Abstract
Objective
To test the hypothesis that platelet-derived growth factor (PDGF) accelerates the formation of allograft vascular disease.
Summary Background Data
Allograft vasculopathy, characterized by myointimal hyperplasia of the coronary arteries in the transplanted heart, is the most common cause of late graft failure and death in heart transplant recipients. The cause of the process is unclear, and no treatment exists. PDGF has been implicated in alterations in vascular endothelial biology and in vascular restenosis, but the role of PDGF in allograft vasculopathy has not been explored.
Methods
An orthotopic heart transplant model was established in the rat mismatched at one class II locus using the PVGR8 and PVGR23 strains. No immunosuppressive regimen was used. Six treatment groups (PDGF-A, PDGF-A antibody, and PDGF-A receptor antibody) using 10 rats per group were examined. An untreated group of 10 rats manifesting chronic rejection as well as the native hearts were used as controls. PDGF-A at 1 ng/dL (10 rats) or 10 ng/dL (10 rats) was administered intraperitoneally to each transplant group. Similar groups were treated with PDGF-A antibody and PDGF-A receptor antibody. The animals were killed after 50 days; transplanted and native hearts were removed and coronary arteries were examined morphometrically. Smooth muscle proliferation was confirmed by immunohistochemistry. Statistical analysis was performed using multivariate analysis of variance.
Results
Coronary myointimal hyperplasia was seen in the chronic rejection group. The PDGF-A groups showed significant myointimal hyperplasia. Administration of PDGF-A antibody did not attenuate the process. Administration of PDGF-A receptor antibody at 1 ng/dL resulted in reduction of the hyperplasia, and 10 ng/dL significantly attenuated the process.
Conclusions
This study establishes a cause-and-effect relation between PDGF-A and coronary myointimal hyperplasia in the rat transplant model. Blockade of the PDGF-A receptor clearly attenuates the process, indicating a potential mode of therapy to be explored.
As the survival of cardiac allograft recipients reaches the 10-year mark, various complications limit the long-term survival of these patients. Graft coronary artery disease or allograft vasculopathy becomes the leading cause of death after the first year and becomes more significant the longer the patient lives. A 30% to 40% incidence of allograft vascular disease has been reported 5 years after transplantation, 1 and these statistics have not changed significantly even with the development of new immunosuppressive regimens. The process is not isolated to cardiac allografts: data from multicenter trials in North America have shown that fewer than 50% of renal allografts survive 6 years, despite the fact that more than 80% are functioning at the end of the first year. 2 This graft attrition is attributed to allograft vascular disease.
The histopathology of allograft vascular disease was first described in 1968 3 and has since been substantiated in various animal models and clinical studies. The consensus from all studies is that the pathology is one of progressive concentric myointimal hyperplasia of the arteries in the affected organ. One of the suggested mechanisms contributing to the process is migration of smooth muscle cells from the media of the vessel wall to the subintimal layer. Evidence supporting this hypothesis is derived from studies examining the formation of atheromatous plaque. 4 The theory was tested in a rat aortic allograft model that clearly demonstrated vascular smooth muscle cells to be an important component of the lesion of intimal hyperplasia in allograft vasculopathy. 5 However, the inciting mechanism has yet to be determined.
Several growth factors, including platelet-derived growth factor (PDGF), have been shown to invoke a mitogenic response in smooth muscle cells and have been implicated in the pathogenesis of atherosclerosis. 6 There is also significant evidence that PDGF receptor is upregulated in allograft tissue involved in chronic rejection. 7 However, to date there has been no experimental evidence to support a cause-and-effect relation between PDGF and allograft vasculopathy. This report explores the hypothesis that PDGF plays an integral role in the formation of allograft vascular disease.
METHODS
Male and female PVGR8 and PVGR23 rats (University of Massachusetts, Worcester, MA) weighing 250 to 300 g were used in the study. The animals were housed in the animal facility with a controlled environment and a 12-hour light/dark cycle. They were kept in ventilated shoebox racks and allowed water and food (rat chow) ad libitum. The animals were inspected daily and were killed if signs of distress were present, according to the guidelines of the Animal Care Welfare Act.
Cardiac Transplant Model
Each donor recipient pair was anesthetized using intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg). The cardiac transplantation procedure used was a modification of the original technique described by Adams et al. 8
In brief, the donor was prepared first in all instances. After the induction of anesthesia, the chest was prepared with povidone–iodine. A median sternotomy incision was made. The inferior vena cava was isolated and the heart-lung block was perfused in a retrograde fashion with Plegiasol {TM}(Abbott, Chicago, IL) at 4°C until cardiac standstill was obtained. The heart and lungs were then excised en bloc and placed in a 4°C Plegiasol bath. The heart was isolated and prepared for transplantation. The recipient, in the meantime, was anesthetized and the neck was prepared. The carotid artery and internal jugular veins were isolated. The donor aorta was anastomosed to the recipient carotid artery, and the donor pulmonary artery was anastomosed to the internal jugular vein. Warm saline was then applied to the graft, and the incision was closed only when satisfactory cardiac activity ensued. The mean time between donor cardiectomy and transplantation was 35 minutes.
Experimental Design
Seven groups, each with 10 animals, were used (Fig. 1). Each animal underwent orthotopic cardiac transplantation, and no immunosuppression was used. Graft function was assessed daily by palpation. Each animal was maintained for 50 days, during which time either no agent was administered or a daily intraperitoneal injection of PDGF-A (1 or 10 ng/dL), PDGF-A antibody (1 or 10 ng/dL), or PDGF-A receptor antibody (1 or 10 ng/dL) was administered, based on the group to which the animal was assigned (in masked fashion). Doses were determined by previously performed cell culture experiments. At the end of the experimental time, each animal was killed. Native and transplanted hearts were removed for morphometric analysis using IP Lab Scientific Imaging Software {TM}(Scanalytics, Inc., Fairfax, VA). Myointimal hyperplasia was confirmed by immunohistochemical staining. The native heart and no-treatment group animals served as controls.
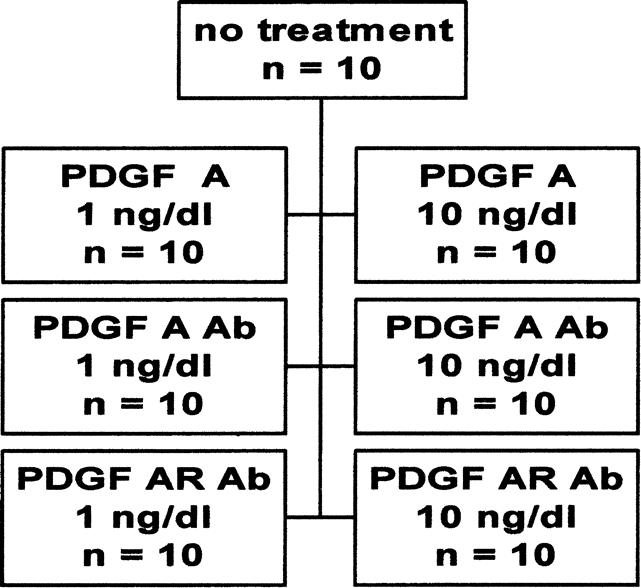
Figure 1. Control and six treatment groups.
Thirty sections were obtained from each heart. To assess the degree of myointimal hyperplasia, each coronary artery in each section was analyzed. Measurements were taken at four quadrants in each vessel. A mean of the measurements from the 30 sections was then calculated.
Statistical Analysis
Results are presented as mean ± standard error of the mean. Multivariate analysis of variance was used to analyze the significance of the data.
RESULTS
PVGR8/R23 Model
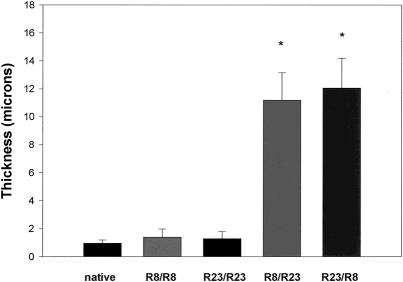
To verify the model, isogeneic as well syngeneic transplants were performed. As shown in Figure 2, little if any myointimal hyperplasia developed in isogeneic allografts. Statistically significant allograft vascular disease developed after 50 days in the syngeneic allografts, regardless of the donor/recipient combination.
Figure 2. Degree of myointimal hyperplasia produced in the rat cardiac transplant model using PVGR8/PVGR23. A statistically significant amount of myointimal hyperplasia was generated in the syngeneic donor/recipient pairs versus the native hearts and isogeneic controls (n = 10 per group;P < .001).
Treatment Groups
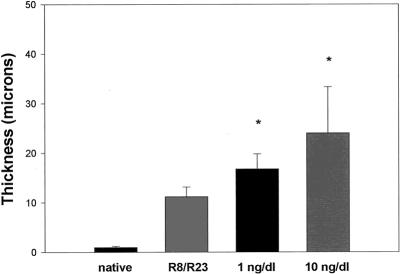
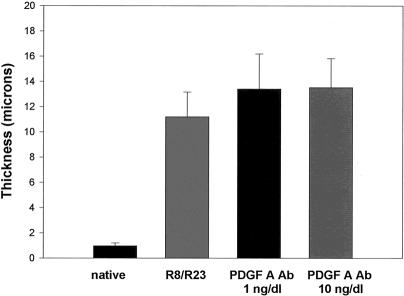
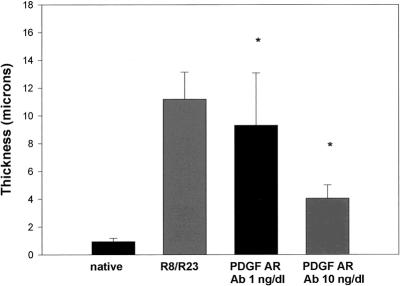
PDGF-A was administered in doses of 1 and 10 ng/dL. Figure 3 shows that PDGF-A significantly potentiated the formation of myointimal hyperplasia in the transplanted groups in a dose-dependent manner. When PDGF-A antibody was administered independently to the transplanted groups, no effect was observed on the degree of vasculopathy generated when compared with the control group R8/R23 (Fig. 4). Administration of PDGF-A receptor antibody produced an interesting result (Fig. 5). When the 1-ng/dL dose was given, the hyperplastic response was diminished from that seen in the control group. The 10-ng/dL dose produced a more pronounced attenuation of the process.
Figure 3. Amount of myointimal hyperplasia induced with the administration of platelet-derived growth factor (PDGF)-A. Myointimal thickness (in microns) was directly related to the dose administered (n = 10 per group;P < .001).
Figure 4. Response to administration of platelet-derived growth factor (PDGF)-A antibody. The amount of myointimal hyperplasia produced was not affected by the treatment, regardless of the dose (n = 10 per group).
Figure 5. With the administration of antibody to platelet-derived growth factor (PDGF)-A receptor, myointimal hyperplasia regressed in a dose-dependent fashion. The thickness of the subintimal level did not return to native levels, however (n = 10 per group;P < .001).
DISCUSSION
Our results strongly suggest that PDGF plays a significant role in the formation of the myointimal hyperplasia seen in allograft vasculopathy in a dose-dependent fashion. Historically, evidence for this hypothesis is gleaned from the literature on atherosclerosis. Fractions of advanced atherosclerotic plaques were analyzed for PDGF genes, and expression of the PDGF-A chain was found. 9 By using in situ hybridization techniques, Wilcox et al 10 observed that PDGF-A was associated with smooth muscle-like cells.
Extrapolation of these observations to allograft vascular disease can be made after investigation as to the origin of PDGF and its function. PDGF is expressed and secreted by several cells involved in the process of vascular injury and rejection, including platelets, T cells, B cells, activated macrophages, and endothelial cells. 11 PDGF-A directly stimulates smooth muscle cell proliferation in culture 12 and is highly chemotactic for fibroblasts, smooth muscle cells, mononuclear cells, and neutrophils. 13,14 These factors lend support to the pursuit of a cause-and-effect mechanism between PDGF-A and allograft vasculopathy.
Our results demonstrate a dose-dependent mitogenic effect of PDGF-A on the development of coronary myointimal hyperplasia in a chronic rejection model of cardiac transplantation. The vasculopathy is clearly attenuated when antibody to PDGF-A receptor is administered. This is a gratifying result, supported by observations that PDGF receptor expression is enhanced in human allografts undergoing chronic vascular rejection. 8
The ineffectiveness of antibody to PDGF-A in altering the process may have several explanations. PDGF functions in an autocrine fashion. Once the mitogenic process is initiated, the presence of the protein may not be required. Therefore, addition of the antibody would not be effective in blunting the hyperplastic response, whereas blockade of the receptor would be. Premature binding of the antibody, breakdown of the protein, and antibody delivery issues must also be considered as possible reasons for the lack of an effect.
Studies from our laboratory have defined damage to coronary junctional proteins during the process of myocardial preservation. 15 This damage may be enough to allow diffusion of the PDGF into the subintimal layers and to contribute to the initiation of the hyperplastic process that is then propagated by rejection episodes. Regardless, the implication of PDGF in the formation of allograft vasculopathy and the effectiveness of PDGF receptor blockade in attenuating the process are significant. These new data contribute to the definition of the mechanism involved in the allograft vascular disease process and raise new areas of investigation for future therapeutic options.
Discussion
Dr. Francis T. Thomas (Birmingham, Alabama): Chronic rejection and vasculopathy are clearly the major problems in organ transplantation today. A number of factors have been identified, and we just heard an elegant presentation of one of these factors. Our group has produced long-term kidney grafts in a preclinical primate model off all immunosuppression for up to one decade or more. Earlier, we demonstrated T-cell tolerance and a complete absence of B-cell tolerance with antibody production in chronic rejection, including the absence of severe vasculopathy, similar to what Dr. Mancini is showing you.
Recently, using a new and novel tolerance induction protocol, we have established a group of primates with clearly no signs of chronic rejection up to 5 years. [Slide] This is a typical biopsy 3 years after a second kidney from the same donor, read by our pathologist as completely normal with normal glomerulae and no vasculopathy. You can see small vessels towards the top of the slide, but no tubular atrophy fibrosis, and no sign of fibrosis, including transforming factor beta stains or other molecular markers of fibrosis. [Slide] This slide shows the equal function of the first kidney and the second kidney, one at 3 years and one at 5 years. And these slides showed a different mass due to the orientation of the kidney, but the quantitative renal blood flow and excretion is similar between the two kidneys. And, most importantly, both kidneys had normal biopsy at 3 and 5 years with no signs of chronic rejection whatsoever.
So my question to Dr. Mancini and Dr. McDonald is simply, can we prevent this pathology with antiplatelet-derived growth factor, growth factor receptor drugs, or with a cocktail of suppressive drugs? Our tolerant animals showed no presence of antidonor antibodies if they had no chronic rejection, and presence of antidonor antibodies if they did have chronic rejection. Therefore, the question in our mind is, is this a matter of B-cell tolerance as the ultimate basic seminal pathology occurring here?
Have the authors looked at any of the endothelial antiplatelet or antidonor antibodies to be found in these animals of long-time posttransplant circulating or within the kidney?
Dr. Irving L. Kron (Charlottesville, Virginia): I should tell the audience that Dr. Mancini was given the Nina Braunwald award a few years back by the Thoracic Surgery Research Foundation for medical research in her career. You can see it has already paid off.
This is a tough model and difficult clinical problem. There is evidence in our own program, and I believe nationally now, that CMV prophylaxis reduces the incidence of this problem. I wonder if Dr. Mancini can correlate her hypothesis regarding platelet-derived growth factor with this particular finding.
Dr. Robert M. Mentzer, Jr. (Lexington, Kentucky): I congratulate Dr. Mancini and Dr. Evans on their work in a very important field that affects not only heart transplant recipients, but patients undergoing solid organ transplantation in general. All too often, we have had the tendency to attribute late graft dysfunction to chronic rejection, whether it occurs in the heart, the lung, the liver, or the kidney. This explains in part our enthusiasm for participating in clinical trials designed to evaluate the efficacy of new antirejection drugs in the prevention of chronic rejection, vascular obstruction, and subsequent graft loss.
A recent example is the mycofenolate mofetil prospective randomized clinical trial that involved more than 600 patients. Approximately 200 of these patients underwent intense evaluation for the development of transplant coronary vasculopathy. The disease was monitored intensely using conventional angiography and intravascular coronary ultrasound at predetermined time intervals. Although the early findings were encouraging, after 2 years, there was no evidence that this relatively new potent antirejection medication had any effect on the progression of myointimal hyperplasia.
In retrospect, perhaps we should not have been surprised if, as Dr. Mancini suggests, an important ingredient in the development of allograft vascular disease is the mitogenic effect of platelet-derived growth factor. In this regard, I would like to ask Dr. Mancini three questions.
First, do you have any data regarding PDGF receptor expression in the transplanted animals, particularly changes in expression over time?
Secondly, can you help us better understand the clinical ramifications, the implications of the dose response findings with the antibody? If an even higher dose of the receptor antibody was used, would you predict that the hyperplastic response could have been prevented altogether?
And finally, would you share with us your plans regarding future preclinical and/or clinical trials?
Dr. Curtis G. Tribble (Charlottesville, Virginia): Dr. Mancini asked me to discuss this paper, I think hoping that I would have some deep understanding of her research, which I do not. I do have a few reflections on this problem from my own perspective as a clinical transplant surgeon of hearts and lungs, and I do have a few questions for her.
I thought it might be of interest to some of you that the first person to write about transplant vasculopathy was Alexis Carrel in a beautifully titled paper called “The Latent Life of Arteries” published in 1910. Sixty years elapsed before someone else took up this banner, and that happened to be Dick Lower, writing about the obliterative changes in the hearts of dogs and people they were transplanting at Stanford.
Despite the advances in all aspects of transplantation, the half-life of hearts after transplantation is only about 7 years. In some programs, it’s a little longer, but it’s never much more than that. And that is surprising. A lot of people think–patients, particularly, and a lot of our doctors—that a patient gets a heart transplant and lives forever. The primary problem is the problem of so-called chronic rejection, which has been discussed already today, and that is really graft vasculopathy. This assault on the arterial wall is thought to be immune-mediated by many, but it is also contributed to by diabetes, hypertension, lipid abnormalities, receptors of various kinds, atherosclerosis and maybe even viral infection, as has been mentioned. This assault probably leads to the chronic diseases we see in all transplant organs: atherosclerosis, biliary atresia, bronchiolitis, et cetera.
The current clinical practice in dealing with this is primarily related to diagnosis and staging. There may be some medical treatment options. We occasionally do coronary bypass surgery in this setting. At our place, after hundreds of heart transplants, we have done only two coronary bypasses on our heart transplant patients. Some people think these patients can be treated with calcium channel blockers with some efficacy. Obviously, cholesterol-lowering agents are standard fare. Some people increase the immunosuppression. But the truth is, it doesn’t seem as though these things affect these patients’ vascular disease very much.
But now, with the sort of research that Dr. Mancini has presented, there is the possibility of manipulating some growth factors such as the one she has found in her very nicely done work. That leads me to a couple of questions that are basically from a clinical perspective.
Dr. Mancini, is there some way that we may soon be able to use some blocking agents for platelet-derived growth factors clinically?
You mentioned in your manuscript but not in your discussion that you had some thoughts about preservation techniques that might influence how these growth factors affect the vessels. I wonder if you would speculate just briefly about what preservation techniques might be better.
And finally, with your deep interest in this area, have you yourself changed your posttransplant care at Shreveport to try to decrease this very significant problem?
Dr. Arnold G. Diethelm (Birmingham, Alabama): Acute rejection has been enormously modified by the use of immunosuppressive therapy. True tolerance should obviate chronic rejection. In spite of the advances of immunosuppressive agents against acute rejection, chronic rejection still persists. Therefore the question is, why does chronic rejection occur? The long-term notion that B-cell antibody causes antigen–antibody interaction at the level of the vascular endothelium has not been well proven. Thus, I don’t disagree with anything that you have said. But if I understand your thesis, once the antigen–antibody interacts at the vascular endothelium, there is a release of cytokines downstream, and that’s where the platelet-derived growth factor or other growth factor inhibitors would play a role. So we are really dealing, if I understand your thesis correctly, with a downstream effect. I think this is a very realistic concept. There is no question that vascular endothelium is injured with long-term preservation. Some forms of preservation are better than others, but with every organ preservation technique there is some element of vascular endothelial injury. This is a very interesting concept. I think that the role of growth factors in chronic rejection is real, and I would like to ask you just a few questions.
One, do you feel that this is the second stage of antigen–antibody interaction at the endothelial cell and you are blocking the downstream effect, if you will, of the cytokine release? That is a very important step, but it doesn’t quite get to the question of the antigen–antibody interaction that occurs at the vascular endothelium, which initiates the original injury that leads to chronic rejection.
As a final comment, chronic rejection is the most difficult challenge to answer today in the field of transplantation, and chronic rejection occurs with different temporal relationships. Some patients proceed very quickly to chronic rejection, and other patients proceed over many years. Chronic rejection is initiated in some patients by acute rejection but not in everybody. So your comments are very interesting.
The paper is extremely important, and I think may relate to a different subject in the future, and that is the release of cytokines from the endothelial cell injury initiated as a result of an antigen—antibody interaction. Furthermore, the cytokine release downstream may be the cause, not only of graft vasculopathy, but also ultimately of fibrosis of the organ.
Dr. Mary C. Mancini (Closing Discussion): I’d like to group Dr. Thomas’s and Dr. Diethelm’s comments together, since they relate somewhat. First, chronic rejection is clearly a multifactorial process, and I believe that part of the problem with approaching it is that we have tried to simplify it to one entity or another. And clearly that is not the case.
As clinical transplanters, we are primarily involved with antibody–antigen reactions, and we are trained to blunt that effect either by drugs or other therapeutic manipulations.
Over the years and historically we have ignored the fact of endothelial injury, preservation injury, and now, at a more subcellular level, what really happens here.
So in response to Dr. Thomas’s question about prevention of the process with just anti-PDGF receptor antibody or some combination coupled with a tolerance induction, I believe that we are going to have to do both. I don’t think that a blockade of the receptor alone will attenuate the process to zero. Clearly, this is a multifactorial situation, and I will discuss that further when I reach Dr. Mentzer’s comments.
Dr. Diethelm, I do think that we are approaching it as a second-stage system. PDGF is an interesting entity, and the receptor is an even more interesting problem. It is a constitutively expressed receptor that is not always present and can be induced—by a drug, by another infection, by rejection. We can even induce it in cell culture. So what we are trying to do is block the step in another manner. I think we will find in the future that blockade of the process will involve either a part of this receptor or a part of the process downstream, probably an NFκβ, which is the ultimate end point of the inciting event.
Dr. Kron, I appreciate your comments. With respect to CMV and chronic rejection, there is a tremendous body of literature out there now trying to implicate CMV. I like to think of it in two ways. First, CMV in and of itself is a virus that incites the immune system. You already have an immunocompromised patient where you are battling with tolerance in these patients and then all of a sudden you hit them with a virus. And the virus will activate various aspects of the immune system.
The other interesting entity about CMV and CMV protein is that it’s a great mimicker. A hypothesis that we already are looking at in the laboratory is that portions of the CMV protein will in and of itself engage the PDGF receptor. I don’t have an answer to that process yet; however, I do believe we will find some interesting result coming out of our cell culture work.
Dr. Mentzer, just to reiterate with the PGDF receptor expression, no, I don’t think increasing the dose will blunt the effect entirely to normal. I believe that we will see that there will probably be two or three growth factors involved here. TGF-β is now hitting the forefront, and we will see that probably a combination of that along with PDGF will really produce some effect as far as correcting the process. I will not be surprised in the future to see that the TGF-β does engage the PDGF receptor.
Future trends for our work: we’d like to carry out the model to the other organ systems and tie the story together. One of the clear directions we will take is genetic manipulation of the receptor, as soon as we learn more about it. There is new data coming out every day from the biochemists, and it seems like monthly there is some paper that says, well, this is the way it looks but, no, really it isn’t, it’s something else. It’s a little bit hard to deal with.
Dr. Tribble, blocking the agent clinically for the PGDF receptor is probably 3 years down the road. There was an interesting drug out of Germany which looked like it produced some good results in cell culture; however, when applied to some initial animal studies, as we all know, didn’t work. We will have to see. That may have been a dose problem; these were very preliminary studies.
Our preservation study that we quoted in the paper and that Dr. Tribble alluded to is a study that I was fortunate to have undertaken by a resident in my laboratory, Dr. Trocha, where we looked at some subcellular entities in endothelial gap junction proteins. We found that regardless of the preservation solutions used in cardiac transplantation, including UW, there is one important protein called occludin that was destroyed, no matter what manipulation we performed. And this cannot be seen histologically. It cannot even been seen at the EM level. We discovered it under a Western blot technique. So we are now targeting preservation studies to see how we can manipulate this and actually improve the preservation of these gap junction proteins, which I think will be important in the future as far as providing better endothelial integrity and thus, preventing the process.
Has this affected my posttransplant care? Yes, I tend to manipulate platelet activity in these patients much more aggressively. Calcium channel blockers do inhibit PDGF to some extent. Aspirin clearly is a good drug. We have been using heparin metabisulphate initially. We can’t continue it chronically because of the expense and because it’s difficult for the patients; however, we have seen some improvement in outcomes.
Footnotes
Correspondence: Mary C. Mancini, MD, Dept. of Surgery, Louisiana State University Health Sciences Center—Shreveport, 1501 Kings Highway, Shreveport, LA 71106.
This work was funded in part by the Thoracic Surgery Foundation for Research and Education. Dr. Mancini received the Nina S. Braunwald Career Development Award for this work.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
E-mail: mmanci@lsumc.edu
Accepted for publication December 1999.
References
- 1.Ardehali A. Heart transplantation: accelerated graft atherosclerosis. Adv Cardiac Surg 1995; 6:195–205. [PubMed] [Google Scholar]
- 2.Cook D. Long-term survival in kidney allografts. In: Terasaki PI, ed. Clinical Transplantation. Los Angeles: UCLA Tissue Typing Laboratory; 1987: 277.
- 3.Kosek JC, Hurley EJ, Lower RR. Histopathology of orthotopic canine cardiac homografts. Lab Invest 1968; 19:97–112. [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis: a defense mechanism gone awry. Am J Pathol 1993; 143:987–1002. [PMC free article] [PubMed] [Google Scholar]
- 5.Geraghty JG, Stoltenberg RL, Sollinger HW, et al. Vascular smooth muscle cells and neointimal hyperplasia in chronic transplant rejection. Transplantation 1996; 62:502–509. [DOI] [PubMed] [Google Scholar]
- 6.Ross R, Bowen-Pope DF, Raines EW. Platelet-derived growth factor and its role in health and disease. Phil Trans R Soc Lond 1990; B327:155–169. [DOI] [PubMed] [Google Scholar]
- 7.Fellstrom B, Dimeny E, Larsson E, et al. Importance of PDGF receptor expression in accelerated atherosclerosis–chronic rejection. Transplant Proc 1989; 21:3689–3691. [PubMed] [Google Scholar]
- 8.Adams DH, Tilney NL, Collins JJ, et al. Experimental graft arteriosclerosis I. The Lewis to F-344 allograft model. Transplantation 1992; 53:1115–1119. [PubMed] [Google Scholar]
- 9.Barrett TB, Benditt EP. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci USA 1988; 85:2810–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcox JN, Smith KM, Williams LT, et al. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest 1988; 72:1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raines EW, Bowen-Pope Dr, Ross R. Platelet-derived growth factor. In: Sporn MB, Roberts AB, eds. Handbook of Experimental Pharmacology, Vol. 95, Part I (Peptide Growth Factors and Their Receptors). New York: Springer Verlag; 1989.
- 12.Raines EW, Dower SK, Ross R. IL-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science 1989; 243:393–396. [DOI] [PubMed] [Google Scholar]
- 13.Seppa H, Grotendorst G, Seppa S, et al. Platelet-derived growth factor is chemotactic for fibroblasts. J Cell Biol 1982; 92:584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams LT, Antoniades HN, Goetzl EJ. Platelet-derived growth factor stimulates mouse 3T3 cell mitogenesis and leukocyte chemotaxis through different structural determinants. J Clin Invest 1988; 72:1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trocha SD, Kevil CG, Mancini MC, et al. Organ preservation solutions increase endothelial permeability and promote loss of junctional proteins. Ann Surg 1999; 230:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]