Abstract
Objective
To determine whether reresection affects survival in patients with inadequately resected, primary extremity soft tissue sarcoma. This study correlates reresection with local recurrence-free survival, metastasis-free survival, and disease-free survival.
Summary Background Data
Soft tissue sarcomas are rare neoplasms, with an incidence of approximately 6,000 per year in the United States. Because these tumors are rare and benign soft tissue tumors are common, many are initially thought to be benign and are excised without wide margins.
Methods
Patients who underwent treatment for primary tumors from July 1982 to June 1999 at a single institution were the subject of study. Two groups of patients were analyzed: those who underwent one definitive resection (one operation) and those whose tumors were previously resected and who were then referred for subsequent reresection (two operations). Patients were given adjuvant radiation or chemotherapy according to the standard of care.
Results
Of 1,092 patients with primary extremity soft tissue sarcoma underwent resection, 685 underwent definitive radical resection and 407 underwent reresection after undergoing excisional resection elsewhere. Median follow-up was 4.8 years. The 5-year disease-free survival rate of the definitive resection (one operation) group was 70%; that of the reresection (two operations) group was 88%. On multivariate analysis, reresection was adjusted and controlled for age, grade, depth, size, histology, and margins. Reresection remained a significant predictor of improved disease-free survival, even after these adjustments. To determine whether this difference was stage- or referral-biased, the patient population was divided by AJCC stage. In all stages there was a trend toward improved outcome; this was most marked for those with stage III disease (>5 cm, high-grade, and deep).
Conclusions
Patients with extremity soft tissue sarcoma who undergo reresection with two “primary” operations have an improved survival compared with those who undergo one operation. The most plausible explanation, referral and selection bias, is questionable given the significance of reresection as a variable after adjusting for stage and other risk factors. This suggests that where indicated and possible, reresection should be liberally applied in patients with primary extremity soft tissue sarcoma.
Soft tissue sarcomas are rare neoplasms, with an annual incidence of approximately 6,000 in the United States. 1 Because these tumors are rare and benign soft tissue tumors are common, many are initially thought to be benign and are excised without wide margins. After the diagnosis of sarcoma is made, these patients are usually referred to a specialist center. A reexcision is performed to obtain an adequate margin, and appropriate adjuvant multimodality therapy, where indicated, is administered. The rationale for reexcision is predicated on the principle that resection margin affects survival, although most of these studies have examined this for local recurrence. 1–4 In addition, severalstudies have documented patient benefit from the improved treatment of rare tumors at a specialist center, and this has been specifically addressed in soft tissue sarcoma. 2–4
We tested the hypothesis that reresection has an impact on survival. The aim of this study was to analyze survival outcome in a large, well-characterized cohort of prospectively followed-up patients with primary extremity soft tissue sarcoma who were initially treated at a cancer specialist center versus those who underwent initial resection elsewhere and were subsequently referred for reresection. In these patients, we analyzed the effect of reresection on local recurrence-free survival, metastasis-free survival, and disease-free survival.
METHODS
A prospective database of adult patients (older than 16 years) with soft tissue sarcoma treated at Memorial Sloan-Kettering Cancer Center (MSKCC) was established in July 1982. Patients who underwent treatment for primary extremity soft tissue sarcoma between July 1, 1982, and June 30, 1999, were the subjects of this study. Patients were treated according to the standard of care at MSKCC. 1 For new patients, biopsy was followed by wide resection and adjuvant radiation, chemotherapy, or both where indicated. For those who had undergone insufficient excision, repeat wide resection was performed and followed by adjuvant radiation, chemotherapy, or both where indicated. Patients who underwent primary amputation in both groups (n = 71) were excluded from analysis.
Patients were classified into two groups. The first, the resection group, comprised patients who were referred before any surgical treatment and who subsequently underwent only one therapeutic operation at MSKCC. The second, the reresection group, comprised patients who were referred after undergoing excision without planned wide margins before referral and who subsequently underwent a second procedure (reresection) at MSKCC.
Surgical, clinical, and pathologic variables were correlated with survival endpoints. Surgical variables were one (resection) versus two (re-resection) and microscopic margins (negative or positive). Clinical variables analyzed included age at diagnosis (younger than or older than 50 years), gender, and presenting symptoms. Tumor variables analyzed included size (<5 cm, 5–10 cm, or ≥10 cm), depth (superficial or deep), site (upper or lower extremity), histologic grade (low or high), histologic subtype, and the presence of bone or neurovascular invasion. Histologic grade was low or high, distinct from other aspects of intermediate transition. 5 Dermatofibrosarcoma protuberans has an inherently good prognosis, 6 and this histologic subtype was excluded from all analysis. Virtually all of these patients underwent reresection, which would have favored this group.
Survival and Statistical Analysis
Actuarial survival was calculated us the Kaplan-Meier method. 7 Disease-specific and disease-free survivals were also modeled with this method. Deaths that were confirmed to be caused by the disease were treated as an endpoint for disease-free survival; other deaths were treated as censored observations. Disease-free survival was segregated into local recurrence-free and metastasis-free survival. Local recurrence was defined as the first recurrence of disease, of the same histologic subtype, at the site of the primary tumor, occurring more than 3 months after resection (where the resection was performed at MSKCC).
Significance between survival curves of populations was evaluated using log-rank testing for univariate influence and Cox stepwise regression for multivariate influence. To arrive at a parsimonious multivariate model, covariates were selected only if they contributed significantly to the fit of the model. Comparison between clinical and pathologic categorical variables in different groups was performed using the Fisher exact test for univariate and logistic regression for multivariate influence.
RESULTS
During the study period, 1,092 patients with primary extremity soft tissue sarcoma were treated at MSKCC. Of these, 685 (63%) had primary disease and underwent a single resection at MSKCC, and 407 (37%) had undergone one resection and underwent a subsequent reresection at MSKCC. The distribution of clinical and pathologic characteristics in these patients is illustrated in Table 1. For the reresection group, the mean time between resection and reresection was 36 days (range 14–88 days). The median follow-up for all patients was 4.8 years. During this time, local recurrence developed as the first recurrence in 161 patients, distant metastasis developed in 277 patients, and 223 patients died of their disease.
Table 1. CLINICAL AND PATHOLOGIC CHARACTERISTICS

A comparison between the groups (Table 2) revealed significant differences in terms of size, depth, and microscopic margins. This was in accordance with our supposition that patients with larger tumors or deep tumors were more likely to be referred, and hence would undergo primary resection at MSKCC. Thus, there were proportionally more large (P = .001) and deep (P = .001) tumors in the resection group. Further, after the best possible resection, by either one or two operations, more patients undergoing reresection had microscopically negative margins (P = .01). There was no difference in histologic grade (P = .1) between the two groups.
Table 2. ASSOCIATION OF REEXCISION STATUS WITH PATIENT, TUMOR, PATHOLOGIC, AND OUTCOME VARIABLES
*P value by chi-square test for association between factors and by log-rank test for association with outcome.
Disease-Free Survival
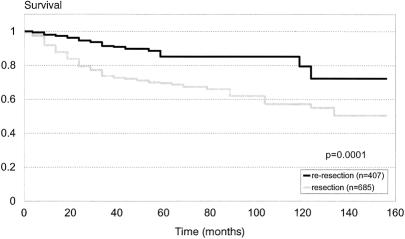
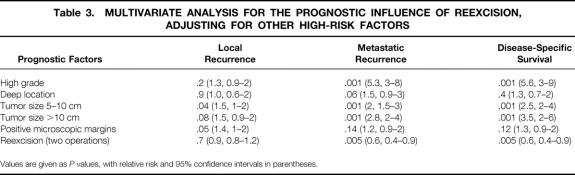
The actuarial disease-free survival is depicted in Figure 1. The 5-year survival rate was 70% ± 6% for the resection group versus 88% ± 5% for the reresection group (P = .0001). Results of Cox multivariate analysis for disease-free survival are summarized in Table 3. Not surprisingly, large size, high-grade histology, deep location, and positive microscopic margins were predictive of death resulting from tumor. After adjusting for these variables, reresection (P = .005; relative risk [RR] = 0.6) was an independent, positive predictor for survival. To determine whether this was stage-related, we divided the patient population by AJCC stage. In all stages there was a trend toward improved survival for the reresection group, and this was most apparent and statistically significant (P = .005) for stage III (>5 cm, high-grade, and deep) disease.
Figure 1. Actuarial disease-specific survival for patients undergoing resection versus reresection for primary extremity sarcoma. Median follow-up for all patients was 4.8 years. The 5-year survival rate was 70% ± 6% for the resection group versus 88% ± 5% for the reresection group (P = .0001).
Table 3. MULTIVARIATE ANALYSIS FOR THE PROGNOSTIC INFLUENCE OF REEXCISION, ADJUSTING FOR OTHER HIGH-RISK FACTORS
Values are given as P values, with relative risk and 95% confidence intervals in parentheses.
Pathologic analysis of the surgical specimens from patients who underwent reresection revealed that 159 patients (39%) had microscopic evidence of residual disease. The remaining 248 patients (61%) had no microscopic evidence of residual disease. However, this did not translate into a difference in the 5-year survival rate between groups (86% ± 6% for the group with microscopic residual disease vs. 89% ± 6% for the group without microscopic residual disease;P = .4).
Because of the greater weight (frequency) of poor prognostic factors in the primary resection group (see Table 2), an analysis of disease-free survival was performed for all the high-risk subsets of the resection and reresection groups. For those with large (≥5 cm) tumors, high-grade tumors, or a positive microscopic margin, survival was always significantly better among patients undergoing reresection versus resection (data not shown).
Metastasis-Free Survival
Actuarial metastasis-free survival is depicted in Figure 2. The 5-year survival rate was 63% ± 6% for the resection group versus 83% ± 5% for the reresection group (P = .0001). Results of a multivariate analysis summarizing prognostic factors are shown in Table 3. Metastasis was predicted in patients with tumors that were larger than 5 cm (P = .001; RR = 2), were initially of high grade (P = .001; RR = 5.3), or had a deep location (P = .06; RR = 1.5). After adjusting for these variables, reresection (P = .005; RR = 0.6) was an independent, positive predictor for metastasis-free survival. Again, to determine whether this was stage-related, we divided the patient population by AJCC stage. In all stages there was a trend toward improved metastasis-free survival for the reresection group, and this was most apparent and statistically significant (P = .005) for stage III (>5 cm, high-grade, and deep) disease.
Figure 2. Actuarial metastasis-free survival for patients undergoing resection versus reresection for primary extremity sarcoma. Median follow-up for all patients was 4.8 years. The 5-year metastasis-free survival rate was 63% ± 6% for the resection group versus 83% ± 5% for the reresection group (P = .0001).
Local Recurrence-Free Survival
Actuarial local recurrence-free survival is depicted in Figure 3. There was no difference (P = .7) between the resection and reresection groups. Results of a multivariate analysis summarizing prognostic factors are shown in Table 3. Tumor size larger than 10 cm (P = .08; RR = 1.5) and positive microscopic margins (P = .05; RR = 1.4) were the dominant factors influencing local recurrence. After adjusting for these variables, reresection (P = .7) was not an independent predictor for local recurrence. In contrast, lower extremity site of disease (P = .003; RR = 0.6) and leiomyosarcoma histologic subtype (P = .04; RR = 0.30) were identified as negative predictors of local recurrence.

Figure 3. Actuarial local recurrence-free survival for patients undergoing resection versus reresection for primary extremity sarcoma. There was no difference between the groups (P = .7).
DISCUSSION
Our study analyzed survival outcomes of two groups of patients with primary extremity soft tissue sarcoma: those who underwent a single primary resection at a specialist cancer institution versus those undergoing reresection after initial resection elsewhere. These data are not population-based and reflect the experience of a major sarcoma referral center. The findings suggest that patients undergoing a second operation (reresection) show as good or better disease-specific and metastasis-free survival compared with those receiving a single operation. One logical explanation for this is referral bias—that is, patients with high-risk tumors are referred before any treatment and will therefore undergo a single procedure. This was verified when comparing prognostic factors between both groups. Surprisingly, even after adjusting for known high-risk variables, 8 reresection was an independent, positive prognostic factor. Analysis of local recurrence-free survival failed to demonstrate a difference for local recurrence between the resection and reresection groups. This was also unanticipated, because it would seem intuitive that reresection represents wider local treatment and that this would reduce local recurrence. These data reflect the patients who were admitted and treated, so patients referred who did not undergo reresection may have had a higher rate of local recurrence.
Soft tissue sarcomas are rare tumors that are often mistaken for common benign soft tissue tumors. Not unexpectedly, in these patients, initial surgical resection is performed as though this were a benign tumor. Thus, tumors are excised without wide margins. Historically, this approach has led to a 70% to 90% local recurrence rate. 9–12 It is therefore widely accepted that patients undergoing initial marginal excision should undergo reexcision with suitable wide margins and appropriate adjuvant therapy.
Further, the benefits of initial treatment in a cancer specialist institution for patients with rare malignancies have been well described. 1–4,13,14 One of the larger studies specifically addressing soft tissue sarcoma analyzed the quality of surgery, defined as the total number of surgical procedures performed for the primary tumor, in a retrospective, population-based series of 375 patients. 2 Comparisons were made between patients referred to the specialist center before any surgery, after surgery, and for patients who were not referred at all. The results revealed that patients referred after an initial surgical procedure required a greater number of total operations and had higher local recurrence rates than did patients undergoing primary treatment at the specialist institution. Moreover, patients who were never referred had the highest number of operations as well as the highest local recurrence rates. However, despite the differences in total operations and local recurrence rates, there was no survival difference demonstrated between the groups. 2
Most patients with high-risk tumors or positive margins receive adjuvant radiation therapy. The potential benefits of local adjuvant radiation therapy are predicated on the quality of the preceding operation in achieving local tumor control. Thus, if the initial operation fails to obtain negative margins, reexcision should be performed before adjuvant radiation therapy. 15,16 In patients with extremity soft tissue sarcoma, the recurring predictors of outcome that have been identified include size larger than 5 cm, high-grade histology, deep location, and microscopically positive resection margins. Of these, margins are the only known factor that can be altered by the quality of surgery. The principle of reexcision after an inadequate or unplanned initial operation has been further evaluated in a retrospective series of 189 patients. 15 In that series, patients undergoing subsequent procedures to achieve clear margins had decreased local recurrence rates.
The unappreciated presence of residual microscopic disease was reviewed in a group of patients referred to a specialist center after reportedly complete resection of extremity soft tissue sarcoma. 12 In that series of patients undergoing reresection, residual tumor was identified in the reresection specimen in 44 of 90 patients. The authors concluded that planned reoperation for inadequately excised disease is nearly always indicated, because the adequacy of tumor removal will influence the likelihood of local recurrence. Although we demonstrated residual tumor in 159 patients (39% of those undergoing reresection), the presence or absence of residual tumor did not affect disease-specific or recurrence-free survival. Despite optimal, limb-sparing multimodality treatment for soft tissue sarcoma, local recurrence occurs in approximately 20% of patients. 17 The association between local recurrence and subsequent survival remains unclear in extremity sarcoma. 18,19 This is in contrast to retroperitoneal sarcoma, where local recurrence may result in death resulting from tumor. 13 In the current study, we observed no difference in local recurrence-free survival for the resection or reresection groups. Prospective studies 20,21 have identified a link between positive microscopic margins at initial resection and local recurrence. The association with subsequent metastasis and tumor-related death is less clear. Thus, although local recurrence may be predictive of metastasis and death, a causal relation cannot be presumed. Our findings suggest that reresection, or a more aggressive local treatment, is an independent predictor of survival. Further, this does not seem to be related to local recurrence. Given previous observations, we would not have expected that a more aggressive local treatment would have changed overall survival, because it is more plausible that this would affect local recurrence.
We have no clear explanation for our findings. It is possible that we unwittingly selected a more favorable group, but our analyses cannot confirm this. Conversely, if these observations are correct, several unproven theoretical mechanisms may be operational. The most plausible explanation is that reresection results in the removal of unappreciated residual local tumor that extends far beyond the pseudocapsule of the primary tumor and is undetectable by the pathologist. The relatively high frequency of residual disease identified after reresection argues that a more complete resection may be achieved after a second operation. This is in accordance with previous observations, 12 but if correct we would expect to demonstrate a difference in local recurrence-free survival, which we did not.
Other speculative explanations for the observed effect of local treatment on distant metastasis include the loss of “dormancy” in satellite lesions or distant subclinical metastasis after the removal of the primary tumor. The inhibitory role of a primary tumor on the development of distant metastases for extremity sarcoma has long been experimentally demonstrated. 22 Recently elucidated potential mechanisms include angiogenic factors associated with primary tumors that may have a significant role in tumor metastasis, disease progression, and tumor dormancy. 23,24 A second speculative explanation includes mediation by endogenous immune activation. Dendritic cells, the most potent antigen-presenting cells, need to be activated to initiate an immune recognition and response. Recent evidence suggests that dendritic cells can be considerably activated by mediators of injury and inflammation. 25–27 Thus, the first operation may markedly activate dendritic cells, which then recognize antigens in the residual tumor, resulting in an immune response with long-term memory.
In summary, our data suggest that patients with extremity soft tissue sarcoma who undergo reresection with two “primary” operations have an improved survival compared with those who undergo one operation. The most plausible explanation, referral and selection bias, is questionable given the significance of reresection as a variable after adjusting for stage and other high-risk factors. Although the mechanistic explanation is unclear, where indicated and possible, reresection (two operations) should be liberally applied in patients with primary extremity soft tissue sarcoma.
Discussion
Dr. Douglas S. Reintgen (Tampa, Florida): This is a provocative paper from the Memorial Sloan-Kettering group, who really have become the leaders in the field in defining the natural history and best treatment for extremity soft tissue sarcoma. This particular paper provides evidence that a second primary operation should always be performed in patients with extremity soft tissue sarcoma when the initial procedure was an excisional biopsy, even though clear margins were reported. The rationale for reresection is that resection margins impact on survival. The Memorial Sloan-Kettering group needs to give the readers and the members here their idea of an adequate margin.
The fact that 39% of patients in the reresection group had microscopic disease identified in their surgical specimens in and of itself is also a strong argument for reresection, although surprisingly this did not translate into a difference in disease-free survival. In addition, the literature suggests that when a nonspecialist center says the margins are clear, on reresection 48% will have residual tumor identified. Perhaps not only the surgery is better handled at a specialist center, but also perhaps the histologic examination. The theory is established that perhaps reresection results in the removal of unappreciated residual tumor foci that extends far beyond the pseudocapsule of the primary tumor and is undetectable by the pathologist. This is likely because routine histology can miss microscopic disease. This fact hits home with the lymphatic mapping and sentinel node biopsy work in melanoma and breast cancer, where 10% to 25% of the patients are understaged with routine histology.
Studies have shown improved survival with the referral of patients with rare tumors to specialist centers. What would be the reason for a patient being referred to Memorial Sloan-Kettering but not undergoing reexcision? Perhaps adequate margins were obtained at the outside institution. Again, what would be considered an adequate margin? Results of previous studies show that patients referred to a cancer center after an initial outside procedure require a greater number of total operations and have a higher local recurrence rate when compared to patients undergoing primary treatment at a cancer center. Moreover, patients never referred had the highest number of operations and the highest local recurrence rate. But no survival advantage has been noted in these studies. Despite optimal limb-sparing multimodality treatment for soft tissue sarcoma, local recurrence remains at 20%.
The association between local recurrence and subsequent survival remains unclear. Salvage therapies after a local recurrence, including amputation, may be effective. What is clear is that a local recurrence puts the extremity in jeopardy and most are treated subsequently with amputation. This is not a great result for a program emphasizing limb salvage, and establishes the fact that local recurrences should be taken seriously and have grave consequences, even if not a survival impact.
Because neither I nor the authors have a convincing hypothesis of why a second operation for clear margins makes such a difference in survival when local recurrence does not seem to affect survival, I want to end by just making one comment about the soft tissue sarcoma database established at Memorial Sloan-Kettering in July 1982.
The gold mine of information that has been generated from this database is tremendous. Clinicians must be aware that not everything we do in medicine is based on a prospective randomized trial. Valuable information about the natural history and best treatments for a disease can be gained with studies performed from an accurate prospectively accumulated database. It is difficult to go to any national meeting today and not see a paper on the program about soft tissue sarcoma generated from this database.
In addition, I want to thank you, Dr. Brennan, for the countless residents and fellows who have had ideas for clinical papers on this population of patients and have not had to be confined to the so-called “bowels of the hospital,” i.e., the medical records department, to get the data for their papers. They can obtain the data that they need in a matter of minutes from this computer-driven database. In addition, I compliment you on the foresight you showed in establishing this database, because your work has helped many of us take care of the patient with this rare tumor.
Dr. Richard Karl (Tampa, Florida): Mr. President, we run the risk of having the Tampa fellows gang up on the folks from the southern hemisphere, but I compliment Dr. Lewis and Dr. Brennan and their colleagues on really an outstanding and astounding paper. This flies in the face of everything we have been taught, mostly by Dr. Brennan, which is that reexcision is good because it’s going to prevent local recurrence, and that’s got to be a good thing. In fact, this paper states that reresection is equally helpful, whether or not the margins are positive. Secondly, resection itself does not appear to decrease local recurrence.
So what are we to make of all this? I asked one of our fellows to review the paper, and she wrote back “this is a really good paper; I can’t find much to say.” And I know exactly how she feels. The paper states that after adjusting for size, depth, high-grade pathology, et cetera, reresection still is an independent favorable variable. Now there is nobody in this room who knows less about statistics than I do, but maybe there is something about these statistics. I have a few questions for Dr. Lewis.
The first is, are there any patients that had two operations done at Memorial Sloan-Kettering?
The second question is, which percentage of patients got adjuvant treatment with chemotherapy or radiation, and could that possibly affect these results?
And was there any difference in the timing of the reresection? That is to say, that if some immune stimulation or depression took place at the time of the second operation, would the time between the first and the second operation affect outcome?
And finally, would you postulate what sorts of immune system perturbations might account for these results?
Of course, it’s entirely possible, I suppose, that you got the reds and the yellows mixed up.
Dr. Edward M. Copeland III (Gainesville, Florida): Would anyone else like to discuss this paper? Dr. Townsend and I have a question for Dr. Lewis, which Dr. Karl mentioned.
I don’t want to be facetious, Murray, but if I understand, you are saying that individuals who are operated on somewhere else and then get reresected at Memorial have a better survival than people who are operated on at Memorial for the initial operation. That would mean that you would operate on people initially at Memorial, wait a period of time and reoperate on them, would it not?
I’m confused. Please have Dr. Lewis address this issue.
Dr. Jonathan J. Lewis (Closing Discussion): Dr. Reintgen asked how we define an adequate margin. The standard that we apply is an approximate 2-cm margin of normal tissue. We do agree with you that there may well be understaging by the pathologist. As you have noted with sentinel lymph node biopsy, there may be microscopic tumor present that the pathologist will not sample. The pathologists at Memorial Sloan-Kettering Cancer Center are methodical in their approach to the microscopic margin on tumors and we do believe they are fairly accurate.
The third question you asked was were there any patients who were referred to us who did not undergo reexcision. The answer is yes, and these patients underwent amputation. They were specifically excluded from this analysis. In both groups, patients who underwent primary amputation were excluded from analysis.
The fourth question you asked was regarding local recurrence. The overwhelming majority of patients with local recurrence do not undergo amputation at Memorial, but undergo a limb-sparing reresection. This is frequently treated with adjuvant radiation therapy.
Dr. Karl, your first question was do any patients who are primarily treated at Memorial undergo two operations. The answer to that is very few. There were less than ten over a 15-year period. Those patients were specifically excluded from the analysis.
Your second question was regarding adjuvant therapy. Adjuvant therapy was applied in a standard fashion and as part of randomized prospective studies that were conducted during this 15-year period. It is our standard to give patients adjuvant radiation therapy for all tumors ≥5 cm in size. In addition, for those patients with tumors less than 5 cm in size who have a positive margin after the best possible resection procedure, adjuvant radiation would be administered. We do not give routine chemotherapy. In patients with tumors that are greater than 10 cm in size, and high-grade, we would offer adjuvant chemotherapy.
The third question you asked was did we find any difference in the timing of reresection. We examined this carefully, looking at time both as a dependent and independent variable, and as a discrete and continuous variable. We found no difference. The average time was 36 days between procedures, and the range was 14 through 88 days.
Your fourth question was what do we speculate as to a possible mechanism. We have no rational explanation. Several possibilities are detailed in the discussion of the manuscript. Your suggestion that the immune system may be invoked is plausible. While there may be some explanations in laboratory models, we have no explanation in the human model and know of no other human cancer where the effect of two operations has been examined. In a variety of research models, a danger signal and/or injury will often hyperstimulate immune recognition. There are many reasons as to why this is happening, and as you suggest, it is a possible mechanism in terms of some of the effects we are seeing.
Dr. Copeland, in answer to your question, we did not mistakenly confuse the two groups. Indeed, the first time I saw the data that too was my initial response, but I have rechecked it and in fact, we have not mixed up the two groups. We first observed this a few years ago, and because of our initial surprise, we have let these data mature and repeatedly come up with the same results.
Your second question was have we undertaken intentionally doing two operations on these patients. No, we have not, and while a prospective randomized trial would address several key questions about these data, obviously this is not something that it will not be possible to do.
In conclusion, I would like to echo the discussants’ comments that the value of this database set up by Dr. Brennan 15 years ago is remarkable. Based on his vision, we are able to continue to generate new biologic and translational questions such as those we have reported today.
Footnotes
Correspondence: Jonathan J. Lewis, MD, PhD, Dept. of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021.
Presented in part at the Annual Meeting of the Society of Surgical Oncology, Atlanta, GA, March 21–24, 1996, and at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Supported by NIH Grant CA-47179 (MFB).
Dr. Espat is currently with the Dept. of Surgery, University of Illinois at Chicago, Chicago, IL.
E-mail: lewisj@mskcc.org
Accepted for publication December 1999.
References
- 1.Lewis JJ, Brennan MF. Soft tissue sarcomas. In: Sabiston DC, ed. The Biological Basis of Modern Surgical Practice. New York: Saunders; 1999: 528–534.
- 2.Gustafson P, Dreinhofer KE, Rydholm A. Soft tissue sarcoma should be treated at a tumor center. A comparison of quality of surgery in 375 patients. Acta Orthop Scand 1994; 65:47–50. [DOI] [PubMed] [Google Scholar]
- 3.Gustafson P. Soft tissue sarcoma. Epidemiology and prognosis in 508 patients. Acta Orthop Scand 1994; 259(suppl):1–31. [PubMed] [Google Scholar]
- 4.Clasby R, Tilling K, Smith MA, Fletcher CDM. Variable management of soft tissue sarcoma: regional audit with implications for specialist care. Br J Surg 1997; 84:1692–1696. [PubMed] [Google Scholar]
- 5.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg 1988; 12:326–331. [DOI] [PubMed] [Google Scholar]
- 6.Bowne WB, Antonescu CR, Katz SC, et al. Multifactorial analysis predicts outcome in patients with dermatofibrosarcoma protuberans (DFSP): a 48-year experience. Cancer 2000 (in press).
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc 1958; 53:457–462. [Google Scholar]
- 8.Pisters PWT, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Onc 1996; 14:1679–1689. [DOI] [PubMed] [Google Scholar]
- 9.Gerner RE, Moore GE, Pickren JW. Soft tissue sarcomas. Ann Surg 1975; 181:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Kent H, Costa J. Prospective randomized evaluation of the role of limb-sparing surgery, radiation therapy and adjuvant chemo-immunotherapy in the treatment of adult soft tissue sarcomas. Surgery 1978; 84:62. [PubMed] [Google Scholar]
- 11.Shiu MH, Castro EB, Hajdu SI, Fortner JG. Surgical treatment of 297 soft tissue sarcomas of the lower extremity. Ann Surg 1975; 182:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giulano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft tissue sarcomas. J Clin Oncol 1985; 3:1344–1348. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JJ, Leung D, Woodruff J, Brennan MF. Retroperitoneal soft tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998; 228:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stiller CA. Population-based survival rates for childhood cancer in Britain. Br Med J 1994; 309:1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zornig C, Peiper M, Schroder S. Re-excision of soft tissue sarcoma after inadequate initial operation. Br J Surg 1995; 82:278–279. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JJ, Benedetti F. Adjuvant therapy for soft tissue sarcomas. Surg Oncol Clin North Am 1997; 6:847–862. [PubMed] [Google Scholar]
- 17.Brennan MF. The management of soft tissue sarcomas. Br J Surg 1984; 71:964–964. [DOI] [PubMed] [Google Scholar]
- 18.Brennan MF, Hilaris B, Shiu MH, et al. Local recurrence in adult soft tissue sarcoma. A randomized trial of brachytherapy. Arch Surg 1987; 122:1289–1293. [DOI] [PubMed] [Google Scholar]
- 19.Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol 1997; 15:646–652. [DOI] [PubMed] [Google Scholar]
- 20.Pisters PWT, Harrison LB, Woodruff JM, Gaynor JJ, Brennan MF. A prospective randomized trial of adjuvant brachytherapy in the management of low-grade soft tissue sarcomas of the extremity and superficial trunk. J Clin Oncol 1994; 12:1150–1150. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Kent H, Costa J, et al. Prospective randomized evaluation of the role of limb-sparing surgery, radiation therapy, and adjuvant chemoimmunotherapy in the treatment of adult soft-tissue sarcomas. Surgery 1978; 84:62–69. [PubMed] [Google Scholar]
- 22.Klein G, Sjorgen E, Klein E, et al. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res 1960; 20:1561–1572. [PubMed] [Google Scholar]
- 23.O’Reilly SM, Holmgren L, Chen C, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Nature Med 1999; 6:689–692. [Google Scholar]
- 24.O’Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nature Med 1996; 2:689–692. [DOI] [PubMed] [Google Scholar]
- 25.Lewis JJ, Houghton AN. Definition of tumor antigens suitable for vaccine construction. Semin Cancer Biol 1995; 6:321–327. [DOI] [PubMed] [Google Scholar]
- 26.Bowne WB, Moroi Y, Wang S, Houghton AN, Lewis JJ. In vivo recruitment of dendritic cells followed by immunization with mutant p53 peptide primes for cytotoxic T-cell response. Proc Soc Surg Oncol 1998; 14:87. [Google Scholar]
- 27.Gallucci S, Lolkema M., Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nature Med 1999; 5:1249–1255. [DOI] [PubMed] [Google Scholar]