Abstract
Objectives
To evaluate melanoma biopsy specimens for human papilloma virus (HPV) and determine the relation between the presence of HPV, in vitro growth, and clinical progression of melanoma in the patients from whom the biopsy specimens were derived.
Summary Background Data
Ultraviolet radiation from sun exposure appears to be the primary causal agent in the development of cutaneous melanoma. However, other agents, including HPV, as observed in different epithelial carcinomas, may also play a role in melanoma development and progression.
Methods
Twelve melanoma biopsy specimens obtained from 12 patients with AJCC stage III and IV melanoma were stained with antibodies against gp-100 (HMB-45) and S-100 protein to confirm melanoma diagnosis and with a polyclonal HPV antibody. After mechanical dissociation, the melanoma specimen cells’ ability to grow in vitro was assessed. Patients were evaluated for melanoma progression with physical examination, complete blood count, and liver function tests every 3 months and a chest radiograph every 6 months.
Results
All biopsy specimens were positive for S-100, and nine (75%) were positive for gp-100. Seven of 12 (58%) were positive for HPV by immunohistochemistry. In vitro, none of the HPV-negative tumor cells grew from the tumor biopsies, whereas five of seven (71%) of the HPV-positive melanoma tumor cells grew very well. All patients with HPV-positive tumor cells had recurrences and died of melanoma progression, whereas four of five (80%) patients with HPV-negative tumor cells remained alive and without melanoma recurrence.
Conclusions
The presence of HPV was found in 58% of the biopsy specimens obtained from patients with stage III and IV melanoma and correlated with rapid melanoma progression. HPV may serve as a cofactor in the development of melanoma and may modulate a more aggressive phenotype in HPV-containing melanoma cells.
Ultraviolet (UV) radiation from sun exposure appears to be the primary causal agent in the development of cutaneous melanoma. 1,2 Indeed, the use of various sun-block lotions and prevention campaigns against sun bathing may have had some positive effects. 1,2 Moreover, several studies in white populations have shown a correlation between the incidence of melanoma and latitude, which is associated with the amount of UV sun exposure. 1,2 From a cellular and molecular standpoint, UV radiation, especially UVB rays, have been shown to be associated with the development of some mutations in vitro. 3 However, these mutation patterns remain inconsistent. Indeed, only a few of these mutations were associated with cell cycle regulation, especially p53 regulatory protein in melanoma. 4–6 These observations suggest that other agents may be involved directly or indirectly in the development and progression of melanoma.
Other agents, especially viruses, play a preeminent role in the development and progression of some cancers, including cervical, 7 gastrointestinal, and laryngeal carcinoma 8,9 and nonmelanoma skin cancers. 10,11 The role of human papilloma virus (HPV) in the development of cervical cancers has been extensively studied. 11,12 HPV also has been detected in, and may play a role in, some lymphomas 13 and eosophageal cancers 8,9,14. Other studies, however, failed to confirm the presence of HPV or other viruses in these cancers. 14,15 Overall, current evidence suggests that the strength of the association between cancer and viral infections ranks from very strong (human herpes virus-8 in Kaposi sarcoma) to inconsistent (HPV in nonmelanoma skin cancer) in immunocompromised patients. 16 Nevertheless, the influence of early HPV proteins E6 and E7 at key mitotic checkpoints during the G1 phase of the cell cycle has been demonstrated in vitro. 12,17 Although the multiple actions of the early proteins E6 and E7 are not completely understood, recent analyses have shown that E6 and E7 from high-risk HPV subtypes (e.g., HPV 16, HPV 18, and HPV 35) interfere primarily with p53 and pRb proteins, both key cell cycle protein regulators. 5
E6 proteins affect the actions of p53, a tumor suppressor protein involved in the repair of UV-type DNA damage. 18 E6 protein promotes the degradation of p53 tumor suppressor protein. 19–21 This results in a potent inhibition of p53 DNA binding activity 22 and in disruption of the cellular response to DNA damage. 23 The E7 protein mainly affects the G1/S transition of the cell cycle, altering the retinoblastoma protein function. 24 The disruption of the G1/S transition in HPV type 16 E7-expressing human cells is associated with altered regulation of cyclin E. 12 E7 protein has a profound effect on several aspects of the cell cycle machinery, including transit through G1, which is shortened by the premature activation of cyclin E-associated kinase activity. This disrupts the G1/S cell cycle progression in addition to the release of E2F transcription factor. 12,25,26
In vitro, similar actions of these two proteins on melanocytes result in the immortalization of these cells 27 and of normal human keratinocytes. 28,29 Finally, recent reports indicate the presence of HPV in some melanoma biopsy specimens. 30,31 These observations, associated with our recent findings of HPV in melanoma cells derived from melanoma biopsy specimens in vitro, 32 suggest that HPV may play a role in melanoma development and progression.
The present study was designed to evaluate melanoma biopsy specimens for HPV and to determine the relation between the presence of HPV, the in vitro growth rate of HPV-negative and HPV-positive melanoma cells, and the clinical progression of melanoma in the patients from whom the biopsy specimens were derived.
METHODS
Patients and Biopsy Specimens
Patients referred to Carolinas Medical Center for melanoma recurrence (AJCC stage III and IV) were evaluated using standard modalities, including physical examination, computed tomography, positron emission tomography, and magnetic resonance imaging. 33,34 Melanoma tumors were removed by surgical resection, rendering all the patients disease-free by clinical examination and staging radiology. A portion of the tumor was used for research after informed consent was obtained. A consent form approved by the human subject committee permitting research use of removed tissue was used. Each biopsy specimen was treated similarly. Biopsy specimens were divided into two parts. One part was fixed for 48 hours in Z-FIX fixative (Anatech Ltd., Battle Creek, MI), dehydrated in ethanol, embedded in paraffin, and sectioned at 5 μm for histology and immunochemistry. The second part was mechanically dissociated through a 450-μm mesh and washed twice in phosphate-buffered saline. After centrifugation, cells were resuspended in complete culture media (RPMI-1640 medium [Sigma, St. Louis, MO], 10% fetal bovine serum [Sigma], 2 mmol/L L-glutamine [Gibco BRL, Rockville, MD], 2 μg/mL Fungizone [Gibco], and 80 μg/mL gentamicin [Gibco]).
Histology and Immunostaining
After deparaffinization, serial sections (5 μm thick) were used for immunohistology. All the different immunostaining steps were done using a semiautomated immunostainer (Ventana Medical System, Tucson, AZ). A mouse monoclonal antibody against gp-100 (clone HMB-45) and a rabbit polyclonal antibody against S-100 protein (both Ventana) were used to determine the presence of melanoma cells. The presence of HPV was determined using a rabbit polyclonal antibody raised against bovine papilloma virus 1 and reacting with all HPV isolates (Ventana). Melanoma antibodies to S-100 and gp-100 and antibodies to HPV were detected using the alkaline phosphatase Vectastain ABC-AP kit (Vector Laboratories, Burlingame, CA). Antibodies were prediluted according to the manufacturer’s recommendations. Sections were counterstained with hematoxylin (Vector Laboratories). Slides were then mounted using a xylene-based mounting medium (Stephen Scientific, Kalamazoo, MI).
Serial sections were stained for S-100, gp-100, and HPV separately. Negative (normal skin biopsy) and positive (condyloma biopsy) 35 controls were included in each stain experiment. Multiple sections were read using a dual-headed Zeiss microscope (Heidelberg, Germany). Photographs of the same fields were taken at magnifications of ×25 and ×125. Two investigators (DD, CC) recorded the number of HPV-positive cells at a magnification of ×250 in five different tumor-positive fields. In addition, when available, up to five (median 4, range 2–5) normal tissue (nontumor) fields were also counted. No significant difference was observed between both counts, and the results were averaged per biopsy and expressed as number of HPV-positive cells per field. HPV-positive tumors were defined as biopsy specimens with several HPV-positive cells per field. The tumor biopsy specimens with an average value of less than one HPV-positive cell per field were defined as negative.
Follow-Up
After surgery, patients were evaluated on a standard melanoma follow-up protocol with examination, complete blood count, and liver function tests every 3 months and a chest radiograph or scans every 6 months. Patients received no treatment (n = 5) or various immunotherapy regimens (n = 9). 34 Melanoma progression was evaluated based on the development of metastatic lesions as determined by radiologic analyses, tumor biopsies, and melanoma-related death.
Melanoma Cell Cultures
After dissociation, melanoma cells were cultured in complete medium as noted above. The culture medium was changed every 3 to 4 days, and the growth of cells was evaluated every week by microscopic observations. Viable melanoma cells were obtained from all biopsy specimens. Cells isolated from the biopsy specimens were cultured for up to 3 months. The cells still dividing after 3 months in vitro were defined as growing cells. Cells that stopped growing or remained alive but without dividing were defined as nongrowing cells.
Statistical Analysis
Patient characteristics, including sex, age, AJCC stage, treatment, disease progression, and death, and the ability of biopsy cell suspension to grow in vitro were tested using an unpaired t test. Differences in in vitro growth and disease parameters (survival) and the HPV status of the tumor tissues were tested using chi-square tests.
RESULTS
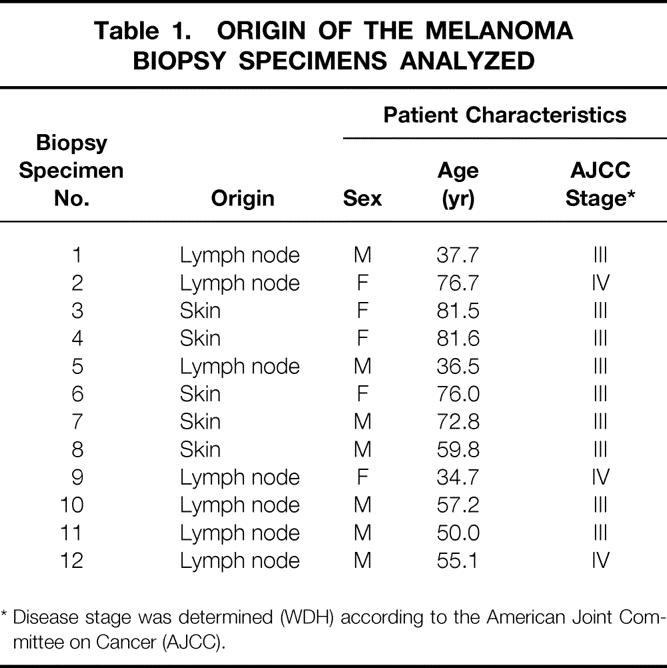
Twelve biopsy specimens were obtained from lymph nodes (n = 7, 58%) or skin (n = 5, 42%;Table 1). The male:female ratio was 7:5. The median age was 58.5 years (range 34.7–81.6). Nine of the biopsy specimens were obtained from patients with AJCC stage III melanoma, and three were from patients with stage IV disease.
Table 1. ORIGIN OF THE MELANOMA BIOPSY SPECIMENS ANALYZED
* Disease stage was determined (WDH) according to the American Joint Committee on Cancer (AJCC).
The biopsy specimens were stained for melanoma using specific antibodies against gp-100 and S-100 proteins. All the samples were positive either for S-100 protein or gp-100. All but one biopsy specimen was positive for S-100 protein (92%). gp-100 was detected in 8 of 12 biopsy specimens (67%). As shown in Figure 1, S-100 and gp-100 were present in melanoma tumors with different intensities and locations. Fewer melanoma cells stained positive for gp-100 than for S-100 protein.
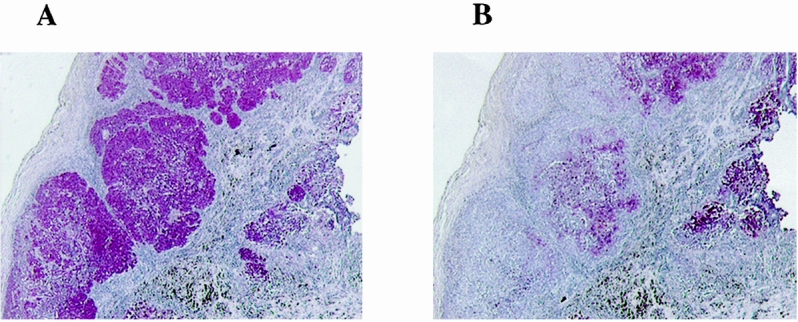
Figure 1. Serial sections of a melanoma biopsy specimen immunostained for S-100 protein (A) or gp-100 protein (B) using specific antibodies. Original magnification ×25.
Cells positive for HPV (Fig. 2) were detected in the tumor areas or the biopsy specimens in cells that were positive for either S-100 or gp-100. Although variations in both the intensity of the staining and the presence of HPV-positive melanoma cells were observed, the presence of HPV was observed in cells stained with melanoma antigens. In the normal tissue areas of the biopsy specimens, regardless of the HPV tumor status, few cells were positive for HPV. The presence of HPV-positive cells in both the tumor and the normal tissue areas of the biopsy specimens was evaluated on multiple sections of each biopsy specimen. HPV-positive cells in the tumor areas of the biopsy specimens were either absent or rare (n = 5) or constantly present throughout the tumor (n = 7) (Table 2). On average, HPV-negative biopsy specimens contained 0.33 ± 0.2 HPV-positive cells per field, whereas HPV-positive biopsy specimens contained 3.9 ± 0.56 HPV-positive cells per field (P = .0002). The number of cells positive for HPV was well below the number of HPV-positive cells recorded in a positive control condyloma biopsy specimen. In the normal tissue, the proportion of cells stained for HPV was similar regardless of the HPV status (range 0–1.3 HPV-positive cells per field; not significant).
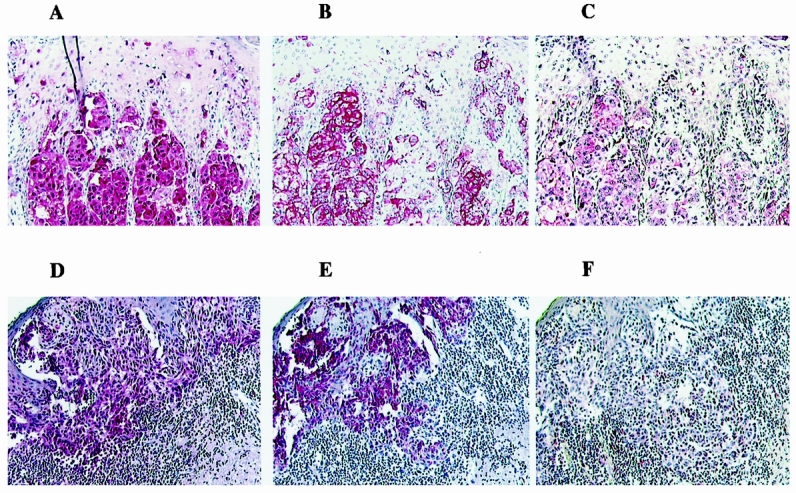
Figure 2. Serial sections of two melanoma biopsy specimens immunostained for S-100 protein (A,D), gp-100 protein (B,E), or human papilloma virus (C,F) using specific antibodies. Original magnification ×125.
Table 2. NUMBER OF HPV-POSITIVE CELLS IN THE TUMOR AND NORMAL AREAS OF THE BIOPSY SPECIMENS
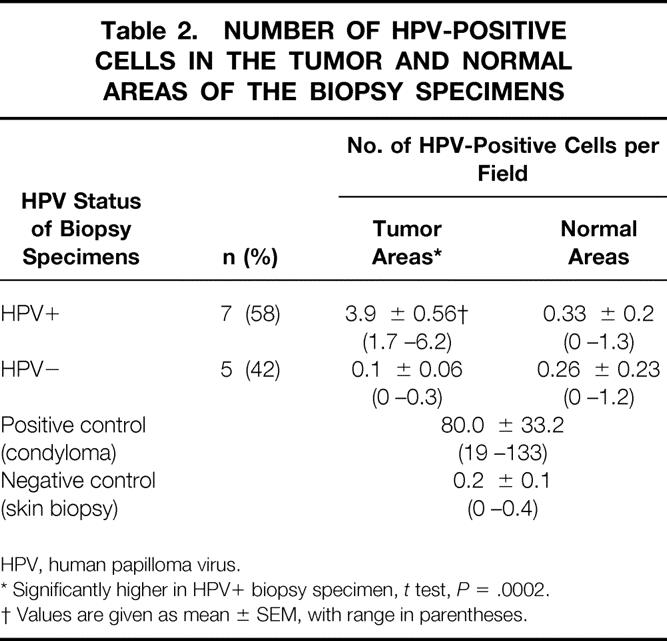
HPV, human papilloma virus.
* Significantly higher in HPV+ biopsy specimen, t test, P = .0002.
† Values are given as mean ± SEM, with range in parentheses.
In vitro, none of the HPV-negative tumor cells grew from the tumor biopsy specimens (Table 3), whereas five of seven of the HPV-positive melanoma specimens grew very well (chi-square test, P = .09, not significant). The sex, age, AJCC stage, and treatment of these patients were not statistically correlated with the HPV status of the biopsy specimens. However, all patients with HPV-positive tumor cells had recurrences and died of melanoma progression. Four of five patients with HPV-negative tumor cells remained alive and without melanoma recurrence. The distribution of HPV-positive biopsy specimens correlated with patient outcome in terms of recurrence (chi-square test, P = .02), death from melanoma (chi-square test, P = .004), and survival time (chi-square test, P = .001).
Table 3. EVOLUTION OF PATIENTS AND CELL CULTURES DERIVED FROM HPV+ AND HPV− MELANOMA BIOPSY SPECIMENS
HPV, human papilloma virus.
* Significant correlation with HPV status, chi-square test, P = .02.
† Significant correlation with HPV status, chi-square test, P = .004.
‡ Significant correlation with HPV status, chi-square test, P = .001. Values are given as mean ± SEM (range).
DISCUSSION
This study was designed to determine whether HPV (or at least HPV proteins) was present in melanoma biopsy specimens, and if so, to correlate the presence of HPV with the clinical outcome and the derivation of long-lasting melanoma in vitro cell cultures. The results indicate that HPV was present in 58% of the melanoma biopsy specimens tested, the presence of HPV was associated with sustained growth of melanoma cells in vitro, and the presence of HPV was associated with a poorer prognosis for patients with AJCC stage III and IV melanoma.
Different approaches may be used to determine the presence of viral oncoproteins in biopsy specimens, including immunohistochemistry, polymerase chain reaction (PCR), and in situ hybridization. PCR detection of different subtypes of HPV has been used on cervical carcinoma samples 36 and nonmelanoma skin cancers. 37 However, because of the sensitivity of this technique, a significant number of false-positive results may occur, as shown in clinical PCR diagnosis of Mycobacterium tuberculosis. 38 Clinical diagnosis using PCR techniques still requires that issues such as control of amplicon contamination, specimen treatment to avoid inhibitors, and interpretation of positive results be addressed. 39 In the present study, an immunohistochemical approach using a specific polyclonal antibody that recognized most HPV types was preferred. Indeed, because the distribution of melanoma cells in the biopsy specimens was heterogeneous, the staining of serial sections allowed for the simultaneous detection of melanoma cells and HPV. The positive (condyloma biopsy) 35 and negative (normal skin biopsy) controls were stained for HPV with the samples and remained positive and negative throughout the experiments. This suggests that the immunostaining technique used here was sensitive in detecting HPV in the melanoma biopsy specimens. Whether this result will be confirmed by in situ hybridization techniques is under investigation.
The presence of HPV in a portion of melanoma cells in some biopsy specimens clearly indicates that HPV is associated with some melanomas, as indicated previously. 30,31 Two types of HPV—HPV 16 30 and HPV 38 31 —have been associated with melanoma in recent clinical cases. In addition, our laboratory has found both HPV 16 and HPV 35 in cell lines derived from melanoma biopsy specimens (unpublished data). HPV has been associated with different nonmelanoma skin cancers such as cutaneous squamous cell cancers from renal allograft recipients, 40 from epidermodysplasia verruciformis, 41 and immunosuppressed patients. 11 The presence of HPV has a well-demonstrated association with the development and progression of invasive cancers of the genital tract, 7 skin, 37 and head and neck. 42 In addition, some recent work suggests that HPV was widespread in more than one third of the skin biopsy specimens tested and often occurred in normal skin. 40 However, others found that HPV type 16 sequences were absent in the DNA of normal skin from patients with cutaneous squamous cell cancer. 42 Our observations indicate that in skin biopsies, HPV was present in some of the normal skin specimens, as reported previously. 40 Different HPV types, including HPV 5, 16, 20, and 38, were found in normal skin and skin lesions. 40–42 The HPV types present in our biopsy specimens, especially HPV 16, 18, 35, and 38, 40 are under analysis.
Five of the seven cell cultures isolated from melanoma biopsy specimens positive for HPV grew in vitro. This observation was in agreement with our previous work on melanoma cell lines derived from fresh biopsy specimens. 32 Indeed, the PCR analysis of 22 melanoma cell cultures showed that HPV was present in 58% of the cell cultures. By sequencing the PCR products, HPV was identified as of type 16 and 35 (unpublished data). The presence of HPV in the cell culture that grew in vitro may be linked to the action of early proteins E6 and E7 on the cell cycle of melanoma cells. Indeed, the introduction of E6 and E7 open reading frames of HPV type 16 into cultured normal human melanocytes resulted in the immortalization of the cells. 27 The presence of these two proteins alone was sufficient to generate a melanocyte cell line in vitro. 27
Mechanisms by which the cell cycle of melanoma cells is altered are likely to be similar to those extensively studied in cervical cancer. 7 The early HPV E6 and E7 proteins have been shown to stimulate cell proliferation by activating cyclins E and A and by interfering with the functions of the cell cycle proteins Rb and p53. 11,43,44 The cellular tumor suppressor gene products, p53 and pRb, were important targets for the viral oncoproteins E6 and E7, respectively, in epithelial cells infected with HPV. 11 The crucial role of E6 and E7 proteins was demonstrated using antisense RNA to E6/E7, which led to growth inhibition from specific E6/E7 downregulation of the tumor cells positive for HPV. 45 Further, few or no mutations of the p53, p16, and p21 genes were found in oral carcinoma lesions associated with HPV. 46 Mutations in p53 were also detected in 48% of nonmelanoma skin cancers in renal allograft recipients and in 63% of sporadic tumors from immune-competent patients, mostly consistent with damage caused by UV irradiation. 47 In both studies, no relation between HPV status and p53 mutation was identified. 46,47 The sequencing of p53 exons 5 to 9 showed that p53 mutations were present in only 2 of 25 cell lines tested (unpublished data). This observation is in agreement with previous studies that indicate a low level of p53 mutation in melanoma. 6 Whether other mutations are involved in the HPV-positive lesions remains to be determined.
Although the interactions between the early protein E6 and E7 with different proteins involved in the cell cycle have been shown, 5,28 the precise sequence of events leading to the immortalization of the cells remains to be determined. Moreover, as in epidermodysplasia verruciformis, 43 and in contrast with anogenital and cervical malignancies, 11 the possible HPV mechanisms remain uncertain and may require UV radiation. Indeed, UV radiation and the presence of HPV were associated with an increased risk of nonmelanoma skin cancer in patients. 11,48 Whether UV alone or the interaction between UV and HPV infection is needed for the development and progression of melanoma remains to be analyzed.
From a clinical standpoint, our results indicate that the biopsy specimens positive for HPV were associated with a poorer clinical outcome, suggesting that the presence of HPV may indicate a melanoma type more prone to recurrence or progression. Although the number of biopsy specimens tested is small, the results clearly indicate a correlation between poor melanoma outcome and the presence of HPV in biopsy specimens obtained from AJCC stage III and IV melanoma. A larger cohort of patients is being evaluated.
Acknowledgments
The authors thank Pam Pitman, LPN, in the Department of General Surgery Research at Carolinas Medical Center for gathering the clinical data, Laura Blacksten, BA, for her technical help, and Cissy Moore-Swartz, MA, for her editing assistance.
Discussion
Dr. J. Patrick O’Leary (New Orleans, Louisiana): I could not help but observe that Dr. Holder sent this manuscript to me, which falls almost completely outside of my sphere of expertise, if in fact I have one. I can only opine that it was to make sure that he got some simple questions to answer, and I will try to comply.
I think this is a fascinating observation. We should not get lost in the nomenclature. What we should focus on is that patients who have HPV virus-positive particles in their tumors died, and those that did not, with the exception of the one patient, lived. Now, the caveats are: the numbers are small, it’s from one center, technology varies—but this is an intriguing observation. My questions are about patient selection.
Why did you choose just stage III and IV patients? Could there have been something else about these patients that might have impacted on their survival?
What about the nutritional state? What about the burden of tumor? Was the burden of tumor the same? Were the majority of the patients who died actually in stage IV disease instead of stage III?
This begs the real question: was the HPV virus infestation causative that pushed the melanoma to become a more aggressive variant, or something that just happened? Was it just happenstance that these tumor variants were susceptible to the infestation by the HPV virus?
Should we go home now and look at all of our melanoma patients, pull their blocks, stain them? And if we find that they have a virus, should we then treat the virus with ganciclovir or some other type of antiviral therapy?
Dr. Douglas S. Reintgen (Tampa, Florida): The authors should be commended for a diligent search for other prognostic factors for melanoma progression. They conclude that biopsies from patients with stage III and IV disease that were HPV-positive correlated with recurrence, death from melanoma, and survival time. However, it is difficult to say anything about cofactors for development of melanoma when the biopsies were performed on metastatic disease, that is, in patients with stage III and IV melanoma. All the biopsies were either from regional nodes or distant skin metastatic sites. UV radiation and HPV infection may be synergistic for the development of melanoma, but certainly this study needs to be performed in premalignant tissue, like dysplastic nevi and primary melanomas, to perform the definitive study.
But this is not unusual, I think, for studies involving HPV. Most epidemiologic studies of HPV infection in cancer have been conducted with the use of samples taken after the cancer is diagnosed, well established, or has metastasized. Such studies that provide no information on the temporal order of events may be biased in their estimation of risk, because the presence of disease itself may increase the detectability of HPV, that is, as the HPV-infected tissue mass grows, sampling is facilitated and the HPV genome is amplified to allow easier detection. In a way, it’s comparable to the cervical cancer story, where cervical intraepithelial neoplasia (a premalignant lesion) and carcinoma in situ is preceded by the persistent presence of a detectable HPV DNA in healthy women.
HPV infection, particularly in the female population that is sexually active, is highly prevalent. Thirty percent of melanoma patients are women of childbearing age. How extensively did the authors test other normal tissue for HPV in these patients with metastatic melanoma?
There is more solid evidence from the study that suggests HPV may be involved in melanoma metastasis, resulting in higher recurrence rate and lower survival. This mechanism is through cell-cycle regulatory steps, and a block in one of the cell-cycle checkpoint proteins would cause immortalization of the cell line. This certainly may be true. There are no good prognostic factors for patients with melanoma that has metastasized. For stage III disease, there is the number of positive lymph nodes and the presence of extranodal disease. For stage IV disease, variables such as skin and soft tissue metastasis have a better survival than patients with visceral metastasis. If this assay can enhance the prognostication once metastasis develops, that can be helpful. However, the more exciting possibility would be that if HPV infection is a cofactor for melanoma development, then treatment strategies such as vaccination or public health measures against this virus may be designed. That is the true potential of this work.
Dr. J. Dirk Iglehart (Boston, Massachusetts): Walter, as the other discussants have indicated, that was a very provocative paper. In other viral systems, such as cervical cancer and penile cancer, where HPV seems to be important, the targets of the HPV are probably p53 and the retinoblastoma gene product. At least in cervical and penile carcinomas, it tends to be those tumors that are p53 mutation-negative that are targeted by HPV. In melanoma, however, the vast majority are p53 mutation-positive. And so you wonder what’s being targeted. Is it the p53-negative tumors similar to cervical cancer and penile cancer that are being targeted?
Dr. Edward M. Copeland III (Gainesville, Florida): Walter, I would assume that in the biopsies you stain for the HPV, you also stain some normal melanocytes in the surrounding tissue. Do you ever find the HPV in normal melanocytes, particularly in those patients who are HPV-positive in the tumor?
Dr. Walter D. Holder, Jr. (Closing Discussion): As you can see, we are very early in evaluating this whole concept of HPV and what’s happening with melanoma patients. As such, I think we certainly need more data to define whether or not HPV is actually involved in the etiology of melanoma, or whether it has consistently negative effect as far as the progression of disease is concerned.
In regard to Dr. Iglehart’s question, are p53-negative tumors targeted by HPV and melanoma: at this point we do not know the answer to that. Hopefully, we will soon be able to tell you.
Unfortunately, we could not evaluate the primary tumor for HPV in most of these patients. We were able to obtain several of the primaries that did correlate with HPV positivity of the metastasis. We are now prospectively evaluating stage I and stage II melanomas and also premalignant tissues, as Dr. Reintgen asked, by immunohistochemistry and also by in situ hybridization for evidence of HPV positivity and to help us clarify the evolution of these HPV-positive cells and correlate this with clinical outcome.
Dr. Copeland asked a question about normal tissues. Normal tissues were removed from all of these patients in addition to the melanoma, and were tested for HPV positivity. Normal tissues included skin and normal melanocytes, subcutaneous tissue consisting of fat, fibroblasts, vessels, and also some lymph node tissue. All these were negative for HPV. We saw none of these patients with condylomata or other evidence of active HPV infection.
Regarding Dr. O’Leary’s question, HPV could possibly just be tagging along for the ride, and not be involved as an etiologic agent or a cofactor at all. We aren’t to the point yet where we could say with any definition that this is or is not the case. However, in every single melanoma primary or established culture that we have evaluated thus far—and we have around 100 of these currently—the presence of HPV in those cells renders those cells much more rapidly dividing and invasive in a highly statistically significant fashion than HPV-negative melanoma cells.
The question is whether or not the viral genome is actually integrated into the host genome. We have looked for E6 and E7 DNA in the established cultures and in melanoma biopsies utilizing PCR and have found evidence of one or the other of these DNA fragments and, actually, both in the majority of cases of the HPV-positive cells—and none in the adjacent normal tissues. At least E6 and E7 are incorporated into the DNA of the cells, and we don’t know as yet if the full viral genome is incorporated or not. We certainly cannot tell you if any infectious virus particles are produced.
Responding to Dr. O’Leary’s questions, should everyone go back and look at all melanoma tissue blocks? I don’t think we would recommend this. I would like to see more data on this and certainly would like to see this evaluated at other centers as well. But again, the implication is very strong that HPV positivity renders a very poor prognosis in patients with melanoma. The choice of using stage III and IV patients was based on our referral patterns and availability of tissues. Finally, we are currently evaluating the effects of antiviral drugs on HPV-positive melanoma cells in vitro.
Footnotes
Correspondence: Didier Dréau, PhD, Dept. of General Surgery Research, Carolinas Medical Center, P.O. Box 32681, Charlotte, NC 28232-2861.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Financed in part by a grant from the Foundation for the Carolinas, Charlotte, NC (DD).
E-mail: ddreau@carolinas.org
Accepted for publication December 1999.
References
- 1.Elder DE. Human melanocytic neoplasms and their etiologic relationship with sunlight. J Invest Dermatol 1989; 92:297S–303S. [DOI] [PubMed] [Google Scholar]
- 2.Robinson JK, Rigel DS, Amonette RA. Trends in sun exposure knowledge, attitudes, and behaviors: 1986 to 1996. J Am Acad Dermatol 1997; 37:179–186. [DOI] [PubMed] [Google Scholar]
- 3.van Elsas A, Scheibenbogen C, van der Minne C, Zerp SF, Keilholz U, Schrier PI. UV-induced N-ras mutations are T-cell targets in human melanoma. Melanoma Res 1997; 7:S107–S113. [PubMed] [Google Scholar]
- 4.Saenz-Santamaria MC, McNutt NS, Bogdany JK, Shea CR. p53 expression is rare in cutaneous melanomas. Am J Dermatopathol 1995; 17:344–349. [DOI] [PubMed] [Google Scholar]
- 5.Swan DC, Vernon SD, Icenogle JP. Cellular proteins involved in papillomavirus-induced transformation. Arch Virol 1994; 138:105–115. [DOI] [PubMed] [Google Scholar]
- 6.Herlyn M. Human melanoma: development and progression. Cancer Metastasis Rev 1990; 9:101–112. [DOI] [PubMed] [Google Scholar]
- 7.zur Hausen H. Papillomavirus infections—a major cause of human cancers. Biochem Biophys Acta 1996; 1288:F55–F78. [DOI] [PubMed] [Google Scholar]
- 8.Sur M, Cooper K. The role of the human papilloma virus in esophageal cancer. Pathology 1998; 30:348–354. [DOI] [PubMed] [Google Scholar]
- 9.van Rensburg EJ, van Heerden WF, Raubenheimer EJ. Langerhans cells and human papillomaviruses in oesophageal and laryngeal carcinomas. In Vivo 1993; 7:229–232. [PubMed] [Google Scholar]
- 10.McGregor JM, Proby CM. The role of papillomaviruses in human non-melanoma skin cancer. Cancer Surv 1996; 26:219–236. [PubMed] [Google Scholar]
- 11.Harwood CA, McGregor JM, Proby CM, Breuer J. Human papillomavirus and the development of non-melanoma skin cancer. J Clin Pathol 1999; 52:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin LG, Demers GW, Galloway DA. Disruption of the G1/S transition in human papillomavirus type 16 E7-expressing human cells is associated with altered regulation of cyclin E. J Virol 1998; 72:975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennig EM, Nesland JM, Di Lonardo A, Venuti A. Multiple primary cancers and HPV infection: are they related? J Exp Clin Cancer Res 1999; 18:53–54. [PubMed] [Google Scholar]
- 14.Poljak M, Cerar A, Seme K. Human papillomavirus infection in esophageal carcinomas: a study of 121 lesions using multiple broad-spectrum polymerase chain reactions and literature review. Hum Pathol 1998; 29:266–271. [DOI] [PubMed] [Google Scholar]
- 15.Atula T, Grenman R, Klemi P, Syrjanen S. Human papillomavirus, Epstein-Barr virus, human herpesvirus 8 and human cytomegalovirus involvement in salivary gland tumours. Oral Oncol 1998; 34:391–395. [DOI] [PubMed] [Google Scholar]
- 16.Mueller N. Overview of the epidemiology of malignancy in immune deficiency. J Acquir Immune Defic Syndr 1999; 21:S5–S10. [PubMed] [Google Scholar]
- 17.Thomas JT, Laimins LA. Human papillomavirus oncoproteins E6 and E7 independently abrogate the mitotic spindle checkpoint. J Virol 1998; 72:1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith ML, Chen IT, Zhan Q, O’Connor PM, Fornace AJ Jr. Involvement of the p53 tumor suppressor in repair of UV-type DNA damage. Oncogene 1995; 10:1053–1059. [PubMed] [Google Scholar]
- 19.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990; 63:1129–1136. [DOI] [PubMed] [Google Scholar]
- 20.Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol 1998; 18:7288–7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keen N, Elston R, Crawford L. Interaction of the E6 protein of human papillomavirus with cellular proteins. Oncogene 1994; 9:1493–1499. [PubMed] [Google Scholar]
- 22.Lechner MS, Laimins LA. Inhibition of p53 DNA binding by human papillomavirus E6 proteins. J Virol 1994; 68:4262–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessis TD, Slebos RJ, Nelson WG, et al. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci 1993; 90:3988–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amellem O, Sandvik JA, Stokke T, Pettersen EO. The retinoblastoma protein-associated cell cycle arrest in S-phase under moderate hypoxia is disrupted in cells expressing HPV18 E7 oncoprotein. Br J Cancer 1998; 77:862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DL, Munger K. Interactions of the human papillomavirus E7 protein with cell cycle regulators. Semin Cancer Biol 1996; 7:327–337. [DOI] [PubMed] [Google Scholar]
- 26.Pagano M, Durst M, Joswig S, Draetta G, Jansen-Durr P. Binding of the human E2F transcription factor to the retinoblastoma protein but not to cyclin A is abolished in HPV-16-immortalized cells. Oncogene 1992; 7:1681–1686. [PubMed] [Google Scholar]
- 27.Le Poole IC, van den Berg FM, van den Wijngaard RM, et al. Generation of a human melanocyte cell line by introduction of HPV16 E6 and E7 genes. In Vitro Cell Dev Biol Anim 1997; 33:42–49. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Han S, Baluda MA, Park NH. HPV-16 oncogenes E6 and E7 are mutagenic in normal human oral keratinocytes. Oncogene 1997; 14:2347–2353. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Salas LM, Cullinan AE, Siwkowski A, Hampel A, DiPaolo JA. Inhibition of HPV-16 E6/E7 immortalization of normal keratinocytes by hairpin ribozymes. Proc Natl Acad Sci USA 1998; 95:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takamiyagi A, Asato T, Nakashima Y, Nonaka S. Association of human papillomavirus type 16 with malignant melanoma. Am J Dermatopathol 1998; 20:69–73. [DOI] [PubMed] [Google Scholar]
- 31.Scheurlen W, Gissmann L, Gross G, zur Hausen H. Molecular cloning of two new HPV types (HPV 37 and HPV 38) from a keratoacanthoma and a malignant melanoma. Int J Cancer 1986; 37:505–510. [DOI] [PubMed] [Google Scholar]
- 32.Dréau D, Hogg M, Foster M, Swiggett J, Culberson C, Holder WD. Presence and effects of human papilloma virus oncoproteins on cell cycle protein expression and growth of melanoma cells in vitro. Proc Am Assoc Cancer Res 1999; 40:573. [Google Scholar]
- 33.Holder WD Jr, White RL Jr, Zuger JH, Easton EJ Jr, Greene FL. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg 1998; 227:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dréau D, Bosserhoff AK, White RL, Buettner R, Holder WD. Melanoma-inhibitory activity protein concentrations in blood of melanoma patients treated with immunotherapy. Oncol Res 1999; 11:55–61. [PubMed] [Google Scholar]
- 35.Mittal K, Demopoulos RI, Tata M. A comparison of proliferative activity and atypical mitoses in cervical condylomas with various HPV types. Int J Gynecol Pathol 1998; 17:24–28. [DOI] [PubMed] [Google Scholar]
- 36.Adams V, Moll C, Schmid M, Rodrigues C, Moos R, Briner J. Detection and typing of human papillomavirus in biopsy and cytological specimens by polymerase chain reaction and restriction enzyme analysis: a method suitable for semi-automation. J Med Virol 1996; 48:161–170. [DOI] [PubMed] [Google Scholar]
- 37.de Villiers EM. Human papillomavirus infections in skin cancers. Biomed Pharmacother 1998; 52:26–33. [DOI] [PubMed] [Google Scholar]
- 38.Beige J, Lokies J, Schaberg T, et al. Clinical evaluation of a Mycobacterium tuberculosis PCR assay. J Clin Microbiol 1995; 33:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baselski VS. The role of molecular diagnostics in the clinical microbiology laboratory. Clin Lab Med 1996; 16:49–60. [PubMed] [Google Scholar]
- 40.Astori G, Lavergne D, Benton C, et al. Human papillomaviruses are commonly found in normal skin of immunocompetent hosts. J Invest Dermatol 1998; 110:752–755. [DOI] [PubMed] [Google Scholar]
- 41.de Villiers EM, Lavergne D, McLaren K, Benton EC. Prevailing papillomavirus types in non-melanoma carcinomas of the skin in renal allograft recipients. Int J Cancer 1997; 73:356–361. [DOI] [PubMed] [Google Scholar]
- 42.McKaig RG, Baric RS, Olshan AF. Human papillomavirus and head and neck cancer: epidemiology and molecular biology. Head Neck 1998; 20:250–265. [DOI] [PubMed] [Google Scholar]
- 43.Franch HA, Shay JW, Alpern RJ, Preisig PA. Involvement of pRb family in TGF beta-dependent epithelial cell hypertrophy. J Cell Biol 1995; 129:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proby CM, Harwood CA. Role of human papillomaviruses in warts and cancer. Hosp Med 1998; 59:33–36. [PubMed] [Google Scholar]
- 45.He Y, Huang L. Growth inhibition of human papillomavirus 16 DNA-positive mouse tumor by antisense RNA transcribed from U6 promoter. Cancer Res 1997; 57:3993–3999. [PubMed] [Google Scholar]
- 46.Heinzel PA, Balaram P, Bernard HU. Mutations and polymorphisms in the p53, p21 and p16 genes in oral carcinomas of Indian betel quid chewers. Int J Cancer 1996; 68:420–423. [DOI] [PubMed] [Google Scholar]
- 47.McGregor JM, Berkhout RJ, Rozycka M, et al. p53 mutations implicate sunlight in post-transplant skin cancer irrespective of human papillomavirus status. Oncogene 1997; 15:1737–1740. [DOI] [PubMed] [Google Scholar]
- 48.Leigh IM, Glover MT. Skin cancer and warts in immunosuppressed renal transplant recipients. Recent Results Cancer Res 1995; 139:69–86. [DOI] [PubMed] [Google Scholar]