 7 using an enzyme-linked immunosorbent assay technique on retrieved wound fluid, showed that levels of platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and transforming growth factor beta (TGF-β) were markedly decreased in chronic pressure ulcers compared with acute wounds. Pierce et al 8 also showed that there was a decrease of PDGF in human pressure ulcers and that the addition of exogenous PDGF resulted in the synthesis of much greater amounts of PDGF by the recruited and activated wound cells.
Based on the demonstrated deficiency of cytokine growth factors in chronic wounds and the successful reversal of impaired healing in many animal models by application of various growth factors, clinical trials have been performed with several cytokines and growth factors. A summary of the results of clinical trials using exogenous application of growth factors in an attempt to accelerate healing appeared in 1996. 9 These trials included patients with chronic wounds such as pressure ulcers, diabetic foot ulcers, and venous stasis ulcers. The trials germane to this report are those performed on patients with pressure ulcers.
Recombinant PDGF-BB was first reported in the treatment of pressure ulcers in 1992. 10,11 In a phase I/II prospective, randomized, masked trial of 20 patients, 100 μg/mL topically applied PDGF-BB produced an increase in the rate of wound closure compared with three other groups. A follow-up multicenter trial by Mustoe et al 12 showed a trend toward healing acceleration, but the results did not reach statistical significance. In a two-center trial of 50 patients, Robson et al 13 reported that bFGF was safe and potentially effective in the management of pressure ulcers. EGF was evaluated in a heterogeneous group of chronic wounds, including pressure ulcers, and was found to be effective compared with the topical antimicrobial silver sulfadiazine. 14 Because of its ability to stimulate monocytes and granulocytes and stimulate macrophages to produce other growth factors, 15,16 interleukin 1-beta (IL-1B) has been tested in a pressure ulcer clinical trial. 16 Although there were no statistical differences in healing rates between cytokine-treated and vehicle-treated patients, patients treated with 1.0 μg/cm2/day rhuIL-1B showed different in vitro fibroblast characteristics than patients in other groups. Fibroblast “senescence” seemed to be overcome after 28 days of treatment with the cytokine. 17 Most recently, Rees et al 18 reported statistically significant improvement in healing of pressure ulcers using a different formulation of PDGF-BB than previously applied to pressure ulcers. This formulation was the same as that approved by the U.S. Food & Drug Administration for the treatment of diabetic neuropathic foot ulcers. That growth factor is the first and only one approved for topical application to wounds for accelerating wound closure.
Because no single growth factor has been shown to be uniformly efficacious in the treatment of pressure ulcers, the possibility of combinations or sequences of growth factors exist. Unfortunately, the combinations and sequences are endless. Several preclinical examples of combining growth factors exist. Lynch et al 19 demonstrated that the combination of PDGF and insulinlike growth factor-I (IGF-1) was more effective than either growth factor alone in healing partial-thickness dermatome-created wounds. Davidson et al 20 reversed the wound healing deficit in diabetic rats by combining bFGF and TGFβ1. Sprugel et al 21 found that a combination of PDGF and bFGF increased the DNA content of rat-implanted wound chambers better than any single growth factor. However, not all of these combination growth factor studies have been successful: Greenhalgh et al 22 reported that the combination of PDGF and bFGF was no more effective than a single growth factor in the genetically diabetic mouse.
The idea of combining growth factors for topical application to wounds is the concept behind the autologous platelet releasate platelet-derived wound healing formula. This releasate from autologous platelets described by Knighton et al 23 contains PDGF, TGFβ, platelet-derived angiogenesis factor, platelet-derived epidermal growth factor, platelet factor-4, and other unknown factors. After the initial uncontrolled trial that suggested efficacy for this combination formula, Knighton et al 24 reported a controlled, double-blind, crossover trial of 32 patients with wounds of mixed etiology. Although no specific trial of pressure ulcers treated with platelet-derived wound healing formula has been reported, five trials have been reported using the combination on lower extremity ulcers, with mixed results. 25–29 Another combination growth factor compound is being investigated. An extract of milk that has been demonstrated to contain IGF-1, PDGF, bFGF, and TGFβ has been reported to be effective in accelerating healing in animal models. 30,31
The availability and function of the various growth factors in normal wound healing appear to be sequential. Wound fluid evaluations suggest that normally the various cytokines are present in the wound at different time periods. 32–35 Platelet-released factors are present in the wound earlier in the healing trajectory than are fibroblast-secreted factors. Because the target cells of various agents are different, it seems reasonable to have a cytokine active on hematopoietic cells (e.g., neutrophils, monocytes, and lymphocytes) present early in the reparative scheme and one more active on regenerative tissue (e.g., endothelial cells, fibroblasts, and epithelial cells) later in the process. Previous experiments in the lead author’s laboratories suggest the granulocyte-macrophage/colony-stimulating factor (GM-CSF) acts early in causing wound contraction and that bFGF acts at a later phase. 36–38 Therefore, it was postulated that the sequential use of GM-CSF and bFGF would result in greater acceleration of healing of a pressure ulcer, which heals largely by the processes of deposition of extracellular matrix and contraction, than would be seen with either agent alone.
The purpose of the study was to perform the first wound healing clinical trial using sequential topically applied cytokines to accelerate healing. In addition to determining the effect on ulcer closure, the study was designed to evaluate the longitudinal molecular and cellular responses of healing wounds.
METHODS
A double-blind, randomized, placebo-controlled trial was performed on inpatients with pressure ulcers to evaluate the safety and efficacy of sequential cytokine therapy. The sequential treatment results were compared with treatment results with each cytokine used alone and with a control placebo arm. The trial involved only patients with pressure ulcers involving any tissue from a bony prominence to the subcutaneous tissue (grade III/IV). All patients were denervated in the area of ulceration because of acquired spinal cord pathology. The investigational protocol was approved by the Institutional Review Boards of the University of South Florida and the Department of Veterans Affairs Medical Center, Bay Pines, Florida.
The cytokines used in this trial were rhuGM-CSF and rhubFGF. The rhuGM-CSF was obtained from the bacterial formulation of a strain of Escherichia coli bearing a genetically engineered plasmid containing an rhuGM-CSF gene. It had a molecular weight of 14,477 daltons and was formulated in an isotonic buffered solution. The GM-CSF and its inert vehicle were provided by Schering Plough Research Corp. (Kenilworth, NJ). The bFGF was produced in E. coli with a molecular weight of approximately 18,000 daltons and an isoelectric point of 9.8. It was in an isotonic buffered solution. The bFGF, with its appropriate inert vehicle placebo, was provided by Scios, Inc. (Mountain View, CA).
Consecutive patients fulfilling the entry criteria with pressure ulcers measuring 10 to 200 cm3 of at least 8 weeks’ duration were randomized to one of four treatment regimens: 2.0 μg/cm2 GM-CSF topically applied daily for 35 days; 5.0 μg/cm2 bFGF topically applied daily for 35 days; 2.0 μg/cm2 GM-CSF applied for 10 days, followed sequentially by 25 days of topically applied 5.0 μg/cm2 bFGF; or the comparative placebos, applied daily for 35 days. These doses were chosen based on previous trials performed with each of the cytokines separately. The amount of topical substance for each week of treatment was based on a volumetrically determined surface area at baseline and on study days 7, 14, 21, and 28. The dosage volume was calculated weekly based on a dose volume of 0.01 mL/cm2 of ulcer surface and was applied as a topical spray. After 15 minutes of air-drying to allow adsorption of the protein, the wounds were dressed with a nonadherent dressing next to the wound surface and dry gauze to fill the ulcer crater.
Patients meeting the study criteria (Table 1) underwent a screening evaluation, including a medical history, physical examination, baseline chest x-ray and electrocardiogram, nutritional assessment, and smoking status before beginning treatment. The ulcer was evaluated by anatomical location, periulcer transcutaneous oxygen tension, periulcer blood perfusion measured with laser Doppler flowmeter, volumetric determination with alginate mold and displacement, 39 and quantitative and qualitative bacteriology. 40
Table 1. STUDY CRITERIA
The endogenous levels of cytokines (GM-CSF, bFGF, PDGF-AB, EGF, TGFB1, TGFB2, IL-1B) present in the ulcers before treatment and on days 10 and 36 were determined using an enzyme-linked immunosorbent assay technique on retrieved wound fluid, as described by Cooper et al. 7 Competition-based Q-reverse transcriptase–polymerase chain reaction (RT-PCR) was used to determine mRNA levels of GM-CSF and bFGF from wound biopsy samples at days 0, 10, and 36. 41,42 Five different RT-PCR reactions were performed per biopsy sample, and the data were used to calculate a best-fit line from which the numbers of copies of mRNA for the specific gene were calculated. This method allows errors that occur in individual RT-PCR reactions to be minimized by the least squares linear regression procedure, and the interpolated value for the average number of copies of mRNA per cell reflects the results obtained from the five separate RT-PCR reactions. Fibroblast cultures were performed on wound biopsy samples at days 0, 10, and 36 to analyze the effect on fibroblast-populated collagen lattice (FPCL) contraction 43 and determination of cell senescence. 44
Electron microscopy was also performed on wound biopsy samples obtained on days 0, 10, and 36. 45 Four random areas from each pressure ulcer biopsy sample were selected to represent a wound from each patient. Under a dissecting microscope, these tissues were sliced further into small 1- to 2-mm pieces, fixed, and embedded in plastic resin (EM-bed 812, EM Sciences, Ft. Washington, PA), as suggested by VandeBerg et al. 45 Eight to 10 tissue pieces were cut into thick sections (1.0 μm), mounted on glass slides, and stained with toluidine blue. Under the light microscope, the orientation of the tissue was observed to select uniform areas from distal cell layers lining the ulcer bed surface to 1 to 2 mm proximally into the dermis. From five selected tissue pieces, 10 to 15 thin sections were cut (60 nm) and mounted on unsupported 300-mesh grids, stained in uranyl acetate and bismuth subnitrate. At least four grids of 15 to 20 sections from each of the selected areas were examined and photographed using a Zeiss EM-10B electron microscope (Carl Zeiss, Inc., Thornwood, NY). All ultrastructural data were obtained by one electron microscopist (JSV) masked to the treatments the wounds received.
After screening and obtaining baseline data, sharp débridement of the ulcer to remove any necrotic tissue and to open all sinuses and tracts was performed. Initial drug administration was delayed for at least 24 hours after débridement. All patients were kept on pressure-relief surfaces for the 35-day treatment period, and fixed turning schedules were maintained. To determine safety, hematology, serum chemistry, and urinalysis values were determined weekly during the 5-week study. Adverse reactions, intercurrent medical events, and concomitant medications were recorded daily.
The pressure ulcer was measured on day 0 and weekly for 5 weeks using planimetry of the ulcer opening and volume determination using alginate molds. 39 Color photography of the ulcer at a set focal distance was obtained weekly to determine ease of closure by two blinded surgeons, as described by Robson et al. 46
The change in difficulty of wound closure was studied in relation to the composite cost, including surgeon’s fee, anesthesia fee, and operating room cost (Table 2). 46
Table 2. EASE OF CLOSURE SCALE/PROCEDURAL COSTS
Modified from Robson et al. 46
Descriptive statistics were computed for demographic characteristics such as age, ethnicity, smoking status, and pressure ulceration duration. The patients’ ages and ulcer duration were compared by analysis of variance, whereas ethnicity and smoking status were compared using chi-square analysis (Sigma Stat 2.03, SPSS, Chicago, IL). Both parametric and nonparametric analyses were used to determine the efficacy of GM-CSF treatment alone, bFGF treatment alone, or sequential GM-CSF/bFGF treatment, depending on the apparent normality of the data. The percentage decrease in volume during the 35 days was compared among patient groups using the Kruskal-Wallis method of analysis of variance on ranks (Sigma Stat). Patients achieving various percentages of healing versus time were compared across treatment groups by Kaplan-Meier survival analysis 47 (JMP software, SAS, Cary, NC) using the general method suggested by Hokanson et al. 48
Because five previous trials encompassing 130 patients with this pressure ulcer model showed a mean placebo response of a 70% ± 2% decrease in ulcer volume 49 and a topical antimicrobial response of 60% reduction during a 4-week period, 50 an arbitrary response rate of at least 85% wound closure during a 35-day period was chosen as indicative of a responder. Proportions of responders at more than 85%, 90%, and 95% wound closure were compared between GM-CSF, bFGF, or sequential cytokine therapy groups and placebo-treated patients using the Fisher exact test (Sigma Stat).
Endogenous cytokine levels at day 0 and cytokine levels at days 10 and 36 were compared by one-way analysis of variance if data passed the normality test. If not, Kruskal-Wallis one-way analysis of variance on ranks was performed. The Tukey or the Dunn method was used as a multiple comparison procedure to isolate the group or groups differing from the others. RNA message determinations of the GM-CSF and bFGF genes at days 0, 10, and 36 in each treatment group were compared with the placebo-treated patients as well as the percentage change in message from day 0 to 10, and day 0 to 36. Chi-square analyses compared the message change between each group and the placebos.
All data obtained longitudinally on ulcer measurements, cytokine levels and changes, and fibroblast activity in FPCLs were evaluated for possible correlations using the Spearman rank order correlation (Sigma Stat). With this test, pairs of variables with positive correlation coefficients and P values < .05 tend to increase together. For pairs with negative correlation coefficients and P values < .05, one variable tends to decrease while the other increases.
RESULTS
Sixty-one patients completed the 35-day acute phase of the trial. Fifteen received 35 days of GM-CSF, 15 received 35 days of bFGF, 16 received sequential therapy of 10 days GM-CSF followed by 25 days of bFGF, and 15 received the placebo vehicles. Demographics disclosed no significant differences in age, ethnicity, smoking status, or duration of pressure ulcer among the four treatment groups (Table 3) . No hematologic, chemical, or urinalysis abnormalities were attributable to the topical administration of the sequential cytokine therapy or to either GM-CSF or bFGF alone. Periulcer Tc02 and laser Doppler perfusion revealed no ulcers to be hypoxic (<30 mm PO2) or ischemic.
Table 3. DEMOGRAPHICS OF PATIENTS AND PRESSURE ULCERS
bFGF, basic fibroblast growth factor; GM-CSF, granulocyte-macrophage/colony-stimulating factor.
* Analysis of variance.
† Chi-square.
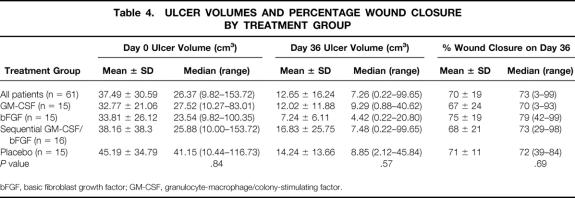
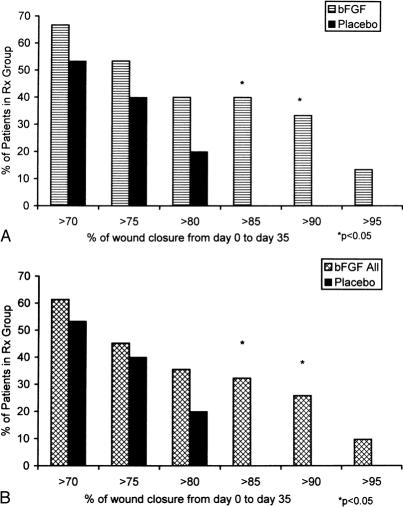
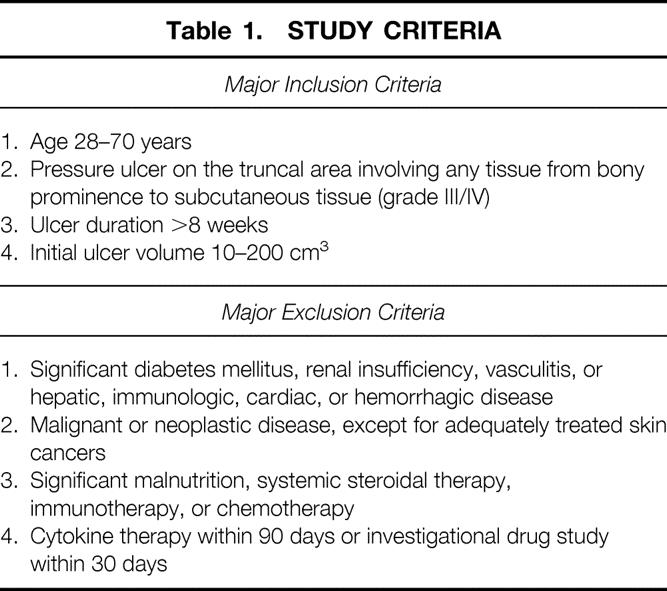
Evaluation of the mean percentage of initial ulcer volume remaining on day 36 among the four treatment groups by analysis of variance showed that the test for normality was not passed. Kruskal-Wallis analysis of variance on ranks showed no significant differences (Table 4). The patients treated with bFGF alone had smaller wound volumes than the other groups on day 36 and had a trend toward greater wound closure. However, the proportion of patients achieving a significant response (>85% wound closure in 35 days of treatment) suggested a Fisher exact test of proportions (Fig. 1). When patients receiving any cytokine therapy (GM-CSF, bFGF, or sequential GM-CSF/bFGF) were compared with patients receiving placebo vehicles, significantly more patients treated with cytokine achieved a more than 85% decrease in ulcer volume (P = .03). Patients treated for 35 days with bFGF alone did the best. The bFGF-alone group had significantly more patients than the placebo group with more than 85% closure (P = .02) and more than 90% closure (P = .04) (Fig. 2). The sequential cytokine therapy reached a significance level of P = .10 compared with placebo at more than 85% healing. The patients treated with GM-CSF alone did not respond significantly better than placebo-treated patients at more than 85% closure (P = .22).
Table 4. ULCER VOLUMES AND PERCENTAGE WOUND CLOSURE BY TREATMENT GROUP
bFGF, basic fibroblast growth factor; GM-CSF, granulocyte-macrophage/colony-stimulating factor.
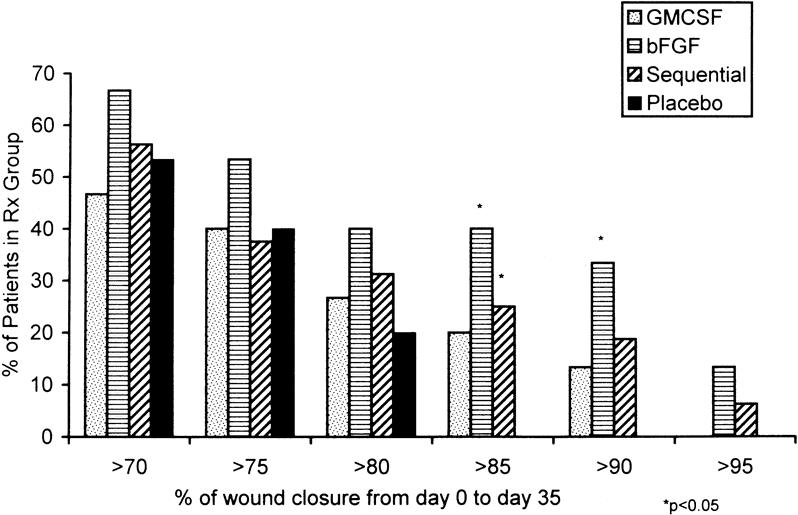
Figure 1. Wound healing response measured by percentage of wound closure after 35 days of treatment for each of the four treatment groups. The group receiving only basic fibroblast growth factor (bFGF) and the group receiving sequential granulocyte-macrophage/colony-stimulating factor (GM-CSF) and bFGF had significantly more patients with more than 85% ulcer closure. The bFGF group had significantly more patients with more than 90% ulcer healing.
Figure 2. (A) Comparison of the group receiving only basic fibroblast growth factor (bFGF) with the placebo group shows that more patients in the former achieved wound closure of more than 85% and more than 90% during 35 days of treatment. (B) The bFGF response can be seen by comparing patients who received any bFGF treatment (those in the bFGF-alone group and those who received sequential granulocyte-macrophage/colony-stimulating factor [GM-CSF] and bFGF) with the placebo group. However, the healing response appeared to be blunted by delaying the onset of bFGF therapy for 10 days.
The positive healing effect of bFGF was further demonstrated when we compared all patients who received bFGF (the bFGF group and the sequential GM-CSF/bFGF group) with the placebo-treated patients. When patients received bFGF at any time in the treatment course, they healed significantly better than placebo-treated patients at more than 85% closure (P = .02) and more than 90% closure (P = .04). Comparing Figures 2A and 2B suggests that delaying the onset of bFGF by 10 days may have diminished its effect on healing.
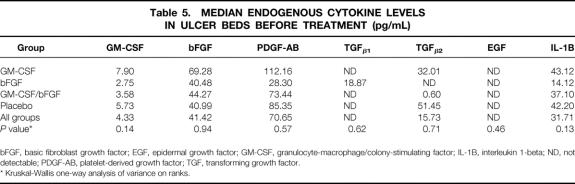
Before treatment, median levels of the various cytokines were evenly distributed among the four treatment groups (Table 5). Patients treated with GM-CSF for 35 days had a threefold increase in GM-CSF; levels in the other three groups did not significantly change (P < .05). The bFGF level on day 36 showed a 20-fold increase over day 0 in both the bFGF 35-day treatment group and the GM-CSF/bFGF sequential therapy group (P < .05). However, the bFGF level on day 36 was not significantly different from day 0 in the patients treated with GM-CSF alone or the placebo-treated patients.
Table 5. MEDIAN ENDOGENOUS CYTOKINE LEVELS IN ULCER BEDS BEFORE TREATMENT (pg/mL)
bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; GM-CSF, granulocyte-macrophage/colony-stimulating factor; IL-1B, interleukin 1-beta; ND, not detectable; PDGF-AB, platelet-derived growth factor; TGF, transforming growth factor.
* Kruskal-Wallis one-way analysis of variance on ranks.
The Spearman rank order correlation demonstrated that a greater increase in the ulcer level of PDGF-AB at day 10 from day 0 was related to a greater degree of healing (r = 0.325, P = .03). A greater increase in TGFβ1 in the ulcer between day 10 and day 36 was also correlated with better wound closure (r = 0.418, P = .03). The higher amounts of TGFβ1 in the wound on day 36 were correlated with greater percentages of wound closure (r = 0.344, P = .01). Interestingly, a large amount of EGF in the ulcer on day 36 tended to be associated with a decreased amount of healing (r = 0.374, P = .008).
Evaluation of mRNA for the GM-CSF gene did not show upregulation with exogenous application of GM-CSF. However, in the bFGF-alone group, 58% of patients had upregulated bFGF message from day 0 to day 10, compared with only 31% of placebo-treated patients (Fig. 3). Similarly, the gene was upregulated in bFGF-treated patients from day 0 to day 36 compared with placebo-treated patients (50% vs. 31%). The sequential GM-CSF/bFGF-treated patients also had upregulated bFGF mRNA from day 10 to day 36.
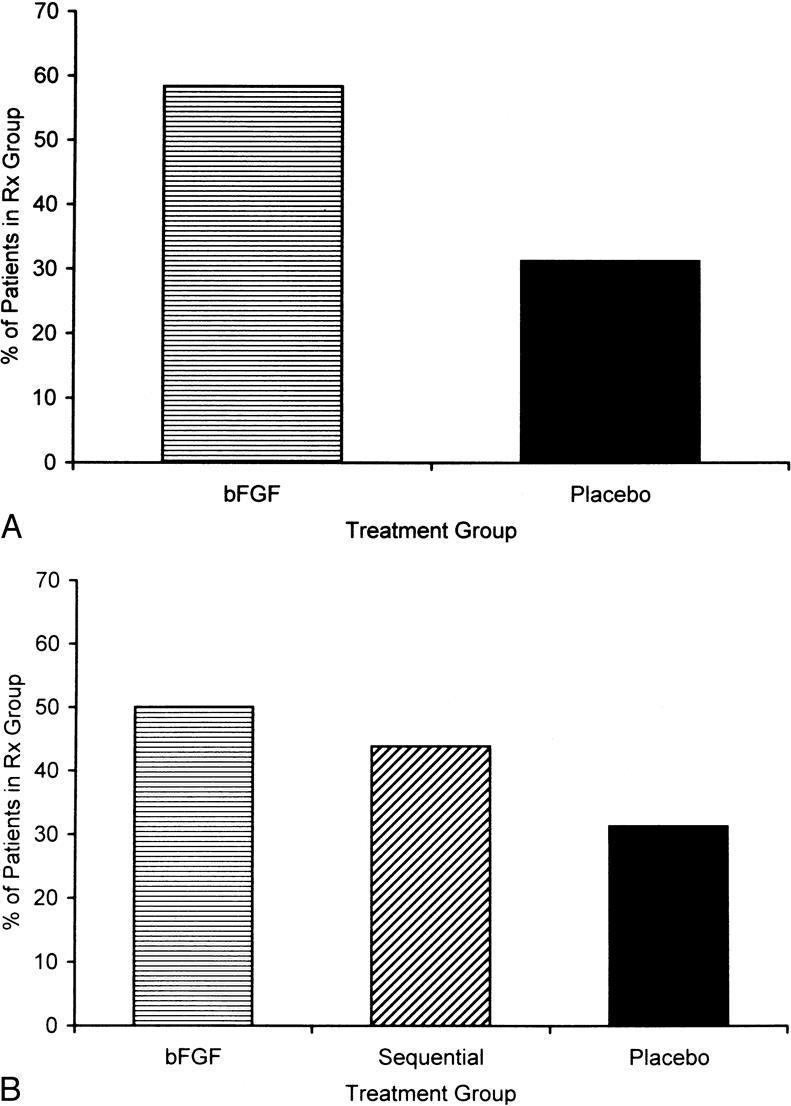
Figure 3. (A) The percentage of patients with upregulation of basic fibroblast growth factor (bFGF) mRNA from day 0 to day 10 in the bFGF-alone group compared with the placebo group. (B) When bFGF was added to the sequential granulocyte-macrophage/colony-stimulating factor (GM-CSF)/bFGF group after day 10, upregulation was noted in the sequentially treated patients when upregulation of bFGF mRNA from day 0 to day 36 was compared in the bFGF-alone patients, the sequentially treated patients, and the placebo group.
Contraction of FPCLs harvested serially from the pressure ulcer beds showed no correlation with the degree of ulcer healing after 10 days or 36 days of treatment in any of the four treatment groups (P > .05). Ultrastructure of the ulcer biopsy samples by electron microscopy demonstrated that although no ulcers exhibited a histology of complete wound repair, cellular integrity among fibroblasts appeared common in most areas of the bFGF-treated wounds (Fig. 4). These cells showed evidence of well-developed endoplasmic reticulum, Golgi apparatus, and numerous ribosomes within the cytoplasm. In the immediate vicinity of the fibroblasts, new collagen synthesis was evident, suggesting development of the extracellular matrix. Vascular development consisting of apparently viable, adjoining endothelial cells and paravascular support cells was observed in approximately 75% of these wounds. These changes were most marked in patients who demonstrated more than 85% wound closure during the 35-day treatment period. Placebo-treated wounds showed some randomly located fibroblasts with development of endoplasmic reticulum and ribosomes; however, matrix development was sporadic even near areas of vascular formation. In these wounds, vascular lumina were incompletely surrounded by endothelial cells. Frequently, arterioles were partially occluded by inflammatory cells, fibrin, and cellular debris related to clotting.
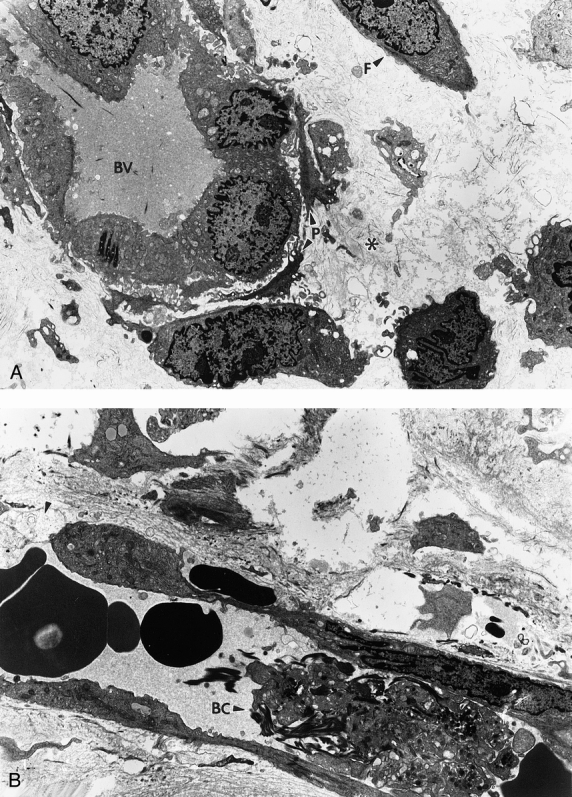
Figure 4. (A) Good blood vasculature (BV) development was commonly observed in pressure ulcers treated with basic fibroblast growth factor. These areas could be defined by adjoining endothelial cells completely enclosing a luminar space. Surrounding paravascular cells (P) and fibroblasts (F) often established sites of new collagen synthesis (*) (×3,600). (B) Wounds in placebo-treated patients showed some new collagen synthesis, but most blood vessels were incompletely formed by absence of surrounding endothelial cells (arrow). Many of these vessels appeared occluded with cellular debris, macrophages, inflammatory cells, and other elements indicative of the clotting process (BC) (×4,800).
Ease of wound closure steadily improved in all groups over time as the ulcer volume decreased. The median ease of closure for all four groups on day 0 was 11, meaning the wounds would best be closed by a somewhat difficult flap at a cost of $10,000 (see Table 2). Patients in the bFGF-treated groups improved 7 points on the ease-of-closure scale (see Table 2). 46 Sequential GM-CSF/bFGF-treated patients improved 5 points on the scale, compared with 4 points in the patients receiving GM-CSF and only 3 points for the placebo controls.
At the end of the trial, the ulcers of patients in the bFGF-alone group could have been closed by a difficult direct wound approximation at a cost of $800 to $1,000. The procedure required for the sequential GM-CSF/bFGF patients would have been a somewhat easy skin graft at a cost of $1,700. The ulcers of patients receiving GM-CSF alone could have been closed with a somewhat more difficult procedure at a cost of $2,200; the placebo group’s ulcers could have been closed for $3,000. Therefore, the procedural cost savings for the bFGF-alone group was $9,000 to $9,200, for the sequential group $8,300, for the GM-CSF group $7,800, and for the placebo group $7,000.
DISCUSSION
The concepts involved in using topical cytokine growth factor to stimulate healing in the chronic wound are sound. The relative deficiency of growth factors in pressure ulcers is well documented. As seen in Figure 1, only in the three groups of patients receiving exogenous growth factors did the ulcers heal at least 85% during the 35-day trial. Of the three groups, the patients receiving bFGF at some time during their treatment healed better than those who did not receive bFGF. Those that received only bFGF for the entire 35 days did significantly better than the placebo-treated patients. Delaying the onset of bFGF treatment appeared to decrease its response (see Fig. 2). This response to topical bFGF confirms the previous report by Robson et al. 13 Both their report and ours found a correlation between the actual decrease in pressure ulcer size and the initial size in all four treatment groups. Slopes of the regression curves were compared with the F test, and bFGF enhanced a decrease in ulcer volume compared with placebo-treated wounds, as was also found in the previous study. 13
The fact that treatment with bFGF alone was more effective than sequential treatment differed from that predicted by animal models. It confirmed LeGrand’s 51 observation that there is no way a priori to determine the relevance of animal models to human wounds. However, both the bFGF-alone group and the sequential GM-CSF/bFGF group had more rapid healing than the patients treated with PDGF-BB reported recently by Rees et al. 18 The bFGF used in the present trial and in the earlier trial 13 may not have been the most ideal formulation. Angiogenesis, an important component of the healing process in pressure ulcers, requires a sustained concentration gradient of a chemotactic, angiogenic factor that can induce the directional migration of capillary endothelial cells into the wound site. 52 The cytokines in this study were applied in an aqueous solution, conferring no protection against wound-associated proteases. The topical application of an angiogenic cytokine, such as bFGF, in a formulation that releases the cytokine over a sustained period and protects against protease exposure might be expected to improve the positive healing responses observed in this study by providing a concentration gradient of a longer duration.
Although more than 85% wound closure appeared to distinguish cytokine-treated wounds from those treated with placebos, and a statistically greater number of bFGF-treated patients achieved more than 85% closure, only 21% of all the patients had more than 85% wound closure. This was probably due to the 35-day treatment period. This time period was chosen from animal experiments suggesting that sequential cytokine therapy would accelerate wound closure to that degree. Previous pressure ulcer trials with single, exogenously applied growth factors have lasted 28 to 112 days. 10–13,16,18 The bFGF-treated group in this study achieved a mean of 75% closure and a median of 79% closure in the 35 days (see Table 4). This suggests that a future trial with this cytokine could be significantly less than 112 days.
Treatment of chronic wounds such as pressure ulcers with exogenous cytokines appears to upregulate cells to produce other growth factors. The significant upregulation of PDGF-AB during the first 10 days and the upregulation of TGFβ1from day 10 to day 36 confirmed preclinical hypotheses that both GM-CSF and bFGF can stimulate cells to produce other factors, because neither PDGF nor TGFβ was exogenously added to the wounds. 53–55
The upregulation of mRNA of bFGF by topical bFGF is the first demonstration in humans that an exogenously applied growth factor can upregulate its gene expression in a chronic wound. This suggests that the gene message for bFGF is autoinduced, as has been suggested in vitro by Alberts et al 56 and Wang et al. 57 Although Thomassen et al 54 demonstrated that exogenous GM-CSF enhanced expression of several cytokines, they did not show autoinduction of the GM-CSF gene, in agreement with the data from this trial.
The FPCL has been an excellent in vitro model for contraction. 58–60 Its failure to correlate with in vivo wound closure in the pressure ulcer model is probably due to the fact that pressure ulcers heal by a combination of processes, such as angiogenesis, deposition of extracellular matrix, contraction, and epithelialization, rather than by contraction alone.
The electron microscopic inspection of pressure ulcer biopsy samples at the end of the clinical trial showed a histology that supported volume measurements. Although pressure ulcers with more than 85% closure did not exhibit the morphology of completely repaired wounds, the proportion of fibroblasts to inflammatory cells and the appearance of new collagen fibers suggest that these significantly responding wounds were directed in a coordinated fashion toward normal repair. Responding placebo wounds demonstrating volume changes toward repair also displayed evidence of good cellular integrity. However, there did not appear to be an overall uniform progress toward repair. For example, the placebo wound in Figure 4B showed new collagen synthesis and some functionally appearing fibroblasts, but it lacked a comparable level of angiogenesis compared with that seen in good-response bFGF-treated wounds. Vascular lumina in placebo wounds were not completely enclosed, nor did they display a clearance for good circulation.
These data suggest that bFGF may synchronize all wound-healing elements so that they are uniform in establishing repair. Further, such synchronicity may be coordinated with the level of bFGF in the wound, or more specifically with the ability of fibroblasts to upregulate their own FGF.
Our assessment of the ease of wound closure revealed that bFGF-treated ulcers would have required a pedicled flap on day 0 and could be closed at the bedside with several sutures after 35 days of treatment. If we convert these surgical procedures into a cost analysis, as reported by Robson et al, 46 the $9,000 to $9,200 cost savings exceeds that reported from a 16-week PDGF-BB pressure ulcer trial. 18
Although the sequential cytokine therapy was not the most effective in this trial, the concept is still of interest. Smith et al 61 have reported that sequential therapy was effective in accelerating healing in acute surgical incisions; they found the effective sequence to be PDGF-BB followed by TGFβ2. This sequence might be worth trying in the pressure ulcer model, because the most effective responses occurred in patients with the greatest increase in PDGF-AB from day 0 to day 10 and the greatest increase in TGFβ1 from day 10 to day 36. From our experience, it appears when testing a PDGF/TGFβsequence or any other cytokine sequence for its ability to accelerate healing in pressure ulcers, the results should be compared with the effect obtained with bFGF therapy.
Acknowledgments
The authors thank the wound service clinical staff and laboratory personnel for their help in making the study possible; G. Schultz, J. Heggers, D. Cooper, H. Browne, D. Meltzer, and R. Mannari for their valuable consultation; and L. Carlotti for manuscript preparation.
Footnotes
Correspondence: Martin C. Robson, MD, Institute for Tissue Regeneration, Repair and Rehabilitation, Bay Pines VAMC (151W), P.O. Box 5005, Bay Pines, FL 33744.
Supported by a grant from the National Institutes of Health (ROI-AR42967).
Schering-Plough Research Institute and Scios, Inc. provided the cytokines used in this study.
Accepted for publication September 27, 1999.
References
- 1.Lazurus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493. [PubMed] [Google Scholar]
- 2.Nwomeh BC, Yager DR, Cohen IK. Physiology of the chronic wound. Clin Plast Surg 1998; 25:341–356. [PubMed] [Google Scholar]
- 3.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg 1993; 165:728–737. [DOI] [PubMed] [Google Scholar]
- 4.Bennett NT, Schultz GS. Growth factors and wound healing. Part II: role in normal and chronic wound healing. Am J Surg 1993; 166:74–81. [DOI] [PubMed] [Google Scholar]
- 5.Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wounds Rep Regen 1996; 4:411–420. [DOI] [PubMed] [Google Scholar]
- 6.Tarnuzzer RW, Macauley SP, Mast BA, et al. Epidermal growth factor in wound healing: a model for the molecular pathogenesis of chronic wounds. In: Ziegler T, Pierce G, Herndon D, eds. Growth Factors and Wound Healing. New York: Springer Verlag; 1997: 206–228.
- 7.Cooper DM, Yu EZ, Hennessey P, et al. Determination of endogenous cytokines in chronic wounds. Ann Surg 1994; 219:688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce GF, Tarpley JE, Tseng J, et al. Detection of increased level of PDGF-AA in actively healing human wounds treated with recombinant PDGF-BB, and absence of PDGF in chronic nonhealing wounds. J Clin Invest 1995; 96:1336–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robson MC. Exogenous growth factor application effect on human wound healing. Progress Dermatol 1996; 30:1–7. [Google Scholar]
- 10.Robson MC, Phillips LG, Thomason A, et al. Platelet derived growth factor-BB for the treatment of chronic pressure ulcers. Lancet 1992; 339:23–25. [DOI] [PubMed] [Google Scholar]
- 11.Robson MC, Phillips LG, Thomason A, et al. Recombinant human platelet-derived growth factor-BB in the treatment of pressure ulcers. Ann Plast Surg 1992; 29:193–201. [DOI] [PubMed] [Google Scholar]
- 12.Mustoe TA, Cutler NR, Allman RM, et al. Phase II study to evaluate recombinant PDGF-BB in the treatment of pressure ulcers. Arch Surg 1994; 129:213–219. [DOI] [PubMed] [Google Scholar]
- 13.Robson MC, Phillips LG, Lawrence WT, et al. The safety and effect of topically applied recombinant basic fibroblast growth factor on healing of chronic pressure sores. Ann Surg 1992; 216:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown GL, Curtsinger L, Jurkiewicz MJ, et al. Stimulation of healing of chronic wounds by epidermal growth factor. Plast Reconstr Surg 1995; 96:251–254. [PubMed] [Google Scholar]
- 15.Kucukcelebi A, Hui PS, Sahara K, et al. The effect of interleukin 1B on the inhibition of contraction caused by bacterial contamination. Surg Forum 1992; 43:715–716. [Google Scholar]
- 16.Robson MC, Abdullah A, Burns BF, et al. Safety and effect of topical recombinant human interleukin-1B in the management of pressure sores. Wound Rep Regen 1994; 2:177–181. [DOI] [PubMed] [Google Scholar]
- 17.VandeBerg JS, Robson MC, Mihail RS. Extension of lifespan of pressure ulcer fibroblasts with recombinant human interleukin-1B. Am J Pathol 1995; 146:1273–1282. [PMC free article] [PubMed] [Google Scholar]
- 18.Rees RS, Robson MC, Smiell SM, Perry BH. Becaplermin gel in the treatment of pressure ulcers: a randomized, double-blinded, placebo controlled study. Wound Rep Regen 1998; 6:A478. [DOI] [PubMed] [Google Scholar]
- 19.Lynch SE, Nixon JC, Calvin RB, Antonaides HN. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci USA 1987; 84:7696–7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson JM, Bradley KN, Quaglino D. Reversal of the wound healing deficit in diabetic rats by combined basic fibroblast growth factor and transforming growth factor-b1 therapy. Wound Rep Regen 1997; 5:77–88. [DOI] [PubMed] [Google Scholar]
- 21.Sprugel KH, McPherson JM, Clowes AW, Ross R. Effect of growth factors in vivo. Am J Pathol 1987; 129:601–611. [PMC free article] [PubMed] [Google Scholar]
- 22.Greenhalgh DH, Sprugel KH, Murray MJ, et al. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 1990; 136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 23.Knighton DR, Fiegel VD, Austin LL, et al. Classification and treatment of nonhealing wounds: successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann Surg 1986; 294:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knighton DR, Ciresi KF, Fiegel VD, et al. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet 1990; 170:56–60. [PubMed] [Google Scholar]
- 25.Atri SC, Mitra J, Bisht D, Misra K. Use of homologous platelet factors in achieving total healing of recalcitrant skin ulcers. Surgery 1990; 108:508–512. [PubMed] [Google Scholar]
- 26.Steed D, Goslen J, Hambley R, et al. Clinical trials with purified platelet releasate. In: Barbul A, Caldwell MD, Eaglestein WH, et al, eds. Clinical and Experimental Approaches to Dermal and Epidermal Repair: Normal and Chronic Wounds. New York: Wiley Liss; 1991: 103–113. [PubMed]
- 27.Krupski WC, Reilly LM, Perez S, et al. A prospective randomized trial of autologous platelet-derived wound healing factors for the treatment of chronic wounds: a preliminary report. J Vasc Surg 1991; 14:526–536. [PubMed] [Google Scholar]
- 28.Steed DL, Goslen JB, Holloway GA, et al. Randomized, prospective, double-blind trial in healing chronic diabetic foot ulcers. CT-102 activated platelet supernatant, topical versus placebo. Diabetes Care 1992; 15:1598–1604. [DOI] [PubMed] [Google Scholar]
- 29.Holloway GA, Steed DL, DeMarco MJ, et al. A randomized, controlled, multicenter, dose response trial of activated platelet supernatant, topical CT-102 in chronic, nonhealing, diabetic wounds. Wounds 1993; 5:198–206. [Google Scholar]
- 30.Rayner TE, Cowin AS, Cooter RD, Belford DA. Wound strength is returned to normal levels in steroid compromised rats by treatment with milk-derived growth factors. Wound Rep Regen 1997; 5:A123. [Google Scholar]
- 31.Belford DA, Rogers ML, Francis GL, et al. Platelet derived growth factor, insulin-like growth factors, fibroblast growth factors, transforming growth factor beta do not account for the cell growth activity present in bovine milk. J Endocrin 1997; 154:45–55. [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka J, Grotendorst GR. Two peptides related to platelet derived growth factor are present in human wound fluid. Proc Natl Acad Sci USA 1989; 86:4416–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratatori DS, Caffee HH, Copeland EM, Schultz GS. Growth factors are present in human wound fluid. Surg Forum 1990; 41:627–630. [Google Scholar]
- 34.Dvonch WM, Murphey RJ, Matsuoka J, Grotendorst GR. Changes in growth factors in human wound fluid. Surgery 1992; 112:18–23. [PubMed] [Google Scholar]
- 35.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am 1997; 77:509–526. [DOI] [PubMed] [Google Scholar]
- 36.Kucukcelebi A, Carp SS, Hayward PG, et al. Granulocyte-macrophage colony stimulating factor reverses the inhibition of wound contraction caused by bacterial contamination. Wounds 1992; 4:241–247. [Google Scholar]
- 37.Stenberg BD, Phillips LG, Hokanson JA, et al. Effect of bFGF on the inhibition of contraction caused by bacteria. J Surg Res 1991; 50:47–50. [DOI] [PubMed] [Google Scholar]
- 38.Hayward PG, Hokanson J, Heggers JP, et al. Fibroblast growth factor reverses the bacterial retardation of wound contraction. Am J Surg 1992; 163:288–293. [DOI] [PubMed] [Google Scholar]
- 39.Resch CS, Kerner E, Robson MC, et al. Pressure sore volume measurement: a technique to document and record wound healing. J Am Geriatr Soc 1988; 36:444–446. [DOI] [PubMed] [Google Scholar]
- 40.Robson MC. Infection in the surgical patient: an imbalance in normal equilibrium. Clin Plast Surg 1979; 6:493–503. [PubMed] [Google Scholar]
- 41.Tarnuzzer RW, Macauley SP, Farmerie WG, et al. Competitive RNA templates for detection and quantitation of growth factors, cytokines, extracellular matrix components and matrix metalloproteinases by RT-PCR. Biotechniques 1996; 20:670–674. [DOI] [PubMed] [Google Scholar]
- 42.Ascroft GS, Horan MA, Tarnuzzer RW, et al. Estrogen accelerates wound healing associated with an increase in TGF-β1. Nature Med 1997; 3:1209–1215. [DOI] [PubMed] [Google Scholar]
- 43.Sahara K, Kucukcelebi A, Ko F, et al. Suppression of in vitro proliferative fibroblast contraction by interferon alpha-2B. Wound Rep Regen 1993; 1:22–27. [DOI] [PubMed] [Google Scholar]
- 44.VandeBerg JS, Rudolph R, Hollan C, Haywood-Reid PL. Fibroblast senescence in pressure ulcers. Wound Rep Regen 1998; 6:38–49. [DOI] [PubMed] [Google Scholar]
- 45.VandeBerg JS, Rudolph R. Pressure (decubitus) ulcer: variation in histopathology. A light and electron microscopic study. Human Pathol 1995; 26:195–201. [DOI] [PubMed] [Google Scholar]
- 46.Robson MC, Maggi SP, Smith PD, et al. Ease of wound closure as an endpoint of treatment efficacy. Wound Rep Regen 1999; 7:90–96. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan E, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958; 53:457–481. [Google Scholar]
- 48.Hokanson JA, Hayward PG, Carney DH, et al. A mathematical model for the analysis of experimental wound healing data. Wounds 1991; 3:213–220. [Google Scholar]
- 49.Cooper DH, Robson MC. In the evaluation of potential wound healing agents a placebo arm is not nontreatment. Wound Rep Regen 1996; 4:A128. [Google Scholar]
- 50.Robson MC, Phillips LG, Heggers JP, et al. Clinical studies on growth factors in pressure sores: preliminary report. In: Barbul A, Caldwell MD, Eaglestein WH, et al, eds. Clinical and Experimental Approaches to Dermal and Epidermal Repair: Normal and Chronic Wounds. New York: Wiley Liss; 1991; 95–102. [PubMed]
- 51.LeGrand EK. Preclinical promise of becaplermin (rhPDGF-BB) in wound healing. Am J Surg 1998; 176(Suppl 2A):485–545. [DOI] [PubMed] [Google Scholar]
- 52.Banda MJ, Dwyer KS, Beckmann A. Wound fluid angiogenesis factor stimulates the directed migration of capillary endothelial cells. J Cell Biochem 1985; 29:183–193. [DOI] [PubMed] [Google Scholar]
- 53.Jyung RW, Wu L, Pierce GF, Mustoe TA. Granulocyte-macrophage colony-stimulating factor: differential action on incisional wound healing. Surgery 1994; 115:325–334. [PubMed] [Google Scholar]
- 54.Thomassen MJ, Ahmad M, Barna BP, et al. Induction of cytokine messenger RNA and secretion in alveolar macrophages from patients with lung cancer receiving granulocyte-macrophage colony-stimulating factor therapy. Cancer Res 1991; 51:857–862. [PubMed] [Google Scholar]
- 55.Fiddes JC, Hebda PA, Robson MC, et al. Preclinical wound healing studies with recombinant human basic fibroblast growth factor. Ann NY Acad Sci 1991; 368:316–328. [DOI] [PubMed] [Google Scholar]
- 56.Alberts GF, Hsu DK, Peiflay KA, Winkles JA. Differential regulation of acidic and basic fibroblast growth factor gene expression in fibroblast growth factor-treated rat aortic smooth muscle cells. Circ Res 1994; 75:261–267. [DOI] [PubMed] [Google Scholar]
- 57.Wang D, Mayo MW, Baldwin AS Jr. Basic fibroblast growth factor transcriptional autoregulation requires EGR-1. Oncogene 1997; 14:2291–2299. [DOI] [PubMed] [Google Scholar]
- 58.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattice by human fibroblasts of different proliferative scar potentiation in vitro. Proc Natl Acad Sci USA 1979; 76:1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrlich HP. The modulation of contraction of fibroblast populated collagen lattices by types I, II, and III collagen. Tissue Cell 1988; 20:47–50. [DOI] [PubMed] [Google Scholar]
- 60.Smith PD, Mosiello G, DeLuca L, et al. TGFβ2 activates proliferative scar fibroblasts. J Surg Res 1999; 82:319–323. [DOI] [PubMed] [Google Scholar]
- 61.Smith PD, Franz M, Wachtel T, Robson MC. Initiating the inflammatory phase of wound healing prior to wounding. Presented at the 41st University Surgical Residents’ Program, New Orleans, LA, Feb. 11, 1999.