Abstract
Objectives
To detail characterization of mutations and uncharacterized variants in the breast cancer susceptibility genes BRCA1 and BRCA2, as observed in a population of breast cancer patients from the southeastern United States, and to examine baseline characteristics of women referred for counseling and testing and provide a preliminary look at how counseling and testing affected intentions toward prophylactic surgery.
Background
Mutations in the BRCA1 and BRCA2 genes give rise to a dramatically increased risk of developing breast or ovarian cancer or both. There are many reports about special populations in which deleterious mutations are present at a high frequency. It is useful to study these genes in more heterogeneous populations, reflecting different geographic regions. Interest in preventive surgery for gene carriers is high in women and their surgeons.
Methods
Women were recruited through a prospective clinical trial of counseling and free genetic testing. BRCA1 and BRCA2 were screened for mutations using standard techniques, and results were given to participants. Baseline questionnaires determined interest in preventive surgery at the beginning of the study. Follow-up questionnaires for those who completed testing surveyed interest in prophylactic surgery after counseling and receiving test results.
Results
Of 213 women who completed counseling and testing, 44 (20.6%) had 29 separate mutations; there were 11 Jewish women carrying three founder mutations. Twenty-eight women (13.1%) had uncharacterized variants in BRCA1 or BRCA2; nine were not previously reported. Women overestimated their chances of possessing a deleterious gene mutation compared to a statistical estimate of carrier risk. A number of women changed their intentions toward preventive surgery after genetic counseling and testing.
Conclusions
Hereditary breast cancer due to mutations in BRCA1 and BRCA2 was a heterogeneous syndrome in the southeastern United States. Most mutations were seen just once, and uncharacterized variants were common and of uncertain clinical significance. In general, positive test results tended to reinforce intentions toward prophylactic surgery. In contrast, women not interested in surgery at the time of entry tended to remain reluctant after testing and counseling.
Interest in familial breast cancer is high among surgeons and their female patients. Both physicians and patients may misinterpret the significance of a familial clustering of breast cancer cases. The occurrence of breast cancer in more than one family member may cause concern about hereditary factors. Conversely, some women may underestimate their breast cancer risk because they have no affected relatives. Overall, women tend to overestimate the likelihood of breast cancer and exaggerate the significance of family history. 1,2 It is possible that misperceptions lead to inappropriate anxiety on the part of patients and unnecessary medical interventions by their physicians.
Hereditary breast cancer is a rare syndrome in most populations of women seen in the United States. The majority of breast cancer cases occur in the absence of a significant family history. Twenty percent of women presenting with primary breast cancer have one or more relatives with a history of the disease. A smaller fraction of affected women have a family history consistent with a genetic cause. 3 However, in some of these rare families, occurrence of breast cancer can be linked to one of two susceptibility genes, BRCA1 or BRCA2. 4,5 Certain mutations in either of these two genes can be inherited as an autosomal dominant trait passed on average in the germline to 50% of a single carrier’s offspring (either father or mother). For women who inherit a mutant allele of BRCA1 or BRCA2, the lifetime risk of getting breast cancer (genetic penetrance) is estimated at 50% to 80%. 6–9 For ovarian cancer, a much less common disease, the penetrance of BRCA1 is probably less than 40%. For BRCA2, the penetrance is less than 20%. 6,7
Disease-associated alleles in BRCA1 and BRCA2 are uncommon in the U.S. population. It is estimated that between 0.01% and 0.1% of people carry mutations in BRCA1 that cause familial breast or ovarian cancer. 10 Similar carrier frequencies are estimated for BRCA2. Certain ethnic and regionally confined populations display a higher frequency of disease-producing alleles, among them Jewish people of Eastern European descent (Ashkenazi Jewish), who carry a limited number of mutations in both genes, which are present at much higher frequency than in the general population. Between 2.0% and 2.5% of Ashkenazi Jewish women carry one of three common mutations in the BRCA1 and BRCA2 genes. 11,12 For women in this group, hereditary breast cancer is a relatively common syndrome. Founding mutations are also present in other regional or ethnic populations, such as African Americans. 13
Reports about the distribution of BRCA1 and BRCA2 mutations and the characteristics of carriers frequently come from special groups, such as the Ashkenazim. Groups with a high frequency of restricted mutations are important for genetic analysis of penetrance, gene-gene interactions, gene-environment interactions, and the interaction with treatment and outcome. However, other studies benefit from less-biased populations. For instance, the spectrum of BRCA1 and BRCA2 mutations frequently come from high-risk clinics in large urban locations, which may not reflect mutations seen in more geographically dispersed populations. Clinical trials, patterns of care studies, and observations about medical decision-making are benefited by access to large and heterogeneous populations. The current study is from Duke University Medical Center, located in North Carolina and serving populations from rural and moderate-sized communities in the southeastern United States. In this study, we present the spectrum of mutations seen in BRCA1 and BRCA2 in our region of the country and present preliminary observations about the reaction of women after receiving genetic testing results.
METHODS
Study Population and Design
The women who are the subject of this report were participants in a randomized clinical trial of educational interventions offered to women suspected of carrying a breast cancer susceptibility gene. This study has been described in detail elsewhere. 14,15 Recruitment methods, registration in a family cancer database, and administration of a medical questionnaire were approved by Institutional Review at Duke University Medical Center. A Certificate of Confidentiality was granted for the results of genetic testing, and testing was done after extensive genetic counseling and approved informed consent procedures.
All participants had a high risk of carrying a breast cancer susceptibility allele. In general, eligible women were affected with early-onset (<56 years of age) breast cancer or ovarian cancer and had at least one first-degree relative with the disease, or they must have had more than three relatives on one side of the family with early-onset breast or ovarian cancer. Exceptions included women with breast cancer before age 31, regardless of family history, and Jewish women with onset of breast cancer before age 40. Other situations were viewed individually and judged to have a prior probability of harboring a deleterious mutation in BRCA1 or BRCA2 of greater than 10%.
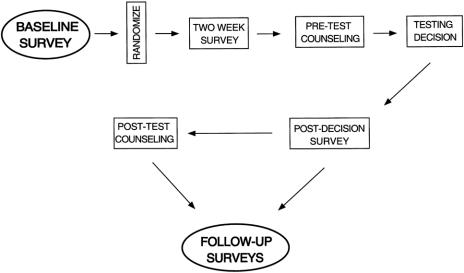
Figure 1 depicts the flow of the clinical trial. Baseline information was collected by a mailed questionnaire, which obtained data about sociodemographic factors, personal attitudes, and knowledge about cancer genetics, genetic testing, and risk. Specific questions asked women to estimate their own chances of carrying a deleterious mutation in BRCA1 or BRCA2 (or a similar potent genetic susceptibility factor) on a Likert scale and by assigning an actual numerical value between 0% and 100%. Women were also asked about their interest in prophylactic surgery (mastectomy or oophorectomy). For instance, the question about prophylactic mastectomy began with a brief description of surgery to prevent cancer and then asked women if they “ever had or considered having a prophylactic mastectomy to reduce your risk of getting breast cancer.” The same question was posed about prophylactic oophorectomy.
Figure 1. Study flow for the randomized clinical trial of counseling strategies in high-risk women considering BRCA1 or BRCA2 testing. The circled survey at baseline and the follow-up survey after receipt of testing results represents data collection used in this preliminary report of 213 women who completed counseling and testing.
After receipt of the baseline survey, participants were randomly assigned to receive two forms of written educational material about breast and ovarian genetics and genetic testing. A 2-week survey collected information after the participants have read the written materials. All participants were offered free genetic counseling prior to making a decision about genetic testing. Finally, follow-up surveys were completed after the women had decided on testing, received results, and undergone posttest counseling. The first of these follow-up surveys was at about 1 month after test results counseling. In this survey, women were again questioned about whether they were still interested in having prophylactic surgery, or whether they actually had surgery during the counseling and testing process. In the current study, women who underwent counseling and received testing results were surveyed before (at baseline, Figure 1) and after counseling and testing (follow-up surveys) about their intentions toward prophylactic mastectomy or oophorectomy.
Carrier Probability Model, BRCAPRO
The statistical algorithms used to calculate carrier probability have been published and are part of a computer program, BRCAPRO (Institute of Statistics and Decision Sciences, Duke University, Durham, NC), 10,16 which estimates the likelihood that a given member of a family is carrying a susceptibility gene with the prevalence and penetrance characteristics of BRCA1 or BRCA2, based on the occurrence of cancer in other family members and position in the pedigree. In practice, BRCAPRO calculates the chance that a woman harbors a deleterious mutation in BRCA1 or BRCA2.
Mutation Screening
The presence of mutations in BRCA1 and BRCA2 was detected by conformational analysis of polymerase chain reaction (PCR)-amplified fragments of coding regions and intron-exon boundaries. Amplified fragments were analyzed by either a combination of single-strand conformational analysis (SSCA) and heteroduplex analysis (HA) or conformation sensitive gel electrophoresis (CSGE). 17 All PCR fragments with aberrant mobility were sequenced on an ABI 377 automated sequencer (Applied Biosystems, San Jose, CA). Results were compared to the Breast Cancer Information Core database. 18
Statistical Analyses
Analyses were performed using SAS version 6.12 (Statistical Analysis Software, Cary, NC). The three-way comparisons of mutation risk distributions in categories of testing results were performed using the Kruskal-Wallis test. Pairwise comparisons were performed using the Wilcoxon rank sum test and the Wilcoxon signed rank test. Correlation between modeled and subject risk estimates was calculated using the Spearman rank correlation statistic.
RESULTS
Characteristics of Participants and Test Results
Accrual to the randomized trial of counseling depicted in Figure 1 was closed in April 1999. At that time, 595 women were invited to participate, and by October 1999, 420 participants had completed the baseline survey. Testing for BRCA1 and BRCA2 mutations was offered to 221 women affected with breast cancer (probands) and to 110 relatives of mutation carriers. Uptake into the genetic testing phase of our study was very high; more than 95% of women who were offered free testing decided to undergo the procedure. Characteristics of 213 probands who completed genetic testing are shown in Table 1; accrual of relatives is incomplete, and their results are not part of this report.
Table 1. CHARACTERISTICS OF PARTICIPANTS
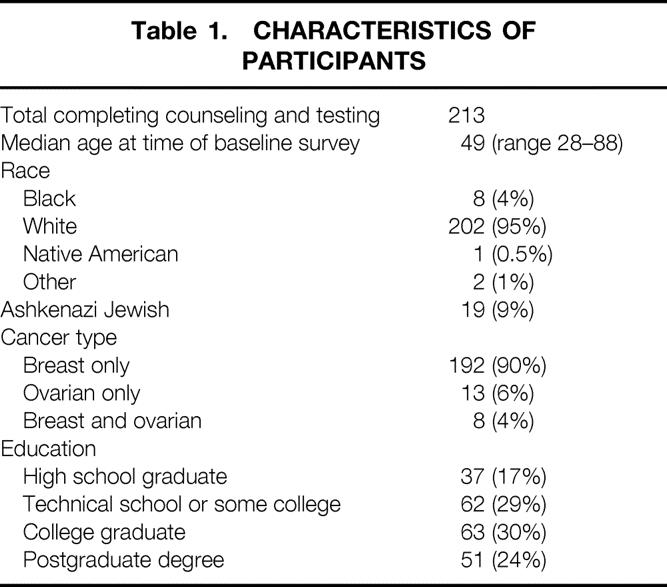
Although our region of the country has a substantial number of African-Americans, only 4% of the women completing genetic testing were black. The majority of women were white. In contrast to high-risk breast cancer clinics in large urban locations, less than 10% of women completing testing in North Carolina were Jewish. Breast cancer was the predominant cancer in probands referred to our clinical trial, although ovarian cancer was present in 10% of the women who completed testing. Finally, the educational levels of women in our study were remarkable. More than 80% completed high school and acquired some college or post-high school education. Other than the proportion of Jewish women, these characteristics are similar to high-risk counseling programs in other areas of the country.
A diverse number of BRCA1 and BRCA2 mutations were observed in the 213 women with breast or ovarian cancer, who were counseled in our clinical trial and completed testing. Forty-four mutations were found in these women, representing 20.6% of the total tested. About two thirds were mutations in the BRCA1 gene, and one third were mutations in the BRCA2 gene. Table 2 summarizes the types of mutations we found. The vast majority were nucleic acid changes that lead to premature termination of the polypeptide product. Deletions and insertions resulting in a shift of the genetic reading frame were the most common mutations. Nonsense mutations, or mutations leading to the appearance of an inappropriate stop codon, were also common alterations. Missense mutations were uncommon. Twenty-nine of 44 mutations were seen only once, and four clearly deleterious mutations were seen for the first time in this population.
Table 2. SUMMARY OF MUTATIONS DETECTED IN 213 PATIENTS
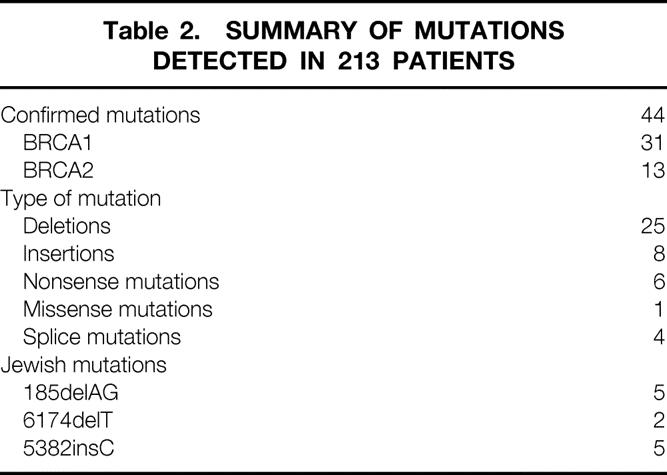
The three founding mutations, commonly encountered in Jewish women, were each represented in our tested cohort. We encountered 11 mutations in 19 Ashkenazi Jewish women, and saw the 5382insC mutation in BRCA1 in one African-American family not known to have intermarried with Jewish antecedents. Four splice junction mutations were all at a single position in BRCA1. This mutation was described in the initial report of BRCA1 mutations in hereditary breast cancer and has been seen in several North Carolina families. 1,18
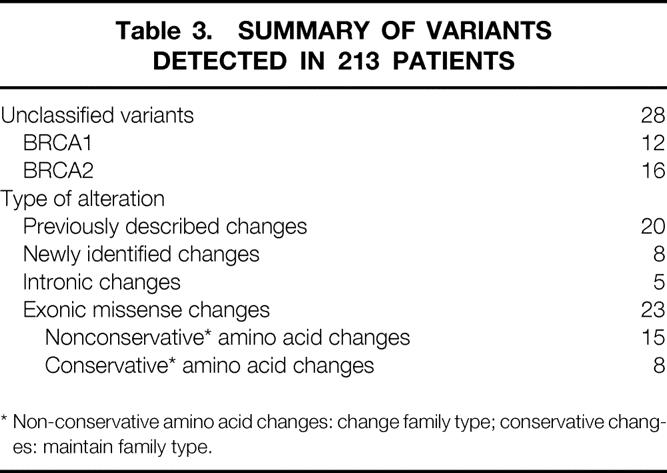
We detected 28 variants in BRCA1 or BRCA2, as shown in Table 3. These variants changed the amino acid sequence of their protein product but have not been previously linked to breast or ovarian cancer in families. Whether they are disease-associated alleles is unknown, and they are reported to patients as changes of unknown significance. Most have been previously reported to the National Institutes of Health (NIH) Breast Information Core (BIC). 18 However, we did find eight variations that were unique, and not part of the BIC. Most of these sequence alterations result in substitution of an amino acid residue in one class (acidic, basic, polar, nonpolar) for an amino acid of another class (Table 3).
Table 3. SUMMARY OF VARIANTS DETECTED IN 213 PATIENTS
* Non-conservative amino acid changes: change family type; conservative changes: maintain family type.
Overestimation of Carrier Risk
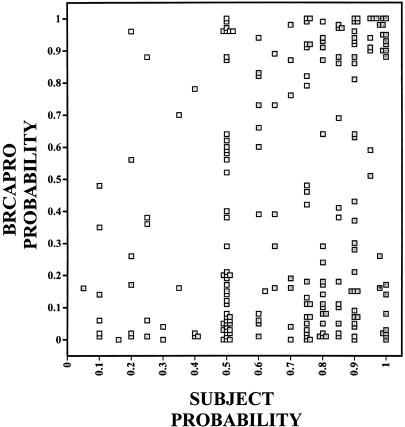
The chance that an individual woman carries a significant BRCA1 or BRCA2 mutation can be calculated using BRCAPRO. 10,16 The statistically derived carrier probability for each individual woman was compared to her own estimate of the likelihood that she was carrying a susceptibility gene for breast or ovarian cancer. This comparison is graphically plotted in Figure 2, where each square represents the individual woman’s self-estimated carrier probability. Overall, subject’s self-estimate and the calculated estimates differed significantly from each other (P < .0001). There was only a weak correlation between a woman’s estimate of her chances of carrying a BRCA1 or BRCA2 mutation and the calculated probability determined by BRCAPRO (Spearman correlation = 0.2597).
Figure 2. Carrier probabilities for 213 women who completed testing. Scatter diagram compares individual calculated probabilities of carrying BRCA1 or BRCA2 mutations with self-estimates, provided in the baseline survey. Data is presented in deciles of calculated or self-estimated risk. Calculated probabilities were provided by BRCAPRO, based on family and personal histories obtained before entry into the trial (prior to baseline).
Figure 2 shows that women in our study who had breast or ovarian cancer and came to a high-risk clinic for genetic counseling, and perhaps genetic testing, overestimated their chances of actually having a genetic susceptibility factor. The median carrier probability estimated by BRCAPRO for the entire cohort was 0.24. In contrast, the median estimate of our study subjects was 0.75. A significant number of women dramatically overestimated their risks, as shown by the cluster of women in the extreme lower right corner of Figure 2 (those with a high self-estimate and a low calculated estimate).
The calculated probability of carrying a BRCA1 or BRCA2 mutation is compared to the results of testing in Figure 3. As expected, a cluster of low BRCAPRO-calculated probabilities is seen in the women who tested negatively. A second cluster of prior high probabilities is seen in the group of women who actually tested positive for a BRCA1 or BRCA2 mutation. Overall, BRCAPRO carrier probability estimates differed according to testing results (P < .0001). Pairwise comparison shows that those with negative test results had lower carrier risk estimates than those with positive results (P < .0001). Also, women with uncharacterized variants tended to have lower prior probabilities of carrying a mutation than those who actually had a mutation (P < .001). However, an examination of Figure 3 shows exceptions to these trends. Twenty-eight women with negative test results had a pretest calculated probability of carrying a susceptibility mutation that was 70% or greater. Seventeen of these women had a BRCAPRO calculation of greater than 90%. Women with uncharacterized variants also fell into two clusters, those with low prior probabilities for carrying mutations and those with high odds. We saw six variants in women with probabilities greater than 90%.
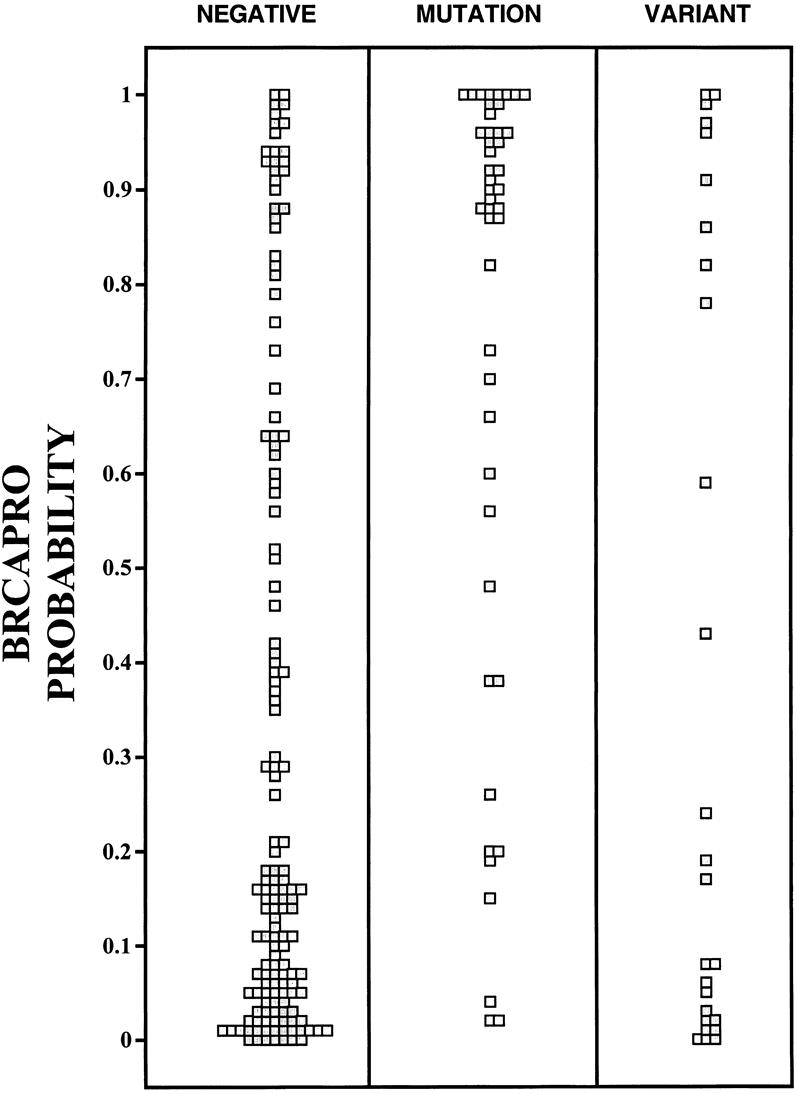
Figure 3. Calculated prior probabilities of carrying a deleterious mutation in BRCA1 or BRCA2. BRCAPRO calculated the chances of carrying a mutation before actual testing for women who tested negatively for a mutation (negative), for women found to have a mutation after testing (mutation), and for women found to have an uncharacterized sequence variation in BRCA1 or BRCA2 (variant).
Treatment Intentions Before and After Counseling and Testing
The purpose of our clinical trial was to determine the effect of tailored counseling, compared to standard information, on the attitudes, decisions, and knowledge base of women with a high baseline risk for familial breast or ovarian cancer. Tailored information was created especially for an individual based on information specific to that person. 19 The trial is now closed, and participants are finishing counseling, making testing decisions, and entering the follow-up phase of the study. The following observational data are preliminary and combines the two arms of the clinical trial.
Figures 4 and 5 depict the actual decision or intention trees followed by women who completed counseling (on either arm of the clinical trial), decided to undergo testing, and received their test results. Ninety-five women answered the questions about prophylactic oophorectomy before and after counseling and testing. Eighty-two women answered questions about preventive mastectomy. Some women did not answer one or both of these questions because they had previous prophylactic surgery. Women were asked at entry (Baseline Intention) whether they had or had considered having prophylactic mastectomy or oophorectomy. As shown in Figure 1, after counseling and testing, the subjects were queried again about whether they had or still considered having prophylactic surgery. Five women failed to answer the posttest questions about oophorectomy, and six women declined to answer the posttest questions about mastectomy (not applicable, NA). In the interval (a maximum of 12 months) between joining the study and answering the posttest questionnaire, some participants were diagnosed with cancer.
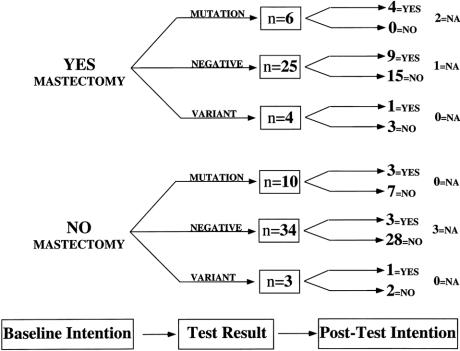
Figure 4. Decision or intention tree for women considering prophylactic mastectomy. Eighty-two women expressed their attitude toward prophylactic mastectomy at baseline (baseline intention); of those, 35 considered a preventive mastectomy (yes, mastectomy) and 47 were not inclined toward prophylactic mastectomy (no mastectomy). After counseling and testing, the attitudes of women were again discerned, shown for mutation carriers (mutation), women testing negative (negative), and women who had an uncharacterized variant (variant) in BRCA1 or BRCA2. NA, did not answer the question or had a therapeutic mastectomy during the study.
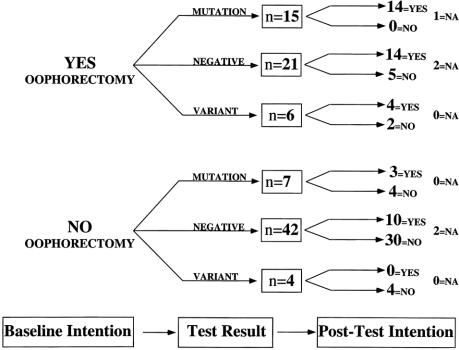
Figure 5. Decision or intention tree for women considering prophylactic oophorectomy. Ninety-five women who completed testing expressed their attitudes toward prophylactic oophorectomy at entry into the counseling study (baseline intention) and after counseling and testing; of those, 42 considered a prophylactic oophorectomy (yes, oophorectomy) and 53 decided against prophylactic oophorectomy (no oophorectomy). After counseling and testing, the attitudes of women were again discerned, shown for mutation carriers (mutation), women testing negatively (negative), and women who had an uncharacterized variant (variant) in BRCA1 or BRCA2. NA, did not answer the question or had a therapeutic oophorectomy during the study.
Figure 4 shows the intentions of women regarding preventive mastectomy before counseling and testing and after posttest counseling. As expected, those interested in the procedure at baseline remained interested if their test result was positive. However, 15 of 25 women (60%) who were interested in preventive mastectomy before counseling and testing, but received a negative test result, changed their intentions and no longer expressed interest in the procedure. Seven of 10 (70%) of women who initially expressed little regard for preventive mastectomy remained uninterested despite a positive test result. Keeping in mind that these women were previously affected with one breast cancer or had a history of ovarian cancer, this reluctance to change attitudes is perhaps not surprising.
Figure 5 depicts plans of women regarding preventive oophorectomy before and after counseling and testing. As with mastectomy, those who were interested at baseline and had a positive test result remained interested in preventive surgery. However, unlike prophylactic mastectomy, interest remained high for 14 of 21 women (66%) even though testing for BRCA1 and BRCA2 revealed no disease-associated mutations. Women not interested in prophylactic oophorectomy before testing generally remained uninterested after testing and counseling. Among women who were not interested in oophorectomy at baseline, only two of seven who tested positive for mutation changed their minds and expressed interest in oophorectomy after testing.
DISCUSSION
The current report presents preliminary data from a project examining the optimal mode of communicating information about breast and ovarian cancer genetics and genetic testing to women at high risk for these diseases. One component of this project is the provision of free genetic testing to participants. Acceptance of free genetic testing is very high in our study; 96% decided to be screened for BRCA1 or BRCA2 mutations. This figure is higher than those reported in other studies of women attending high-risk clinics 20,21; although cost is undoubtedly a factor, in the study by Lerman et al, the results of genetic screening was free to patients. 20 In that study, 43% of women from hereditary breast and ovarian cancer families requested BRCA1 results when they were offered. The study included a large number of unaffected men and women, which may have altered the decision to receive test results. In this report, only women affected by breast or ovarian cancer were examined. Women in our sample may be less concerned with insurability, confidentiality, and the psychological detractions of genetic testing.
Free genetic testing offered in the context of a clinical trial removes the need to request insurance coverage for the cost of the test, or to require that people interested in testing pay out of pocket. Beyond concerns about the cost of testing, our participants may have felt a higher degree of confidence about confidentiality and privacy. The ability to cover the cost of testing, done with some guarantee of confidentiality, may allow greater and more unbiased participation in genetic research of a particularly sensitive nature.
Our counseling project was limited to women affected with breast or ovarian cancer and with a family history of these diseases. Relatives of gene carriers were invited to participate in later phases of the study, and will be reported subsequently. Other entry criteria allowed participation of affected women with very early-onset breast cancer or Ashkenazi Jewish women with breast cancer before the age of 40 years. Overall, entry measures were designed to recruit affected women whose probability of carrying a mutation in BRCA1 or BRCA2 was greater than 10%. In fact, the average carrier probability of the affected participants exceeded 20%. At the time of this report, 213 affected women have been screened for mutations resulting in 44 carriers (20.6%). This proportion of gene carriers approximates the expected number, based upon estimates of carrier frequencies in our population. The proportion of carriers is smaller than a similar tested cohort reported by Myriad Genetic Laboratories. 22 In that study, women were eligible if they were affected with invasive breast cancer before age 50 or had ovarian cancer at any age and had at least one first- or second-degree relative with breast or ovarian cancer. Ninety-four of 238 tested women had BRCA1 or BRCA2 mutations (39%). Roughly, two thirds of the mutations were found in BRCA1 and one third in BRCA2. Therefore, the proportion of BRCA1 and BRCA2 mutations is similar in our study, but the total number of mutations is less. It is likely that some of the discrepancy is due to the use of mutation screening in our study and the provision of full sequencing by Myriad Laboratories.
Women in our study overestimated their chances of carrying a mutation in BRCA1 or BRCA2. This was similar to findings in our first 100 patients, reported earlier, 14,15 and to findings from other investigators. 20,23,24 In a previous study, we examined determinates of overestimation and found that neither race (white vs. nonwhite), age, nor previous testing in a family member predicted overestimation. The only predictor of a distorted self-estimate of carrier probability was the presence of two or fewer affected relatives. Women with three or more affected relatives were significantly less likely to overestimate their own risks of carrying a mutation. 15
There are few studies of decision-making after testing for breast and ovarian susceptibility genes. In the study by Lerman et al cited earlier, carriers and noncarriers reported their intentions to obtain either prophylactic mastectomy or oophorectomy: 17% of carriers intended to have prophylactic mastectomies, and 33% reported intention to obtain prophylactic oophorectomy. None of the noncarriers were interested in preventive surgery. 20 However, the noncarriers were all members of families with hereditary breast and ovarian cancer and passing a definite mutation. As noted previously, a negative test in the circumstance of a known mutation in the family can prevent unnecessary prophylactic surgery. 3,25
In our study, gene carriers who were initially interested in prophylactic surgery were nearly uniform in their intention to undergo preventive surgery after a positive test result. Even a positive result, however, did not persuade the majority of women, who entered with little or no interest in prophylactic surgery. Because our study included probands, without a prior mutation ascertained in their families, a negative result is less helpful. Most women who underwent genetic counseling and testing, and tested negatively, decided against preventive mastectomy at the time of follow-up. This includes 15 of 25 women initially interested in mastectomy who changed their minds after receiving a negative test result and at least two counseling sessions. In contrast, the majority of women who expressed interest in oophorectomy at baseline maintained their interest in the procedure, despite a negative BRCA1 or BRCA2 test.
Because screening for breast cancer is an effective means to reduce mortality among women in general (although there is still no data on its efficacy in mutation carriers), counseling included a discussion of the proven benefits of mammography. 25,26 There are no screening strategies proven to reduce the mortality from ovarian cancer. 27 Counseling for both proven carriers and those who test negatively included a review of the limitations of ovarian cancer screening. 3,27 As Haber 28 noted, we still know far too little about the clinical interventions that may reduce risk or improve diagnosis and treatment among women with known BRCA1 or BRCA2 mutations.
Moreover, there has been far too little research on appropriate strategies for helping women make decisions about possible preventive strategies after the determination of a genetic mutation. In the only experimental study we identified in the literature, Stalmeiere et al 29 showed that a shared decision-making program increased women’s accurate risk perception and knowledge about prophylactic surgery to prevent breast and ovarian cancer. It also reduced decision uncertainty. A vignette study recently reported by Stefanek et al 30 found that women at increased risk for breast cancer wanted to be informed about options for preventive surgery. When given the choice of prophylactic mastectomy or careful surveillance, however, most (75%) said they would opt for the latter. Additional research is needed in this area in order to guide clinical practice and facilitate informed patient decision-making.
It is important to recognize that women who test negatively may still harbor genetic factors responsible for the high incidence of breast or ovarian cancer in their families. Current tests, even complete sequencing, can miss mutations in noncoding regions of the BRCA1 or BRCA2 genes. Large deletions of genetic material within these genes can be missed by both sequencing and conformational methods of screening for mutations. And undiscovered genes, beyond BRCA1 or BRCA2, may exist and be responsible for certain families with many cases of breast cancer. 31–33 The distribution of BRCAPRO-calculated carrier probabilities among women who test negative for BRCA1 or BRCA2 mutations or contain uncharacterized variants (Figure 3) clearly reveals a group of individuals, and their families, who are candidates for more intensive investigation.
Discussion
Dr. Walter D. Holder (Charlotte, North Carolina): This is a very interesting study done by Dr. Iglehart and his colleagues of the BRCA1 and BRCA2 gene mutations and genetic counselling at Duke.
This study once again emphasizes the difficulty in predicting who are BRCA1-positive or BRCA2 mutation carriers without actually doing the genetic studies on all these individuals. It also shows the regional differences that one sees in doing genetic analysis and looking at risks. A critical aspect of this study is that testing was done without charge to these patients.
We continue to see patients who have a high risk of developing breast cancer and ovarian cancer, and we have encountered a number of women who really did not want to be tested. A variety of reasons are usually expressed, including, number one, insurance companies use the information against them to deny coverage. Number two, the lack of trust in doing the testing: “If it’s negative, I can still get breast cancer” or “I can still get ovarian cancer.” And number three, “I simply can’t put my family through this testing and all the uncertainty and stress of doing this.” And, finally, the cost of doing the testing. Dr. Iglehart, would you comment on these issues and how you deal with them?
Finally, how do you deal with a patient who really, in your perception, is a very high risk for breast cancer and ovarian cancer but refuses to have testing or refuses to consider prophylactic surgery?
Dr. Kirby I. Bland (Birmingham, Alabama): I want to congratulate the group from Duke for their continued approach in investigation of this model, particularly the BRCA1 and 2 gene.
For those of you in the audience who aren’t familiar with this approach, BRCA1/2 is a guardian, if you will, of the human genome as a tumor suppressor gene, much like the p53 is in colorectal carcinoma. We see these same mutations in breast carcinoma as well as in the BRCA1/2 genome for germline mutations.
Dirk, I would like to know, however—for those of us in clinical practice—typically when we have tested for a genetic profile, this obviously brings a group of patients into the situation where they may or may not want to consider testing. But if their risk profile is great enough with indications, they actually go forward and have this done. It is not only expensive, but once you have completed testing and it is positive, it is highly probable that the circumstance would lead to loss of insurability in certain states. Further, there are actually actions being taken in Congress to protect patients from this important event. So my question would be, how far should we go with the profiled individual who actually has not considered genetic profile testing for breast or ovarian carcinoma—a patient who comes into an office and finds that she has significant high-risk parameters—in what direction should we go with this presentation relative to testing the genetic coding?
Secondly, I think the surgeons in this audience would like to know, if the patient tests BRCA1- or 2-positive, what should we actually have done thereafter? Would you do bilateral prophylactic mastectomies in patients with normal mammograms if they should have such a confirmatory genetic profile?
This is very important subject matter. It really opens the door as to how best to treat these individuals and their genetic relations, because as you know in the next 3 years, very possibly the entire human genome will be categorized by Francis Collins and his group at the National Cancer Institute. So I think there is a great opportunity for future stratification of these patients relative to their risk for primary and comorbid disease (such as ovarian cancer). Please give us your thoughts on these issues.
Dr. Edward M. Copeland III (Gainesville, Florida): Dirk, I think I can summarize the things I want to know about BRCA1 and 2 testing in two or three categories: Number one, does insurance pay? Number two, who should be tested? Number three, if you have a strong family history of breast cancer but you are BRCA1- and 2-negative, what are your risks of getting breast cancer? There must be some other gene out there that also causes breast cancer.
And if you have breast cancer and you are BRCA1- or 2-positive, what operation should you have? Can you do breast-conserving therapy in a patient who is BRCA1- and 2-positive?
Dr. J. Dirk Iglehart (Closing Discussion): These questions go to the heart of a new era in medical care. We can test people for susceptibility to a variety of different diseases, whether it’s atherosclerosis or heart attack, stroke or cancer. But what do you do with the information once you get it?
Let me go one by one. Walter Holder asked about the negative aspects of testing, and Dr. Bland and Dr. Copeland also wondered what the negative aspects of testing might be.
For some people, this is information they don’t want. Some people don’t want to know what their chances might be of getting a disease 5 or 10 years down the road. This is information that is particular to families. If your sister decides to have a BRCA1 or 2 test, and if she tests positively, then you and all your siblings have a 50% chance of carrying that mutation. That may be information your family does not want to have, but they are committed to having it if the individual goes ahead and is tested.
The second question, and it’s a related question that I think all the discussants brought up, is the issue of the approach you take, both to those people who come from a high-risk family and test negative, as well as the approach to those people who test positively.
First, let’s take those who test negatively but come from a high-risk family. There are a number of possibilities. One, the test missed the mutation, which is possible. For our screening method, we estimate that we pick up at least 75% of the deleterious mutations in these two genes. We definitely don’t pick up 100%. So it may be that we just missed the mutation.
The other possibility is another gene, BRCA3 or a BRCA4. It’s possible that some uncharacterized variants we discovered might actually be disease-producing. So there are a number of reasons why people may test negatively in the presence of a strong family history. I don’t think it eliminates a genetic risk.
The other question that everybody wants to know is what to do with patients who are positive. Perhaps you can tell from the study, we tend toward favoring prophylactic oophorectomy, and we probably tend to dissuade people from having prophylactic mastectomy.
I think the reasons are clear. Ovarian cancer is a deadly disease, and there is no proven and worthwhile screening procedure for ovarian cancer, whereas breast cancer is a very curable disease for which there is a very good screening modality. In fact, from the Breast Cancer Detection Demonstration Program, 80% of women with breast cancer found as a result of screening will be cured of the disease. Furthermore, it is well known that even if you carry a mutation in BRCA1 or 2, your lifelong chances of getting breast cancer are probably about 50%. So you have an 80% chance of being cured of a disease that you have about a 50% chance of getting. I think for an individual woman who is facing those numbers, most of them are going to decide against having both of their breasts removed. The general approach would be to think about oophorectomy, probably in a woman who has had children, who perhaps is entering her forties. Those women probably should have hormone replacement therapy after they have their oophorectomy. And I probably would be less inclined towards recommending a bilateral prophylactic mastectomy.
The final question that was brought up by President Copeland, and I also think by Walter and others, was the question about insurability. Right now, I think three quarters of insurance carriers will pay for a BRCA1 or 2 test if you ask them to pay for it. There is only one provider of testing, Myriad Genetics. We did it for free at Duke, but if you are going to pay for it, it costs about $2500 by Myriad Genetics.
It’s not just the $2500—it’s letting your insurance company know you are going to be tested for a genetic disease. I think that’s the issue. Our 96% uptake in the testing is high, but it’s probably high because we did it for free and could be entirely confidential. However, if a woman who has had a genetic test is asked if she has ever been tested for a genetic disease, and she answers no, she may have committed insurance fraud. So this is information that is going to get out there, and I think everybody has to realize that.
We are trying to do testing in as confidential a manner as possible so we can learn everything we can. The fact is, all of us will face this issue in all aspects of clinical practice.
Footnotes
Correspondence: J. Dirk Iglehart, MD, Dept. of Surgery, Brigham and Women’s Hospital, 75 Francis St., Boston, MA 02115.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Supported by the Duke Specialized Program of Research Excellence (SPORE) in Breast Cancer from the National Cancer Institute.
E-mail: jiglehart@partners.org
Accepted for publication December 1999.
References
- 1.Lerman C, Kash K, Stefanek M. Younger women at increased risk for breast cancer: perceived risk, psychological well-being, and surveillance behavior. J Natl Cancer Inst 1994; 16:171–176. [PubMed] [Google Scholar]
- 2.Lerman C, Daly M, Masny A, et al. Attitudes about genetic testing for breast-ovarian cancer susceptibility. J Clin Oncol 1994; 12:843–850. [DOI] [PubMed] [Google Scholar]
- 3.Biesecker BB, Boehnke M, Calzone K, et al. Genetic counseling for families with inherited susceptibility to breast and ovarian cancer. JAMA 1993; 269:1970–1974. [PubMed] [Google Scholar]
- 4.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994; 266:66–71. [DOI] [PubMed] [Google Scholar]
- 5.Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995; 378:789–792. [DOI] [PubMed] [Google Scholar]
- 6.Easton D, Ford D, Bishop T, et al. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Am J Hum Genet 1995; 56:265–271. [PMC free article] [PubMed] [Google Scholar]
- 7.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408. [DOI] [PubMed] [Google Scholar]
- 8.Thorlacius S, Struewing J, Hartge P, et al. Population-based study of risk of breast cancer in carriers of BRCA2 mutations. Lancet 1998; 352:1337–1339. [DOI] [PubMed] [Google Scholar]
- 9.Warner E, Foulkes W, Goodwin P, et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenzai Jewish women with breast cancer. J Natl Cancer Inst 1999; 91:1241–1247. [DOI] [PubMed] [Google Scholar]
- 10.Parmigiani G, Berry DA, Aquilar O. Determining carrier probabilities for breast cancer susceptibility genes BRCA1 and BRCA2. Am J Hum Genet 1998; 62:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roa B, Boyd A, Volcik K, Richards C. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet 1996; 14:185–187. [DOI] [PubMed] [Google Scholar]
- 12.Szabo CI, King M-C. Population genetics of BRCA1 and BRCA2. Am J Hum Genet 1997; 60:1013–1020. [PMC free article] [PubMed] [Google Scholar]
- 13.Panguluri RCK, Brody LC, Modaly R, et al. BRCA1 mutations in African Americans. Hum Genet 1999; 105:28–33. [DOI] [PubMed] [Google Scholar]
- 14.Iglehart JD, Miron A, Rimer BK, et al. Overestimation of hereditary breast cancer risk. Ann Surg 1998; 228:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluman LG, Rimer BK, Berry D, et al. Attitudes, knowledge and risk perceptions of women with breast and/or ovarian cancer considering test for BRCA1 and BRCA2. J Clin Oncol 1999; 17:1040–1046. [DOI] [PubMed] [Google Scholar]
- 16.Berry DA, Parmigiani G, Sanchez J, et al. Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. J Natl Cancer Inst 1997; 89:227–238. [DOI] [PubMed] [Google Scholar]
- 17.Korkko J, Annunen S, Pihlajamma T, et al. Conformation sensitive gel electrophoresis for simple and accurate detection of mutations: comparison with denaturing gel electrophoresis and nucleotide sequencing. Proc Natl Acad Sci U S A 1998; 95:1681–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breast Cancer Information Core. Online BRCA 1/2 mutation database: http://www.nhgri.nih.gov/Intramural research/Lab transfer/Bic/
- 19.Rimer BK, Glassman B. Is there a use for tailored print communications in cancer risk communication? J Natl Cancer Inst Monogr 1999; 25:140–148. [DOI] [PubMed] [Google Scholar]
- 20.Lerman C, Narod S, Schulman K, et al. BRCA1 testing in families with hereditary breast-ovarian cancer: a prospective study of patient decision making and outcomes. JAMA 1996; 275:1885–1892. [PubMed] [Google Scholar]
- 21.Lerman C, Seay J, Balshem A, Audrain J. Interest in genetic testing among first-degree relatives of breast cancer patients. Am J Med Genet 1995; 57:385–392. [DOI] [PubMed] [Google Scholar]
- 22.Frank T, Manley S, Olufunmilayo I, et al. Sequence of analysis of BRCA1 and BRCA2: correlation of mutations with family history and ovarian cancer risk. J Clin Oncol 1998; 16:2417–2425. [DOI] [PubMed] [Google Scholar]
- 23.Hallowell N, Statham H, Murton F. Women’s understanding of their risk of developing breast/ovarian cancer before and after genetic counseling. J Genet Couns 1998; 7:345–352. [DOI] [PubMed] [Google Scholar]
- 24.Hallowell N, Statham H, Murton F, et al. “Talking about chance”: the presentation of risk information during genetic counseling for breast and ovarian cancer. J Genet Couns 1997; 6:269–275. [DOI] [PubMed] [Google Scholar]
- 25.Clark S, Iglehart JD. Genetic counseling for breast cancer. Adv Surg 1999; 33:199–215. [PubMed] [Google Scholar]
- 26.Smart C, Byrne C, Smith R, et al. Twenty-year follow-up of the breast cancers diagnosed during the breast cancer detection demonstration project. CA Cancer J Clin 1997; 47:134–149. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal A, Jacobs I. Ovarian cancer screening. Semin Oncol 1998; 25:315–325. [PubMed] [Google Scholar]
- 28.Haber DA. Breast cancer in carriers of BRCA1 and BRCA2 mutations: tackling a molecular and clinical conundrum. J Clin Oncol 1999; 17:3367–3370. [DOI] [PubMed] [Google Scholar]
- 29.Stalmeier PFM, Unic IJ, Verhoef LCG, et al. Evaluation of a shared decision-making program for women suspected to have a genetic predisposition to breast cancer: preliminary results. Med Decis Making 1999; 19:230–241. [DOI] [PubMed] [Google Scholar]
- 30.Stefanek M, Enger C, Benkendorf J, et al. Bilateral prophylactic mastectomy decision-making: a vignette study. Prev Med 1999; 29:216–221. [DOI] [PubMed] [Google Scholar]
- 31.Phelan CM, Lancaster JM, Tonin P, et al. Mutation analysis of the BRCA2 gene in 49 site-specific breast cancer families. Nat Genet 1996; 13:120–122. [DOI] [PubMed] [Google Scholar]
- 32.Schubert EL, Lee MK, Mefford HC, et al. BRCA2 in American families with four or more cases of breast or ovarian cancer: recurrent and novel mutations, variable expression, penetrance, and the possibility of families whose cancer is not attributable to BRCA1 or BRCA2. Am J Hum Genet 1997; 60:1031–1040. [PMC free article] [PubMed] [Google Scholar]
- 33.Vehmanen P, Friedman LS, Eerola H, et al. Low proportion of BRCA1 and BRCA2 mutations in Finnish breast cancer families: evidence for additional susceptibility genes. Hum Mol Genet 1997; 6:2309–2315. [DOI] [PubMed] [Google Scholar]