Abstract
Objectives
To examine the safety of transthoracic esophagogastrectomy (TTE) in a multidisciplinary cancer center and to determine which clinical parameters influenced survival and the rates of death and complications.
Summary Background Data
Although the incidence of cancer at the gastroesophageal junction has been rising rapidly in the United States, controversy still exists about the safety of surgical procedures designed to remove the distal esophagus and proximal stomach. Alternatives to TTE have been proposed because of the reportedly high rates of death and complications associated with the procedure.
Methods
Data from 143 patients treated by TTE by one author (1989–1999) were entered into a computerized database. Preoperative clinical parameters were tested for effect on death, complications, and survival.
Results
The patient population consisted of 127 men and 16 women. One hundred twenty-one patients had a history of tobacco abuse, and 118 reported the regular ingestion of alcohol. One hundred fifteen patients had adenocarcinoma, 16 had squamous cell cancer, 6 had another form of esophageal tumor, and 6 had high-grade dysplasia associated with Barrett epithelia. Fifty-six patients had adenocarcinomas arising in Barrett epithelium. Twenty-eight patients were treated with neoadjuvant chemoradiation before surgery. Three patients died within 30 days of surgery (mortality rate 2.1%). Five patients (3.5%) had a documented anastomotic leak; three died). Overall, 42 patients had complications (29%). Twenty-six had pulmonary complications (19%). The mean length of stay in the intensive care unit was 3.35 days; the mean hospital length of stay was 13.54 days. The overall 3-year survival rate was 29.6%.
Conclusions
A high ASA score and the development of complications predicted an increased length of stay. The presence of diabetes predicted the development of complication and an increased length of stay. None of the other parameters tested predicted perioperative death or complications. Only disease stage, diabetes, and blood transfusion affected overall survival. From these results with a large series of patients with gastroesophageal junction cancers, TTE can be performed with a low death rate (2.1%), a low leak rate (3.5%), and an acceptable complication rate (29%).
The incidence of carcinoma at the gastroesophageal (GE) junction, its histologic type, and its management have changed remarkably in the past two decades. In the United States, the incidence of adenocarcinoma of the distal esophagus in the setting of Barrett epithelium, first described in the summer of 1957 and thought to be a curiosity until the 1970s, has increased dramatically. 1,2 At the same time, a slight decrease in the incidence of squamous carcinoma of the esophagus has been noted. 3–5 Further, although the overall incidence of gastric carcinoma has diminished during several decades, the incidence of adenocarcinoma in the proximal stomach has increased. 5,6
During the same period, the management of carcinoma near or at the GE junction has evolved. Many surgeons have adopted the transhiatal approach as originally described by Dent in 1913, first performed by Turner in 1933, and subsequently popularized by Orringer. 7,8 In several studies, this procedure has been found to be equivalent in terms of death and complication rates and subsequent survival to the combined abdominal and thoracic approach originally described by Ivor Lewis in 1946. 7,9,10
While the disease and its surgical management were changing, so were the methods of diagnosis and preoperative evaluation. Endoscopy, endoscopic ultrasound, and computed tomography have replaced the upper gastrointestinal series as the imaging methods of choice. Preoperative pulmonary and cardiac assessments have become more sophisticated. Each of these techniques has led to better patient selection and improved surgical death rates. Finally, reports in some studies of improved survival in patients undergoing preoperative chemoradiation therapy followed by surgical resection compared with those undergoing surgery alone have stimulated the use of neoadjuvant treatment and the institution of prospective randomized trials to examine this question. 11,12
Given these changing variables in cancer at the GE junction, we sought to examine our results during a 10-year period using a consistent surgical approach by one surgeon. We were particularly interested in which clinical parameters affected the rates of death and complications and survival after Ivor Lewis resection.
METHODS
Data from 143 patients with neoplasia at or near the GE junction undergoing Ivor Lewis resection were entered into a computerized database. Data collected included patient demographics, history of weight loss, history of smoking and/or alcohol use, presence of comorbid disease, and preoperative nutritional history. Preoperative laboratory studies included serum albumin, pulmonary function tests, routine chemical and complete blood count panels, and anesthesia risk assessment by the American Society of Anesthesia (ASA) criteria. 13 Twenty-eight patients underwent preoperative neoadjuvant chemoradiation therapy with a combination of 5-fluorouracil and cisplatinum or a continuous infusion of 5-fluorouracil and 45 to 60 Gy radiation.
The surgical procedure was begun through a bilateral subcostal incision. The stomach was completely mobilized, the right gastric and right gastroepiploic vessels were preserved, a Kocher maneuver was performed, and a feeding jejunostomy was inserted. Either a pyloromyotomy or a Heineke-Mikulicz pyloroplasty was used as a gastric drainage procedure. The abdomen was then closed, the right side of the chest was prepared and draped, and the chest was entered through the right sixth intercostal space. The esophagogastrostomy was performed above the level of the azygos vein using a single layer of interrupted 3-O silk sutures.
Surgical data, including operative time, blood loss, transfusions (during and after surgery), and type of drainage procedure (pyloroplasty or pyloromyotomy), were collected. Tumor histology and tumor stage were determined by the TNM classification system. 14 On day 7 after surgery, an esophagogram was performed with meglumine diatrizoate followed by barium. 15,16 Complications, the 30-day death rate, the length of stay (LOS) in the intensive care unit and the hospital, and evidence for anastomotic leaks were tabulated. Follow-up was available for 139 patients. They were assessed for strictures with need for dilatation, need for enteral feeding by a jejunostomy tube after discharge (as assessed by the clinical dietitian), and survival.
Survival and LOS estimates were generated using Kaplan-Meier survival analysis methodology. The log-rank test was used to test for differences in survival distributions; the Fisher exact test was used for differences in complication rates. 17–19
RESULTS
Patient Population
From 1989 through March 1999, 143 patients underwent Ivor Lewis resection. During this same period, 40 patients were treated for GE junction cancers with other types of surgical procedures, either a transhiatal approach for patients with high extension of tumor or Barrett epithelium, or abdominal-only resections for palliation. In the Ivor Lewis study group, there were 127 men and 16 women. Age at time of surgery averaged 63.7 years (range 33–83, median 65.9).
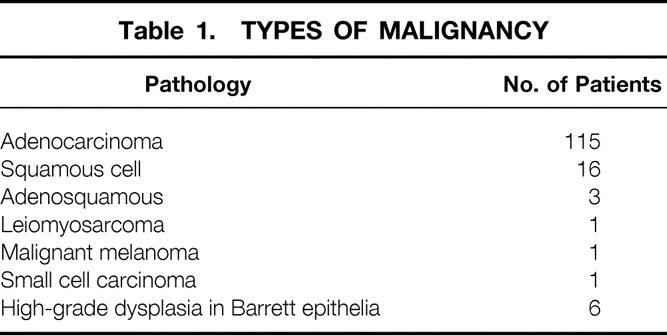
One hundred fifteen patients had adenocarcinoma at the GE junction: 56 were in the setting of Barrett epithelium and 59 were thought to arise in the cardia. Sixteen patients had squamous cell carcinoma and six patients had other types of esophageal malignancies (Table 1). Six patients underwent resection for high-grade dysplasia in Barrett esophagus and no malignancy was found in the surgical specimen; these patients were excluded from survival analysis.
Table 1. TYPES OF MALIGNANCY
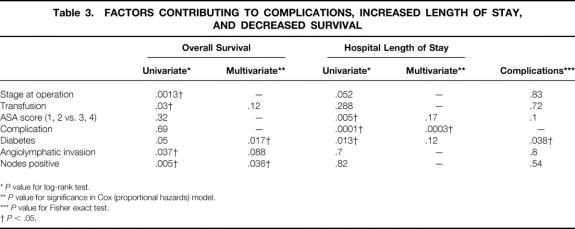
The pathologic stage for resected tumors is listed in Table 2. Angiolymphatic invasion was identified in 25 patients and lymph node metastasis in 79 patients. These findings significantly correlated with poor survival (P = .037 and .005, respectively;Table 3).
Table 2. TNM STAGING CLASSIFICATION
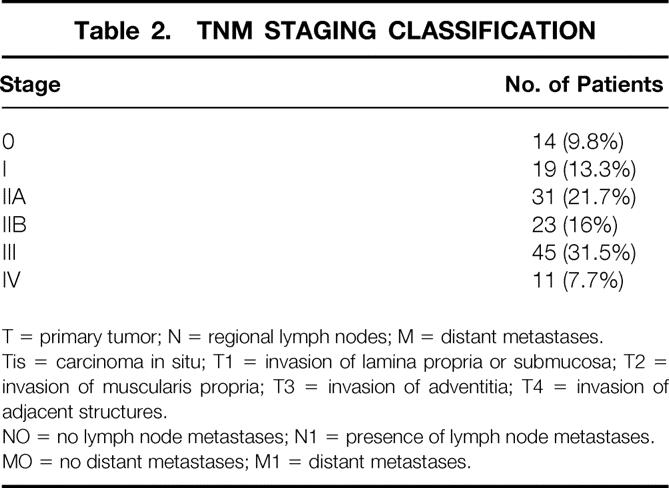
T = primary tumor; N = regional lymph nodes; M = distant metastases.
Tis = carcinoma in situ; T1 = invasion of lamina propria or submucosa; T2 = invasion of muscularis propria; T3 = invasion of adventitia; T4 = invasion of adjacent structures.
NO = no lymph node metastases; N1 = presence of lymph node metastases.
MO = no distant metastases; M1 = distant metastases.
Table 3. FACTORS CONTRIBUTING TO COMPLICATIONS, INCREASED LENGTH OF STAY, AND DECREASED SURVIVAL
*P value for log-rank test.
**P value for significance in Cox (proportional hazards) model.
***P value for Fisher exact test.
†P < .05.
One hundred twenty-one patients had a history of tobacco abuse, and 118 reported regular ingestion of alcohol. Forty had a history of weight loss greater than 10% of normal body weight before the onset of symptoms. Fifteen patients had type II diabetes. Of these variables (age older than 70 years, weight loss more than 10%, smoking and alcohol history, diabetes), only diabetes was predictive of complications, increased LOS, and decreased long-term survival. The median LOS was 13 days versus 11 days for patients with diabetes versus those without diabetes (P = .013); median survival was 0.64 years for patients with diabetes and 1.6 years for those without (P = .05).
Preoperative Testing
Serum albumin was less than 3.5 mg% g/dL in 10 patients. Eleven patients had a forced expiratory volume (FEV1) less than 1.5 L/min. Neither variable was predictive of pulmonary complication or the need for prolonged enteral nutrition after discharge from the hospital. An ASA score greater than 2 was predictive of an increased hospital LOS (high ASA = 13 days, low ASA = 11 days;P = .005).
Neoadjuvant Therapy
Preoperative neoadjuvant treatment was delivered to 28 patients. There was no difference in the rate of complications in these patients, and none of them died in the immediate postoperative period. There were complete pathologic responses in eight patients (28.6%). This treatment did not appear to affect the rate of complications (P = .46) or death (P = .69). Survival is discussed below.
Surgical Parameters
Operative times, measured from induction of endotracheal anesthesia until transfer to the recovery room, averaged 327 minutes (range 160–560, median 315). There was no correlation between the length of surgery and complications, death, or long-term survival. Blood loss was a mean of 481 mm3 (median 400, range 100–2,800). Transfusions were given to 10 patients. The need for transfusions did not affect death or complication rates but decreased survival (median survival with transfusions 0.4052 years, median survival without transfusions 1.62 years;P = .03). One patient required reoperation for bleeding from a short gastric vessel on the greater curvature of the stomach. Two patients underwent splenectomy, one to remove enlarged nodes in the hilum of the spleen and the other as a result of injury to the splenic artery during dissection around the left gastric vessels. Neither patient had a postoperative complication or death.
Deaths
Three patients (2.1%) died within 30 days of surgery. Two had diabetes. There were no in-hospital deaths after 30 days.
One patient died of infarction of the right colon. Jejunostomy tube feedings were started on day 2 after surgery, and an acute abdomen became evident on postoperative day 4. A Gastrografin swallow was interpreted as showing a small contained leak at the esophagogastric anastomosis. A right hemicolectomy for infarction was performed, but the patient died of multisystem organ failure. At the post mortem examination, no leak was found at the anastomosis.
Another patient died on postoperative day 23. She had unexpected advanced cirrhosis at the time of resection. An esophagogram was negative on day 7 after surgery. Sepsis, liver failure, and then multisystem organ failure developed. The findings on repeat esophagograms were negative until postoperative day 17, when a leak was detected. The patient died the same day.
A third patient died of a massive cerebrovascular accident. The patient did well for 4 days, then neurologic symptoms developed. No leak was identified. Nutritional support was ultimately discontinued on postoperative day 20, and the patient was allowed to die.
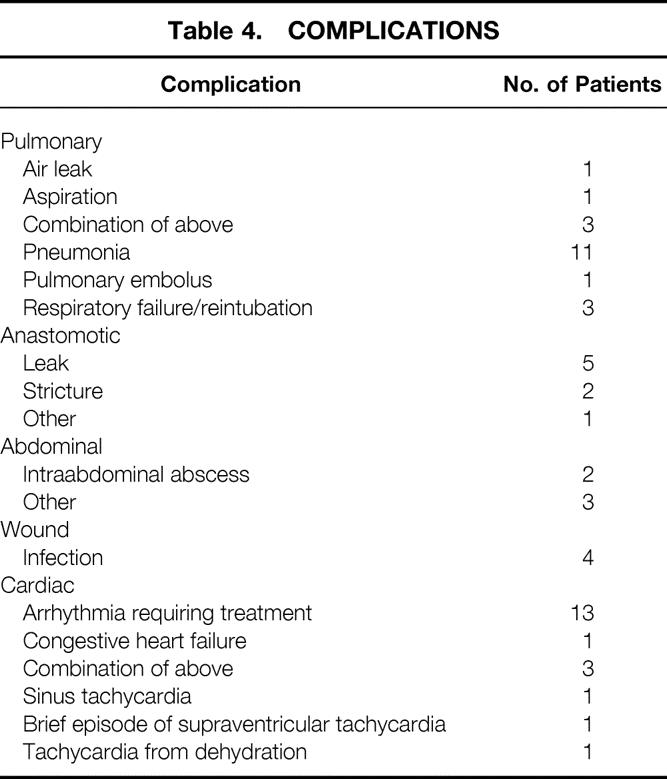
Complications
Fifty-seven complications developed in 42 patients (29%) (Table 4). Twenty (14%) patients had pulmonary complications. Days receiving respirator assistance for patients with pulmonary complications ranged from 3 to 30 days. Only diabetes was predictive of complications (pulmonary:P = .006; cardiac:P = .004).
Table 4. COMPLICATIONS
Anastomotic Leaks
A routine esophagogram on postoperative day 7 showed that four patients had anastomotic leaks. Another leak was detected on postoperative day 17. As discussed above, leaks were diagnosed in two patients who died. One was not confirmed at autopsy and the other developed just as the patient died of multiple organ failure.
Three leaks were more “radiographic” than physiologic. These patients had small controlled leaks into the mediastinum without evidence of sepsis. They were treated by delay in oral feeding, jejunostomy feedings, and antibiotic coverage. Liquids were started on postoperative day 10 to 12 and the patients were discharged home on days 11, 15, and 16.
Thus, five patients (3.5%) had radiographic evidence of a leak sometime during their postoperative course. If one includes the patient who died of a right colon infarction, in whom a leak was originally diagnosed but not confirmed at autopsy, then four patients had leaks that were seen on x-ray that did not affect the patient’s course.
Length of Stay
Mean hospital LOS was 13.54 days (median 11, range 7–55). Mean intensive care unit LOS was 3.35 days (median 2, range 1–31). Both a high ASA score and complications were predictive of an increased hospital LOS. Diabetes was predictive of both complications and increased LOS (see Table 3).
Enteral Feeding
A clinical nutritionist judged that 31 patients (21.7%) required enteral feeding supplementation after hospital discharge. These feedings were discontinued when adequate oral intake was achieved, usually within 3 weeks after discharge. None of the preoperative or intraoperative parameters recorded, including weight loss, albumin, and neoadjuvant therapy, were predictive of the need for enteral feeding after discharge.
Type of Gastric Emptying Procedure
There was no significant difference between pyloromyotomy and pyloroplasty in terms of LOS, need for postoperative enteral tube feedings, or survival (p = .57, .68, and .21, respectively).
Strictures
Fourteen patients (10%) required subsequent dilatation of their anastomosis at our institution. Our follow-up for dilatations at other institutions is incomplete, so the true stricture rate cannot be calculated.
Survival
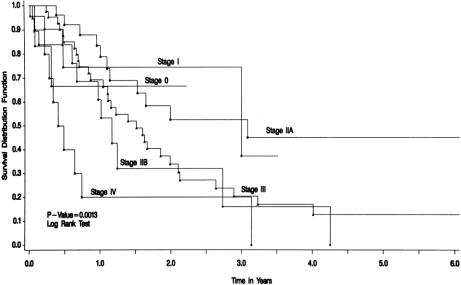
Median survival (excluding the six patients who underwent surgery for high-grade dysplasia only, in whom no invasive malignancy was found) was 1.60 years (25% = 0.69 years, 75% = 3.23 years; mean 2.02 years) (Fig. 1).
Figure 1. Survival by stage for patients with adenocarcinoma and squamous cell carcinoma. Patients with high-grade dysplasia only were excluded. Median survival was 1.60 years (stage I = 3.0 years, IIA = 3.08 years, IIB = 1.17 year, III = 1.51 years, and IV = 0.44 years). Mean survival was 2.02 years.
Only disease stage and blood transfusion affected overall survival. Median survival for patients with stage I disease was 3.0 years, IIA was 3.08 years, IIB was 1.17 years, III was 1.51 years, and IV was 0.44 years. Median survival was 0.41 years for patients receiving blood transfusions and 1.62 years for those not transfused (P = .03).
Median survival was 2.0 years for patients treated with neoadjuvant therapy and 1.5 years for those not treated with preoperative chemoradiation (P = .69). Mean survival for patients who had a complete response to neoadjuvant therapy was 2.7 years (P = .93). These differences were not significant.
DISCUSSION
The Ivor Lewis operation was first described in 1946, 9,20 and we have used it predominantly for the past 10 years. Like others, we have found the procedure to be a safe, practical “middle ground” between the aggressive en bloc resections described by Skinner 21 and Akiyama et al 22 and the transhiatal resection, where some of the procedure is performed by blunt dissection. 8
We sought to determine factors that might predict poor outcome in patients with GE junction cancers. Specifically, we were interested in factors leading to leaks, the need for postoperative nutrition delivered by tube, and surgical death. In addition, we hoped to identify factors affecting long-term survival.
Our patient population emphasizes the shift in demographics reported by others in the Western world. Most patients had a history of smoking and drinking, and most had adenocarcinomas. Alcohol intake has remained stable during this century in this country, but cigarette smoking rose steadily until the early 1970s. 23 Since then, the incidence of obesity has steadily increased. 23,24 The large percentage of patients reporting smoking and alcohol intake emphasizes the possible role of these factors in the increase of GE junction cancers now seen in the United States. Interestingly, smokers have a two- to threefold increase in the risk of esophageal adenocarcinoma, but the risk does not decrease after cessation of smoking, as it does for squamous cell carcinoma of the esophagus. 5,25–27 Obesity may be related to gastroesophageal reflux disease and the increased incidence of Barrett epithelium. 5
Of the preoperative parameters tested, only diabetes was predictive of complications, increased LOS, and decreased survival. Because diabetes was associated with complications, and two of the three hospital deaths, this disease had a significant effect on LOS and survival. Weight loss greater than 10% of initial weight, serum albumin levels of less than 3.5 mg% g/dL, and neoadjuvant therapy did not have an increased statistical risk for complications, perioperative death, the need for prolonged enteral nutrition, or decreased long-term survival.
The operation itself can be performed with safety and predictability. Operative times are not short, but the length of operation did not correlate with an adverse short- or long-term outcome. Transfusions were required in only 10 patients. Some patients had had significant bleeding from their primary tumors before surgery. Although perioperative transfusion was not associated with an increased complication rate or surgical death, it was a significant predictor of decreased overall survival. Some studies have found this effect in other gastrointestinal cancers; others have not. 28–33 Authors who have found an adverse effect of transfusion on survival after resection of gastrointestinal cancers have cited the immunomodulatory effect of allogeneic blood transfusion as an explanation. An interesting study in patients undergoing resection of gastric cancers found that blood transfusions had an adverse effect on patients with minimal residual disease in the bone marrow, but not in patients with negative bone marrow aspirations. 30 A study of patients undergoing esophagogastrectomy for cancer concluded that perioperative allogeneic blood transfusion had an adverse effect on short-term survival but not long-term survival. 34
The relation between tumor stage and outcome was highly significant. This fact underscores the essential conundrum in the management of patients with GE junction cancers: so many have advanced disease at the time of presentation that the likelihood of demonstrating improved results with a particular operation or treatment regimen is small.
Although only a few patients were treated with neoadjuvant therapy, several did have complete responses (28.6%). Nonetheless, there was no statistical difference between these patients and those who underwent surgery without preoperative therapy in terms of complications, death, or survival. In our experience, accrual of patients in prospective randomized trials to evaluate the efficacy of neoadjuvant therapy has been disappointingly low. This important biologic question deserves an early answer.
The three deaths that occurred after surgery are particularly vexing. The causes (infarcted intestine, cerebrovascular accident, liver failure) were different in each patient and were not directly related to a pulmonary complication or an anastomotic leak, although two of the patients had evidence of a leak on radiographic examination. In only one patient was anastomotic disruption related in any way to death, and even then it was a late contributory factor at best.
The role of routine postoperative Gastrografin meal in the diagnosis of leak is important, but evidence of extravasation in a patient who is not sick may not have much clinical relevance. 20 In the one patient who died on the day that a leak was identified (postoperative day 17, when all the other major organ systems had failed and the patient was hypotensive), the leak seemed to be more a sign of death rather than the cause of it. Conversely, four patients had leaks identified radiographically that seemed unrelated to their course. One died of another cause and no leak was found at autopsy. The others were not sick and had no significant delay in discharge. We conclude that patients doing well should be started on oral alimentation when the gastrointestinal tract becomes active, and that Gastrografin/barium swallow studies should be reserved for patients with signs of sepsis, pleural effusion, or hemodynamic instability. In this subset of patients, the study will show whether a leak is the cause of the deterioration.
The variety of complications encountered in this group of patients serves as a reminder of the relatively high risk of surgery in (generally) elderly patients with a history of smoking and alcohol use. In this study, angiolymphatic invasion and lymph node metastasis correlated with poor overall survival. This finding is in agreement with a similar observation in comparable studies of esophageal carcinomas. 35,36
Despite our hypothesis that we could predict which patients would be dependent on jejunostomy feedings after hospital discharge, neither weight loss history, serum albumin, type of gastric emptying procedure, nor neoadjuvant therapy was predictive of the need for enteral feedings. In the end, 21.7% of patients were dependent on tube feedings for survival. Patients generally disliked the jejunostomy tube unless they were dependent on it. We could not identify factors that might allow us to insert feeding tubes selectively.
There was no difference in the incidence of delayed discharge resulting from gastric emptying problems when pyloromyotomy was compared with pyloroplasty. This has encouraged us to use myotomy more commonly in recent years. It takes less time, we have had no leaks, and it can be converted to pyloroplasty if the mucosa is violated.
Overall survival was disappointing, although not different from that in most other reported series. 6,7,10,20,25,37,38 Because tumors in this part of the gastrointestinal tract are becoming more common, new ways to discover cancers at an earlier stage, more effective multimodal therapy, and better adjuvant therapy are necessary. From this study, we learned that the Ivor Lewis resection is safe, that leaks were seldom a cause of death, that we could not predict which patients would need jejunostomy tube feedings after surgery, and that diabetes and blood transfusion were associated with adverse outcomes. We hope to avoid blood transfusion in the perioperative period and to accrue more patients to protocols that examine the effectiveness of neoadjuvant therapy.
Discussion
Dr. Murray F. Brennan (New York, New York): Dr. Karl and his colleagues examined 143 patients treated by the Ivor Lewis transthoracic esophagectomy in a 10-year period. The Ivor Lewis procedure was described by Dr. Lewis in a Hunterian Lecture at the Royal College of Surgeons in London on January 10, 1946, predominantly for midesophageal tumors, but has been widely adapted for lesions in the mid- and distal esophagus. I encourage you to read the seven case histories he describes; four were 2-stage operations, two were 3-stage operations, and one was a single-stage operation. Two died postoperatively.
Dr. Karl’s personal series emphasizes the male dominance in this disease, with 88% of the patients being male, and a heavy predominance of adenocarcinoma (80%), 49% of which developed in association with Barrett’s esophagus. Dr. Karl has an impressively low mortality of 2%. In a recent analysis of 500 GE junction tumors from our own institution, our median length of stay was 15 days and our mortality 3.5%, not as good as Dr. Karl.
There are several issues:
One, this series reinforces the value of a focused endeavor by a single individual and/or single institution; this is not an operation for the casual operator. Working on a combination of the SEER and Medicare databases, we recently reviewed 6,782 esophageal cancer patients, of which 503 had an esophagectomy. It is impressive as to how few patients (7.4%) presenting with the disease ever came to operation. In addition, there were 126 hospitals in that database that performed only one operation and one esophagectomy in an entire 7-year period. As is shown in the slide, operative mortality was clearly volume-dependent. However, a low mortality was relatively quickly reached. This emphasizes not only that volume is important, but also that the actual number of patients required for low mortality can, in some situations, be quite low. It behooves us, I believe, to look not at the high-volume but rather at the casual operator.
Two, the second issue is the question of operative approach. Many GE junction lesions may be able to be resected from an abdominal-only approach. I would appreciate Dr. Karl’s commentary on the relative merits of the Ivor Lewis versus the extrathoracic esophagectomy or, for the low lesions, a proximal gastrectomy and limited esophageal resection. Dr. Karl appears to reserve that for palliative procedures, but when we analyzed those patients able to be resected transabdominally, the results were not improved by an additional thoracic incision.
Three, technically Dr. Karl prefers to close the abdomen and then re-prep the abdomen. We have found it comfortable to prep the entire field initially, with the use of a vacuum-pack support, and then, once the abdominal procedure is completed, to roll the already prepped patient up onto the left side and make the right-sided thoracic incision. Personally, I continue to use running monofilament absorbable sutures on the anastomosis, but Dr. Karl prefers interrupted 3–0 silk. (I wondered if it was appropriate to ask why in 1999 he still puts worm excreta in his patients, but I thought that was too indelicate, and so I will not ask it.)
Dr. Karl performs an esophagogram on the seventh postoperative day, but in the absence of clinical symptoms, we have essentially abandoned that. It does appear that the five leaks identified by radiology did not essentially contribute to any morbidity or mortality. I think we would all appreciate his comments on the clinically asymptomatic radiological leak and the need for routine esophagograms.
Four, given the poor survival and the fact that GE junction adenocarcinomas commonly spread intraperitoneally, is there a role for laparoscopy to rule out M1 disease, either peritoneal or lymphatic? In our own experience, we continue to detect 20% to 30% of unsuspected M1 disease.
Five, what are your indications for palliative procedures in this difficult disease, Dr. Karl? How important is a positive microscopic margin? Do you obtain frozen sections at the time? We have found that a positive margin only influences survival in patients who have T1 and T2 disease with less than five nodes positive.
Six, we have previously reported a prospective randomized study on patients such as this, showing that the routine application of jejunal feeding was not accompanied by any change of morbidity, and it appears that Dr. Karl has come to this conclusion. Is that correct? In the light of Dr. Copeland’s thoughtful address, who should receive the pre- or postop nutritional support?
Dr. John C. Bowen (New Orleans, Louisiana): Dr. Brennan, in his usual thorough fashion, has already raised a lot of very pertinent questions, and I will focus on just a few more aspects of this. I congratulate Dr. Karl and his associates for presenting their results in a prodigious series of 143 patients upon whom Dr. Karl performed an Ivor Lewis-type resection. Because of the substantial and increasing incidence of adenocarcinoma in this area, frequently in association with Barrett’s epithelium, the safety and efficacy of the 2-stage abdominal and thoracic procedure are of more than academic interest to all of us. Dr. Karl’s data clearly show that in his hands the procedure can be performed with a low mortality and low anastomotic leak rate, and the overall complication rate of 29% is well within the expected range for this patient population.
Dr. Brennan has already pointed out the importance of experience in all of these very complicated operations. Such success, however, begs the question of patient selection. Richard, you mentioned in your manuscript that during the same 10-year period, some patients with Barrett’s epithelium were treated with transhiatal esophagectomy. Could you further enlighten us as to what factors lead you to choose one procedure over the other, especially in the patients with Barrett’s esophagus? For example, would you use a transhiatal approach for selected patients in whom a computed tomographic scan suggests the absence of extramural extension or nodal involvement?
Are there any comorbid conditions that would push you preferentially toward the transhiatal approach, for example, COPD?
Are there any postoperative problems or symptoms to lead you to believe that a thoracic anastomosis is preferable to the cervical one?
I also would ask: in your series, did you have transthoracic procedures in which cancer or Barrett’s epithelium could not be cleared microscopically due to proximal extension not known to you at the time you began the operation. If so, how many and did you accept that finding and perform the intrathoracic anastomosis anyway? Or did you convert to a 3-stage procedure with a cervical anastomosis? Dr. Brennan has suggested that in some patients it doesn’t make any difference. I’d like to hear your opinion, please.
Lastly, do you feel there is a role for postoperative adjuvant therapy for adenocarcinoma of the esophagastric junction? You have mentioned preoperative, but you didn’t say anything about postoperative. Thank you.
Dr. Don M. Morris (Albuquerque, New Mexico): Dr. Karl, I will just ask simple questions.
I’m from New Mexico, where we have a large Hispanic population, and we have found that the incidence has increased in Hispanic males as rapidly as non-Hispanic males. I wonder if that’s true in Florida.
You also did not tell us why the diabetics died. Did they die of cancer or of complications of cardiovascular disease?
And finally, was the incidence of pulmonary complications greater in patients who had preoperative therapy, chemotherapy, or radiation therapy?
Dr. E. Armistead Talman (Richmond, Virginia): I did not expect to ask this question, but I put Dr. Copeland on notice that one of the consequences, so to speak, of early retirement is you get to play more tennis. That’s the good news. The bad news is that one of my four tennis partners has esophageal cancer. My questions are related to his particular problem.
He is diabetic, but he is not an insulin-dependent diabetic. Is that in the same category?
My second question is, he is currently undergoing preoperative chemotherapy and radiation. If he does not have evidence of gross disease at the conclusion, what is the appropriate approach?
Dr. Richard C. Karl (Closing Discussion): As to the role of transhiatal esophagectomy or abdominal-only resection: we will do the latter if the tumor is small and it’s in the stomach or right at the junction and we can get an adequate margin. We do freeze all the margins to be sure they are negative. I don’t stand before you to get into the debate about transhiatal versus transthoracic esophagectomy.
As to the worm excreta comment, I will let that be. I don’t know how they make the other stuff; it may be even worse. Mark Twain said people shouldn’t see how we make our laws nor our sausage.
We have largely abandoned getting the esophagograms, simply because, as you saw from the data I presented to you, the results really didn’t much affect how we managed the patients. If the patients are sick, a contrast study is helpful in figuring out if a leak is the cause of the patient’s deterioration. But if the patient is not sick, we just worry ourselves by seeing these small leaks.
We have not done routine laparoscopy in these patients. We find that endoscopic ultrasound pretty much tells us which patients are going to be resectable and which ones aren’t. The added cost and time of laparoscopy has not been studied by us, but we doubt there is going to be much indication for it.
We do do palliative operations. We sometimes find patients who have unexpected liver metastases, small ones. If it appears that they are going to live a while, and they are going to swallow better if we take out the tumor, we do it. We work hard to get negative margins, although we agree with Dr. Brennan’s statement that if the patient’s got more than a T2 lesion or more than five nodes positive, the outlook is so poor that the influence of the margins on their course is probably not important.
And this gets to a question of Dr. Bowen’s, which is if you are high up in the chest and you can’t get a negative margin, will you work to get one by going to the neck? The answer is, if it appears that the tumor falls into that favorable category grossly—that is to say, relatively small tumor and no favorable evidence of multiple involved nodes—we will go to a 3-field operation.
We don’t do routine jejunostomy feedings postoperatively, but they have been life-saving in a small percentage of patients. We were unable to figure out which patients would benefit from it, so we still end up putting tubes in all patients. The enthusiasm for giving jejunostomy feedings, especially in light of President Copeland’s speech, is largely dependent upon the residents.
As to Dr. Bowen’s questions, some of which overlapped with Dr. Brennan’s, we will consider a transhiatal resection for patients whose Barrett’s has a proximal extension, who appear to have no nodes and a favorable-looking cancer on CT scan. That is the minority of patients. It’s amazing how frequently patients are referred to you without adequate documentation as to where the Barrett’s is. The endoscopist tends to focus in on the location of the cancer and forgets to tell you where the Barrett’s begins or ends. We will rescope them in order to determine that.
I think patients who have thoracic anastomoses swallow better than those with anastomoses in the neck, but the anastomosis has to heal before one can make that statement.
Postop adjuvant therapy: there have been a number of studies, most of which have failed to demonstrate any salutary effect of postoperative chemotherapy or radiation therapy or combined therapy.
As to Dr. Morris’s question, we have surprisingly few Hispanic patients in this study. I can’t remember how many, but not many.
The diabetics mostly died of cancer, although I can’t tell you exactly how many.
The pulmonary complications after neoadjuvant therapy were no more frequent in the group that got preoperative chemoradiation than in the other group.
Dr. Talman’s question about his friend is, of course, what makes this a very personal disease. I have become a student of these matters, wondering what I will do should this happen to me, so if he is not an insulin-requiring diabetic and is playing tennis, I would recommend that he get maximum therapy. If he is free of disease by endoscopic ultrasound after his neoadjuvant therapy, I think that I would, if it were me, still undergo a resection.
I want to thank the Association for this stimulating discussion and for the opportunity to answer just a few hundred questions.
Footnotes
Correspondence: Richard C. Karl, MD, Dept. of Surgery, University of South Florida, 12901 Bruce B. Downs Blvd., Box 16, Tampa, FL 33612.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Accepted for publication December 1999.
References
- 1.Barrett NR. The lower esophagus lined by columnar epithelium. Surgery 1957; 41:881–894. [PubMed] [Google Scholar]
- 2.Hesketh PJ, Clapp RW, Doos WG, Spechler SJ. The increasing frequency of adenocarcinoma of the esophagus. Cancer 1989; 64:526–530. [DOI] [PubMed] [Google Scholar]
- 3.Ellis FH, Heatley GJ, Krasna MJ, et al. Esophagogastrectomy for carcinoma of the esophagus and cardia: a comparison of findings and results after standard resection in three consecutive eight-year intervals with improved stage criteria. J Thorac Cardiovasc Surg 1997; 113:836–846. [DOI] [PubMed] [Google Scholar]
- 4.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991; 265:1287–1289. [PubMed] [Google Scholar]
- 5.Devesa S, Blot W, Fraumeni J. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998; 83:2049–2053. [PubMed] [Google Scholar]
- 6.Siewert JR, Stein HJ, Sendler A, Fink U. Surgical resection for cancer of the cardia. Semin Surg Oncol 1999; 17:125–131. [DOI] [PubMed] [Google Scholar]
- 7.Pommier RF, Vetto JT, Ferris BL, et al. Relationships between operative approaches and outcomes in esophageal cancer. Am J Surg 1998; 175:422–425. [DOI] [PubMed] [Google Scholar]
- 8.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 1999; 230:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis I. The surgical treatment of carcinoma of the esophagus, with special reference to a new operation for growths of the middle third. Br J Surg 1946; 34:18–31. [DOI] [PubMed] [Google Scholar]
- 10.Gluch L, Smith RC, Bambach CP, Brown AR. Comparison of outcomes following transhiatal or Ivor Lewis esophagectomy for esophageal carcinoma. World J Surg 1999; 23:271–276. [DOI] [PubMed] [Google Scholar]
- 11.Bossett JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous cell cancer of the esophagus. N Engl J Med 1997; 337:161–167. [DOI] [PubMed] [Google Scholar]
- 12.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996; 335:462–467. [DOI] [PubMed] [Google Scholar]
- 13.Barash P, Cullen B, Stoelting R. Clinical Anesthesia, 2nd ed. Philadelphia: JB Lippincott; 1992:560.
- 14.American Joint Committee on Cancer. Manual for Staging of Cancer, 4th ed. Philadelphia: JB Lippincott; 1992: 75–79.
- 15.Owen JW, Balfe DM, Koehler RE, et al. Radiologic evaluation of complications after esophagogastrectomy. AJR 1983; 140:1163–1169. [DOI] [PubMed] [Google Scholar]
- 16.Anbari MM, Levine MS, Cohen RB, et al. Delayed leaks and fistulas after esophagogastrectomy: radiologic evaluation. AJR 1993; 160:1217–1220. [DOI] [PubMed] [Google Scholar]
- 17.Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 1951; 38:141–149. [PubMed] [Google Scholar]
- 18.Cox DR. Regression models and life tables. J Royal Stat Soc 1972; 34:187–220. [Google Scholar]
- 19.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–748. [PubMed] [Google Scholar]
- 20.Lozac’h P, Topart P, Perramant M. Ivor Lewis procedure for epidermoid carcinoma of the esophagus. A series of 264 patients. Semin Surg Oncol 1997; 13:238–244. [DOI] [PubMed] [Google Scholar]
- 21.Skinner DB. En bloc resection for neoplasms of the esophagus and cardia. J Thorac Cardiovasc Surg 1983; 85:59–71. [PubMed] [Google Scholar]
- 22.Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Systemic lymph node dissection for esophageal cancer: effective or not? Dis Esoph 1994; 7:2–13. [Google Scholar]
- 23.National Center for Health Statistics. Health, United States, 1996–97 and Injury Chartbook. Hyattsville, MD: Public Health Service; 1997 DHHS Pub. No. (PHS) 97–1232.
- 24.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. JAMA 1994; 272:205–211. [DOI] [PubMed] [Google Scholar]
- 25.Kabat GC, Ng SKC, Wynder EL. Tobacco, alcohol intake, and diet in relation to adenocarcinoma of the esophagus and gastric cardia. Cancer Causes Control 1993; 4:123–132. [DOI] [PubMed] [Google Scholar]
- 26.Brown LM, Silverman DT, Pottern LM, et al. Adenocarcinoma of the esophagus and esophagogastric junction in white men in the United States: alcohol, tobacco, and socioeconomic factors. Cancer Causes Control 1994; 5:333–340. [DOI] [PubMed] [Google Scholar]
- 27.Gammon MD, Schoenberg JB, Ahsan H, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 1997; 89:1277–1284. [DOI] [PubMed] [Google Scholar]
- 28.Quintiliani L, Buzzonetti A, DiGirolamo M, et al. Effects of blood transfusion on the immune responsiveness and survival of cancer patients: a prospective study. Transfusion 1991; 31:713–718. [DOI] [PubMed] [Google Scholar]
- 29.Edna TH, Bjerkeset T. Perioperative blood transfusions reduce long-term survival following surgery for colorectal cancer. Dis Colon Rectum 1998; 41:451–459. [DOI] [PubMed] [Google Scholar]
- 30.Heiss MM, Allgayer H, Gruetzner KU, et al. Prognostic influence of blood transfusion on minimal residual disease in resection gastric cancer patients. Anticancer Res 1997; 17:2657–2661. [PubMed] [Google Scholar]
- 31.Molland G, Dent OF, Chapuis PH, et al. Transfusion does not influence patient survival after resection of colorectal cancer. Aust NZ J Surg 1995; 65:592–595. [DOI] [PubMed] [Google Scholar]
- 32.Donohue JH, Williams S, Cha S, et al. Perioperative blood transfusions do not affect disease recurrence of patients undergoing curative resection of colorectal carcinoma: a Mayo/North Central Cancer Treatment Group Study. J Clin Oncol 1995; 13:1671–1678. [DOI] [PubMed] [Google Scholar]
- 33.Fong Y, Karpeh M, Mayer K, Brennan MF. Association of perioperative transfusions with poor outcome in resection of gastric adenocarcinoma. Am J Surg 1994; 167:256–260. [DOI] [PubMed] [Google Scholar]
- 34.Craig SR, Adam DJ, Yap PL, et al. Effect of blood transfusion on survival after esophagogastrectomy for carcinoma. Ann Thorac Surg 1998; 66:356–361. [DOI] [PubMed] [Google Scholar]
- 35.Tachikawa D, Inada S, Kotoh T, et al. An evaluation of malignancy and prognostic factors based on mode of lymph node metastasis in esophageal carcinoma. Surg Today 1999; 29:1131–1135. [DOI] [PubMed] [Google Scholar]
- 36.Sugimachi K, Matsuura H, Kai H, et al. Prognostic factors of esophageal carcinoma: univariate and multivariate analyses. J Surg Oncol 1986; 31:108–112. [DOI] [PubMed] [Google Scholar]
- 37.Roth JA, Putnam JB. Surgery for cancer of the esophagus. Sem Oncol 1994; 21:453–461. [PubMed] [Google Scholar]
- 38.Watson A. Operable esophageal cancer: current results from the West. World J Surg 1994; 18:361–366. [DOI] [PubMed] [Google Scholar]