Abstract
Objective
To determine surgical, postoperative, and postdischarge complications associated with percutaneous dilational tracheostomy (PDT) in an 8-year experience at the University of Kentucky.
Summary Background Data
There are known risks associated with the transport of critically ill patients to the operating room for elective tracheostomy, and less-than-optimal conditions may interfere with open bedside tracheostomy. PDT has been introduced as an alternative to open tracheostomy. Despite information supporting its safety and utility, the technique has been criticized because advocates had not provided sufficient information regarding complications.
Methods
A prospective database was initiated on all patients who underwent PDT between September 1990 and May 1998. The database provided indication, procedure time, duration of intubation before PDT, and intraoperative and postoperative complications. Retrospective review of medical records and phone interviews provided long-term follow-up information.
Results
In the 8-year period, 827 PDTs were performed in 824 patients. Two patients were excluded because PDT could not be completed for technical reasons. There were 519 male and 305 female patients. Mean age was 56 years. Prolonged mechanical ventilatory support was the most common indication. Mean procedure time was 15 minutes, and the average duration of intubation before PDT was 10 days. The intraoperative complication rate was 6%, with premature extubation the most common complication. The procedure-related death rate was 0.6%. Postoperative complications were found in 5%, with bleeding the most common. With a mean follow-up of greater than 1 year, the tracheal stenosis rate was 1.6%.
Conclusions
On the basis of this large, single-center study, the authors conclude that when performed by experienced surgeons, PDT is a safe and effective alternative to open surgical tracheostomy for intubated patients who require elective tracheostomy.
Tracheostomy is performed frequently on critically ill patients to facilitate weaning from mechanical ventilatory support and to prevent the complications associated with prolonged translaryngeal intubation. 1–3 Open tracheostomy performed in the operating room or at the bedside has been the standard of care for the past 25 years. 4 Although transport risks for critically ill patients may preclude the use of the operating room, suboptimal lighting and exposure as well as lack of appropriate equipment may compromise open tracheostomy performed at the bedside. 5–7 Consequently, alternative approaches to the open procedure need to be explored.
Shelden et al, 8 in 1955, were the first to describe a technique for percutaneous tracheostomy. Unfortunately, blind cannulation of the trachea with a bladed instrument limited the usefulness of this device. Toy and Weinstein 9 introduced the use of a guide/dilator device to allow safe passage of the percutaneous cannula. Several other guidewire-based percutaneous tracheostomy techniques have been described but have not gained widespread popularity. 10,11 Most recently, a translaryngeal tracheostomy technique has been introduced by Fantoni. 12 In 1985, Ciaglia introduced a modification of Toy’s technique that involved serial dilation of the trachea over a Seldinger wire. 13,14 This technique has achieved considerable success and widespread clinical use. 15 Percutaneous dilational tracheostomy (PDT) has been criticized, however, because advocates of the technique have not provided sufficient information regarding procedural complications. Also, long-term follow-up as to the incidence of tracheal stenosis has not been reported, although there is evidence that PDT can be performed safely. 16–19
At the University of Kentucky, PDT is the preferred method of tracheostomy for critically ill patients requiring prolonged mechanical ventilatory support. The purpose of this study was to review our 8-year experience with the procedure. 20,21
METHODS
Between September 1990 and May 1998, a prospective database was initiated to evaluate all patients undergoing PDT. This included documentation of indications, procedure location, procedure time, duration of intubation before PDT, and intraoperative and postoperative complications. Retrospective reviews of the hospital chart, clinic records, and phone interviews provided long-term follow-up information. All intubated adult patients with indications for elective tracheostomy were considered candidates for PDT. Patients who required an emergent surgical airway or patients who required tracheostomy as part of a larger head and neck operation were not considered candidates for PDT and were excluded from the study. The protocol was reviewed and approved by the University Institutional Review Board.
The Ciaglia technique was used exclusively; the procedure has been described in detail elsewhere. 14,15 There are three essential elements: percutaneous puncture of the trachea and guidewire insertion, placement of a guide sheath to prevent wire bending and pretracheal dilation, and progressive controlled dilation of the trachea to accommodate an appropriate-sized tracheostomy tube. Local anesthesia was supplemented with sedation, analgesia, and paralytic agents when necessary. The Ciaglia Percutaneous Tracheostomy Introducer Set (Cook Inc., Bloomington, IN) was used from September 1990 to March 1996; the Sims Per-Fit Kit (Sims Inc., Keene, NH) was used after March 1996. The latter kit contains a tracheostomy tube specifically designed for percutaneous placement. Completion chest radiographs were performed only when difficulty was encountered during the procedure. Bronchoscopic guidance was not routinely used; it was reserved for patients with difficult anatomical landmarks, when a problem was encountered during the procedure, or as a teaching guide. All procedures were performed by attending physicians with PDT experience or by surgical house staff under the direct supervision of the attending faculty.
RESULTS
During the 8-year period, 829 PDTs were attempted and 827 were completed on 824 patients (Table 1). One patient had a calcified trachea, and after percutaneous puncture, sequential dilation of the trachea could not be performed. In the second patient, the initial tracheostomy tube chosen was not the proper length. By surgeon choice, open tracheostomy was completed in the operating room. The mean patient age was 56 (range 15–87) years. Most patients were male. There were 31 different admitting diagnoses. Multisystem trauma (221/827, 27%) and head injury (90/827, 11%) were the most common diagnoses, followed by peripheral vascular disease (60/827, 7%), chronic obstructive pulmonary disease (51/827, 6%), and coronary artery disease (41/827, 5%). Respiratory failure requiring prolonged mechanical ventilatory support (578/827, 70%) was the most common indication. Pulmonary hygiene (216/827, 26%) and airway compromise (33/827, 4%) accounted for the remainder.
Table 1. PATIENT DEMOGRAPHICS
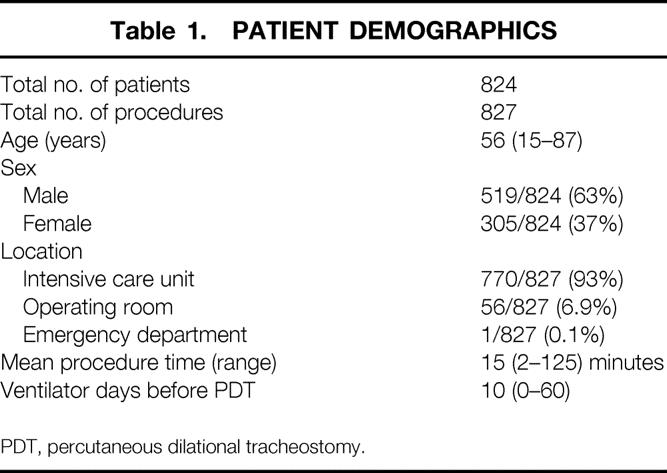
PDT, percutaneous dilational tracheostomy.
The vast majority of the procedures were performed in the intensive care unit (see Table 1). Fifty-six patients had PDT performed in the operating room in conjunction with another surgical procedure. One procedure was performed in the emergency department. The average procedure time was 15 (range 2–125) minutes. The average duration of translaryngeal intubation before PDT was 10 days.
Procedural complications are shown in Table 2. Most procedures were free of complications. There were 49 procedural complications identified in the study. Premature extubation during endotracheal tube withdrawal was the most common complication, followed by bleeding not requiring transfusion, creation of a false passage, and incorrect tracheostomy tube size. There were two tracheal lacerations, one of which required emergent thoracotomy and repair. Two patients sustained a tracheoesophageal fistula; one of these patients underwent successful repair.
Table 2. SURGICAL COMPLICATIONS IN 829 PROCEDURES
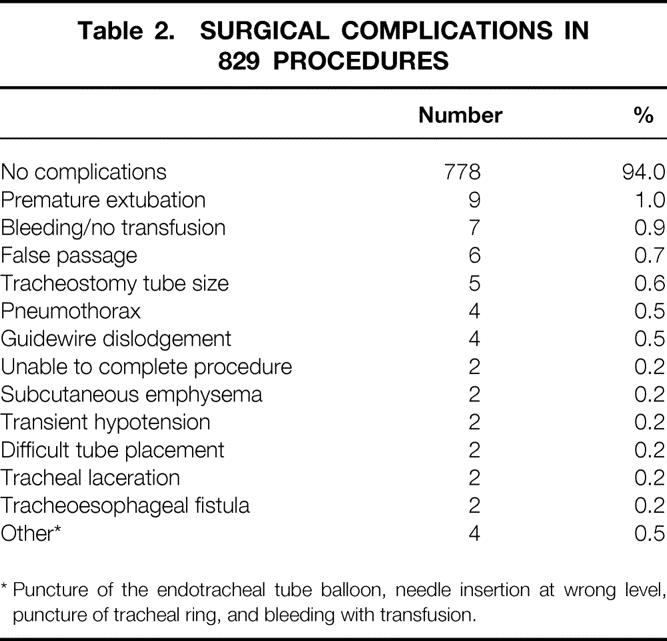
* Puncture of the endotracheal tube balloon, needle insertion at wrong level, puncture of tracheal ring, and bleeding with transfusion.
Table 3 shows the distribution of early postprocedural complications. Although most patients had no postprocedural complications, 41 did have complications. Bleeding was the most common, occurring in 18 patients for an overall incidence of 2.2%. Five of these 18 patients required transfusion, and all had a significant coagulopathy at the time of PDT. There were no tracheoarterial fistulas identified in this series of patients.
Table 3. POSTOPERATIVE COMPLICATIONS IN 827 PROCEDURES
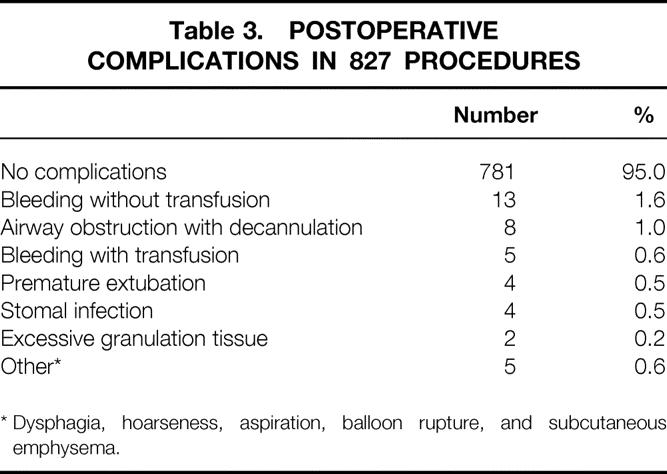
* Dysphagia, hoarseness, aspiration, balloon rupture, and subcutaneous emphysema.
Reflecting the acuity of the patient population, one quarter died during the hospital stay (Table 4), most as a result of their underlying illness. There were five deaths directly related to PDT for a procedural death rate of 0.6%. One death resulted from intense bronchospasm after guidewire placement. A second patient died when the tracheostomy tube dislodged several hours after PDT. Extensive subcutaneous emphysema ensued, and an airway could not be reestablished. Most likely, this was related to incorrect tracheostomy tube selection. A third patient died as a result of sepsis and multiorgan failure from a procedure-related tracheoesophageal fistula. Two patients on the medical service died within 36 hours of the procedure. On chart review, there was evidence that clinical deterioration resulted from inability to ventilate the patient adequately. In both patients, PDT could not be excluded as a proximate contributing cause of death.
Table 4. POSTPROCEDURAL OUTCOMES
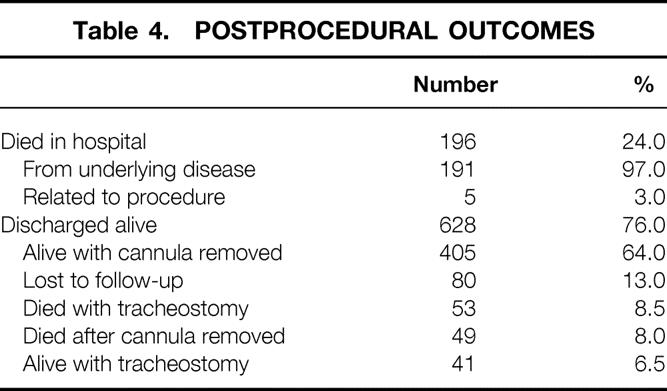
As shown in Table 4, 75% of the patients were discharged alive; a much smaller number were lost to follow-up. Forty-one patients still had a cannula in place at the time of this follow-up. A few patients died either before or after cannula removal. Two thirds of the patients were alive and decannulated. The average follow-up for decannulated patients was 461 days. Table 5 lists the postdischarge complications. Most patients had no complication after discharge. Symptomatic tracheal stenosis/malacia was identified in nine patients with adequate follow-up data. Five patients underwent surgical correction of the tracheal stenosis. Another four patients could not be decannulated secondary to obstruction. These patients were chronic care patients with significant neurologic impairment, and there was no attempt at surgical correction. The tracheal stenosis rate was 1.6% (9/548).
Table 5. POSTDISCHARGE COMPLICATIONS
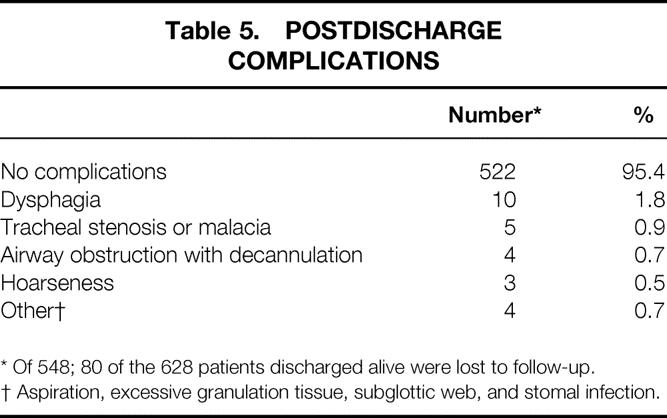
* Of 548; 80 of the 628 patients discharged alive were lost to follow-up.
† Aspiration, excessive granulation tissue, subglottic web, and stomal infection.
The overall complication rate in this series was 15%; the majority of these (80%) were minor. Major complications of tracheal stenosis, death, bleeding requiring transfusion, tracheal laceration, and tracheoesophageal fistula accounted for 20% of the complications and occurred in only 3% (23/827). Procedural complications such as premature extubation, guidewire removal, false passage, and incorrect tracheostomy tube selection occurred early in our experience and were not encountered in the last 471 procedures.
There were no discernible differences in complication rates between the Cook Percutaneous Tracheostomy Kit used in the first half of the series and the Sims Per-Fit Kit that we currently use.
DISCUSSION
Elective open tracheostomy performed in the operating room should no longer be recommended for patients requiring prolonged mechanical ventilatory support. Transport risks for this patient population are real and significant. 5–7 Until the recent introduction of percutaneous tracheostomy, open bedside tracheostomy was the only alternative to the operating room. The latter can be performed at the bedside in the intensive care unit with good results. 22,23 Bringing the operating room personnel to the intensive care unit eliminates the transport risks but negates some of the cost advantages for bedside tracheostomy.
Any new procedure or technique must undergo rigorous evaluation to determine safety and efficacy. For percutaneous tracheostomy, this evaluation should include an adequate sample size, detailed data regarding death and complications, and long-term patient follow-up. We believe this study meets these criteria and provides sufficient evidence to conclude that PDT is the preferred method of surgical airway for intubated critically ill patients who require elective tracheostomy.
As with all procedures, there are several different techniques for percutaneous tracheostomy. The original technique described by Shelden et al 8 in 1955 involved transfixing the trachea with a barbed needle, followed by blind placement of a bladed instrument. The potential for injury limited the usefulness of the technique. Toy and Weinstein 9 in 1969 were the first to incorporate both a guide and a dilator for percutaneous placement. This technique was not used in our study, but improvements have been made in the device and a kit is commercially available. The Rapitrac system described by Schachner and the dilator forceps method of Griggs use a Seldinger wire to gain safe entry into the trachea. 10,11 Both of these methods use a spreading device to create a tracheotomy, increasing the risk of tracheal injury. 10,11 Recently a translaryngeal percutaneous technique has been described by Fantoni. 12 Early results are good, and a comparative study with PDT and open tracheostomy has been reported. 24 We used the Ciaglia method because the early reports were excellent and it allowed for controlled dilation of the trachea, thereby minimizing the potential for tracheal damage.
Early in our experience, difficult anatomy (obesity, indistinct landmarks) or a contraindication to neck extension (e.g., spine fracture) were considered contraindications to PDT. 20 Over time, we have expanded the use of the Ciaglia technique to include these patients and now believe it is the preferred approach. Procedure time has remained constant during the 8 years, and prolonged mechanical ventilatory support has remained the most common indication. 20,21 The duration of translaryngeal intubation before percutaneous tracheostomy has diminished from 12 days to 10 days. The reduction in days of translaryngeal intubation is consistent with recent reports that early tracheostomy results in reduced mechanical ventilator days, intensive care unit days, and the incidence of nosocomial pneumonia. 25,26 Overall, the complication rates have declined steadily, from 19% in our earlier publications to the present level of 15%. 20,21 Most of the complications were minor; major complications were encountered in only 3% of patients. The absence of common procedural complications in the last 471 procedures reflects our increased expertise with the technical aspects of the operation.
Despite several reports, including our own, the use of PDT has not been universally accepted. This is due in part to publication of small case series with poor results or larger series that collated results from several authors using different percutaneous techniques. 27,28 Variations in open surgical technique, improvements in equipment, and differences in the definition of complications limit the usefulness of comparisons between the recent PDT literature and older open tracheostomy literature. 29–31 Nevertheless, there are now four published prospective trials that compare PDT to open tracheostomy. 16–19 There were no deaths in patients undergoing PDT, whereas patients undergoing open tracheostomy had a death rate ranging from 0% to 7.4%. 16–19 Our death rate of 0.6% (5/829) compares favorably with these reports. At the very least, it appears that the death rates for open tracheostomy and PDT are equivalent. 32
With respect to prevention of bleeding complications, every effort should be made to correct significant coagulopathy before undertaking PDT to minimize the risk of hemorrhage. In our experience, bleeding rarely interrupted the procedure because the dilators and subsequent tracheostomy tube placement controlled the hemorrhage. As to the concern for potential development of tracheal stenosis after PDT, this does not appear to be a major problem. Powell et al’s 33 comprehensive review comparing open and percutaneous tracheostomy reported an overall tracheal stenosis rate of 0.5% for open tracheostomy and 1.0% for PDT. There are reports that tracheal stenosis rates are actually lower for PDT than for open tracheostomy. 16,21,29–31 In our study, four of the nine tracheal stenoses could be related to technical error, trauma from repeated translaryngeal intubation, and prolonged translaryngeal intubation before tracheostomy. Eight of the nine tracheal stenoses that we identified occurred in our first 356 patients. 21 Shorter duration of intubation before tracheostomy and greater technical expertise may account for the marked reduction in the stenosis rate.
With respect to cost savings, it is still unclear whether PDT is less expensive than open tracheostomy. Although there is literature that documents cost savings for PDT versus open tracheostomy, comparisons between bedside PDT and open tracheostomy performed in the operating room are of limited value. 29,34,35 This is because the incremental costs associated with anesthesia and operating room time do not apply to tracheostomy performed in the intensive care unit. In fact, the actual costs of open bedside tracheostomy and PDT may be quite similar. Bernard and Kenady 36 have reported that PDT is actually more expensive because of the cost of the kit.
Early in our experience, we used the Ciaglia Percutaneous Tracheostomy Introducer Set. This kit was more than adequate, and we were able to achieve good results. 20,21 One drawback with this kit was the lack of a tracheostomy tube designed for percutaneous placement. An appropriate-size tracheostomy tube had to be selected and loaded on the proper dilator. Also, there were frequent problems with dilator–tracheostomy tube interface, leading to difficult placement or buckling of the anterior tracheal wall. We now use the Sims Per-Fit Kit because it contains a tracheostomy tube specifically designed for percutaneous placement. The tracheostomy tube is beveled and has a low-profile cuff to facilitate percutaneous entry. Cook has recently introduced a simplified PDT kit with a better tracheostomy tube–dilator interface. Although we have used only a few of these new Cook kits, our initial experience has been positive. Because manufacturers are making constant product improvements in kit design, ultimately the decision on which products to use will depend on utility, clinical results, and cost.
The PDT procedure has undergone and continues to undergo rigorous evaluation. As demonstrated in this large series of patients, excellent, consistent, and reproducible results can be achieved. Like percutaneous endoscopic gastrostomy and laparoscopic cholecystectomy, PDT is a technical improvement over the open surgical technique. On the basis of our study, we believe that PDT is the preferred method for intubated critically ill patients who require elective tracheostomy.
Discussion
Dr. R. Neal Garrison (Louisville, Kentucky): This is a clearly written and very honest and straightforward presentation of an 8-year experience with this procedure. They report 3% to 4% major complication rate, depending on how you count them, and five deaths, or an incidence of 0.6%, which compares favorably with reports in the literature for the open technique.
I have several comments or questions for the authors.
In your opinion, could any of the major complications or resultant deaths that you report have been prevented or better handled in an OR environment? It appears to me that percutaneous tracheostomy is not safer than the open technique but is done because of convenience in the intensive care unit, particularly in institutions where OR time is tight. Before you can conclude that the percutaneous technique is the preferred technique, you need to show a clear improvement in care or outcome, not just equivalent results.
Secondly, are there any hard and fast contraindications to this technique? You mention in your manuscript that C-spine stabilization and obesity were relative contraindications in your early experience, but now are not considered as such. Should a complication occur in such a patient that needs C-spine stabilization or obesity, I would personally feel more prepared to deal with this in the operating room where visual control with an open procedure can be readily accomplished.
Thirdly, one of my major concerns with any tracheostomy is postoperative dislodgement in the intensive care unit about 3 o’clock in the morning. With the open technique, traction sutures in the trachea can be placed to help stabilize the trachea and bring it back to the surface where replacement of the tube can be done. Do you have any hints from your experience that would help with reintubation if needed on an emergency basis?
And finally, would you care to comment on the role of the nonsurgeon intensivist with this procedure? A concern that comes to mind is that these individuals will adopt this technique as less invasive and therefore better for their patients, and coincidentally for their wallets, because it can be done within their domain. Yet, most of them are not prepared to handle the majority of complications that clearly need surgical intervention.
Dr. Lewis M. Flint, Jr. (Tampa, Florida): I must admit I was in general support of everything that I heard in the presentation up until the final slide. But I’d like to just comment on a couple of areas, some of which are similar to the concerns that Neal Garrison raised.
First, we are taught nowadays to think that if there are two procedures that are equally efficacious, that we should choose the one that is less expensive. Some of the manuscripts in the literature relating to percutaneous tracheostomy have dealt with the issue of cost. But I don’t think any of the currently available literature, including this presentation, has really dealt with the value of the procedure. The data that were presented here this afternoon certainly support that the procedure is safe and that the time investment on the part of the surgeon and the ancillary staff in the intensive care unit are small. But the question of value has not been addressed.
When we look at the cost of the procedure in terms of mortality and morbidity, those are clear costs. But when we look at financial costs, these analyses sometimes break down because of the wide interinstitutional variability in how costs are accounted.
In our institution, what is very clear to me, from a fairly detailed analysis of our cost of percutaneous tracheostomy, is that what we simply do is transfer the cost from the operating room to the intensive care unit, and that in terms of financial benefit, we don’t really have that much of a difference for percutaneous tracheostomy.
I also have a concern, as did Dr. Garrison, about the impact of percutaneous tracheostomy on outcome. Some authors have suggested that percutaneous techniques might lead to more tracheostomies being done overall, and tracheostomies being done at an earlier time in the patient’s course, say, within the first 3 or 4 days of ventilator therapy. Do you have any data on this? In your patients who were done in an early phase, what was their outcome with regard to days in the ICU on the ventilator, incidence of pneumonia, and so on?
Third, there is the real problem of complications. Of these, accidental decannulation seems to be a particularly troublesome complication. Loss of airway is a hazard in the percutaneous tracheostomy that may not be present when tracheostomies are done by the open technique. I have personally seen one accidental decannulation with inability to recannulate 3 weeks after the original tracheostomy. So it is clear to me that these patients don’t form the same kind of mature tracts that open tracheostomy patients form, which is possibly due to the process of cannulation and dilatation that occurred with the technique.
You have a very low rate of tracheal stenosis, but you didn’t tell us how you made the diagnosis. Were only symptomatic patients evaluated or were all of the patients screened for this complication?
Finally, what about training for this procedure? Have you compared the cost in attending time, bronchoscope usage, et cetera, for your residents you train? At what level are residents taught the procedure, and how many procedures are necessary before you allow the residents to serve as operating surgeon or as teaching assistant?
Dr. C. James Carrico (Dallas, Texas): I will try not to repeat the previous comments but would like to summarize a couple of important points about this series.
Number one, the authors performed over 100 procedures per year, so there was quite good experience and expertise. Number two, the majority of these procedures were done in the ICU. Indication was prolonged ventilation. So that patient population is important to keep in mind.
Number three, mentioned in the manuscript but not in the presentation, and I think extremely important, these procedures were done either by an attending surgeon or under the direct supervision of an attending surgeon. So these were done basically at a high level, as tracheostomies should be done.
The authors confirm the observation of others, that with these requirements—that is, extensive experience, attending surgeons involved—tracheostomies can be done in the intensive care unit in critically ill patients with a very reasonable complication rate and very reasonable results and, as a matter of fact, probably more safely than transporting these complicated patients to the operating room. So I would agree with that point.
I also agree with Dr. Flint that you were doing great until you put that last slide up there. That slide basically says that percutaneous tracheostomy should become the standard or, in the abstract and in the manuscript, that it should replace open tracheostomy. And I feel constrained to quibble with that conclusion.
The complication rates were low, but as you pointed out, some of the complications were devastating and a direct result of the cannula. There were four tracheal or tracheoesophageal lacerations. What’s pointed out in the manuscript, two patients with tracheal lacerations were taken to the operating room for thoracotomy for repair of the trachea. One of the patients with a TE fistula died from sepsis. So one of the five deaths was directly related to the cannula.
We do not know what would have been the outcome had the same requirements been applied to a series of patients with open tracheostomy in the ICU—the requirements being extensive experience, an attending involved in every case, and patients selected primarily for prolonged ventilation. So I would maintain that, number one, you have confirmed the observations of others, that these procedures can be done outside the operating room in critically ill patients. Number two, you have demonstrated excellent results when attendings are involved and tracheostomy is treated as a major operative procedure.
But, number three, you have not established its superiority over open tracheostomy, and it would require a very carefully done prospective study to do that.
Dr. George M. Watkins (Tampa, Florida): This group has produced an excellent paper. In spite of what some of the authors have said, I, an open tracheostomy person in the operating room for my entire career, feel that as a result of this study, there will be a new standard. I think this will be the way we’ll be doing tracheostomies in intubated patients.
I have several comments and questions.
What were the five procedural deaths due to? And how have you altered your technique so that those do not recur?
I understand that you have controlled bleeding in part by watching the patient’s coagulation status before and, hopefully, after the operation. However, I would caution, there is one particular instance in which this is contraindicated, and that’s the superior caval syndrome. Those people have tremendous hypertension of the venous side. They have a tremendous complex of veins that are covering the trachea, and I think that would be one place that you shouldn’t study; you should just go ahead and do an open procedure in the operating room.
I can see that ER physicians will eventually perform this procedure, not just for intubated patients alone, but for emergency procedures.
We presently teach cricothyroidotomy in ATLS. Many of you have been experienced, and many of you know I have been extremely experienced, in this. However, I have been impressed that emergency room physicians cannot really learn, for the most part, cricothyroidotomy, or they would be sitting here discussing this paper rather than a bunch of surgeons. And I mean that. So one has to look—and the question I want to raise—is this procedure such that Dr. Kearney thinks it should be added to ATLS protocol or not? Because it is going to be there once you talk about it being done outside the operating room.
Do you still keep an open tracheostomy set by the bedside each time a percutaneous tracheostomy is performed? Do you also keep a tracheostomy set taped to the bedside after the tracheostomy is performed?
Finally to the authors, this procedure calls—dramatically, I think—for video-assisted surgery. The thing that this does, potentially, will eliminate half to two thirds of your complications. You can see the trachea being compressed, you can see the needle is not in the right place, the wire doesn’t come up, and so forth. It can be done, even in a small tube, with a ureteral fiberoptic cannula through the side port. It’s not that big a deal, and it’s not that big a deal to get the monitoring device up. From my standpoint, more importantly than just preventing complications to the patient, it’s preventing complications of heart attacks in attendings, because nothing beats video-assist in a blind procedure such as this.
Dr. Frank C. Spencer (New York, New York): My comments are similar to those already made. As they say, I enjoyed it very much until the last slide came up. My caveat is, not yet—let’s have a bit more data.
But I read it with a lot of interest because I have been a skeptic about the procedure for a long time. It implies it’s safer to tear it blindly rather than to incise it while looking at it. But instincts are one thing, data are something else.
In the data here, as Dr. Garrison pointed out, I think the complications are too high for everybody to say let’s go do it. But why in the world don’t you take them to the operating room? If somebody is bleeding, he’s got subcutaneous emphysema, I don’t think you should do a coagulation workup and then transfuse him, assuming that there couldn’t be anything surgically wrong. I think he should go to the operating room. But as commented by Dr. Watkins, I suspect this is here to stay, and it will probably become much more popular because it’s simpler, it’s quicker, it’s safe, and it’s cheaper. It has a lot of attraction.
Most of my comments are not relating to the technique, but I have long pushed for more liberal use of tracheostomy as opposed to long-standing intubation. I have been in the minority for a long time about that, but my practice for years has been to recommend that if you can’t extubate a patient within 3 or 4 days, do a trach. The properly performed trach—in my penchant for jargon—is about like a handshake if well done. You get lots of complications otherwise. But the tracheostomy not only has the advantage of patient comfort, it greatly facilitates the removal of bronchial secretions. This is the way the minority approach. It’s the way I have practiced and taught for a long time. In the excellent textbook on critical care, which just came out, by Hall and associates, University of Chicago, two volumes, second edition, a tracheostomy is mentioned very, very briefly as almost obsolete.
This carries the basic premise that you can remove secretions as clearly through a long endotracheal tube as you can through a tracheostomy by suctioning or by bronchoscopy. The important clinical question is how often is so-called ventilator-associated pneumonia due to retained secretions that then become purulent tracheal bronchitis and spreads through the parenchyma than vice versa? You simply don’t know. What makes it a tough problem, if someone has good ciliary action, you don’t have to do anything except leave them alone. He will lie on his back, his cilia will sweep it up like an escalator. If he’s got bad cilia, needs a lot of help with suctioning, tracheostomy, bronchoscopy, you don’t know except in retrospect. So I think, like a lot of things, you have different practices and strong opinion, but you don’t have any P values. Hopefully, if this evolves, one can consider with Dr. Mentzer, where this is properly performed, what about randomization? You take a group at day 4, day 5, comparable groups, do a percutaneous tracheostomy on some, on the others leave them intubated and then look at your data over a time and look at the so-called ventilator-associated pneumonia. In my experience, which is distorted because I have no alternative, severe pneumonia in a patient who was a tracheostomy and suction is very rare. It occurs, but it is briefly and promptly resolved.
Dr. Michael L. Hawkins (Augusta, Georgia): I appreciate your letting me talk after this long list of questions, and I will not be repetitious.
Percutaneous tracheostomy covers the gamut from everything from a skin incision and then, as Dr. Spencer said, rip and tear into the trachea, all the way to dissecting down to the trachea and then dilating the tracheal ring instead of excising. My question is exactly what do you mean in your procedure, and do you do this with a bronchoscope in place for direct vision from inside at the same time?
Dr. Paul A. Kearney (Closing Discussion): Let me start with Dr. Garrison. His specific question was, could the major complications have been handled better in the operating room. If you look at our major complications, the only one that really would—and I know the surgeon that staffed that particular one with the resident—I am sure they would have much preferred to have been in the operating room. They had a tracheal laceration which led into precipitous clinical deterioration, and I think they would answer yes, that they would much rather have been in the operating room. But I think overall, in the entire series of patients, I don’t think that any of the major complications would have been averted had we been in the operating room—it just would have been a better place to be.
In terms of doing this in the ICU, that was a question regarding doing them in the ICU versus the operating room. It’s just more than doing them at the bedside. It really gets into the issue, with this group of patients, for us who take care of these critically ill patients all the time, of transport risks. Many of these patients do require tracheostomy, and some of them are quite ill, and there is very nice literature on the transport risks associated with moving these patients around. It is particularly frightening, sometimes, to hand them over to the anesthesia staff, as you all know. So it’s much easier for us to do this in a controlled situation in the ICU and not expose these patients to transport risks.
Regarding the tube dislodgements or cannula dislodgements, we only had four patients where the tracheostomy tube became dislodged after it was placed, and one of those patients was a death. The patient had the tube dislodged when he was being turned in the ICU. It was unrecognized. Initially, the patient developed subcutaneous emphysema. We were not contacted; actually, they called the anesthesia department to come intubate the patient, and they were unable to intubate the patient. Then we were called, but by then it was a lost cause.
The other three tube dislodgements—two of them occurred moderately late, 3 to 4 days after the procedure, and actually, in both cases, you can reestablish the tract and get in. We had one that occurred early, and in fact the patient was an obese gentleman who had been operated on for an orthopedic procedure. We did the tracheostomy tube simultaneously in the operating room, and we noticed the tube had become dislodged during the transport of the patient back to the operating room. That was a little bit more of a dicey operation, but we were able to get the guide sheath back through the tracheostomy and then just reintroduce a longer tracheostomy tube without much difficulty.
So I think that the incidence of tracheal cannula dislodgement is no larger with this percutaneous technique than it is with open bedside tracheostomy. The risks are the same.
As far as nonsurgeons doing this, this is a difficult question to answer. We have participated in the training of some of our pulmonologists, and while some of my colleagues are not happy about that, one of the problems that we were faced with is that these gentlemen were going to go out and go to the “Acme” percutaneous training classes and come back with some sort of piece of paper and begin doing these. We felt that it was probably better to teach them the proper technique from the beginning and supervise them, and we have done that and have done it safely.
There is no question that their understanding of anatomy does influence their training period, and you certainly have to be much more cautious about certification and permitting them to do these procedures independently, but they are doing them anyway. You have to remember that Pat Hazard was not a surgeon—he was a pulmonologist and had pretty good results in a very small series.
With regard to Dr. Flint’s questions regarding the value of this procedure and cost, we relied very heavily on our own personal bias, but also the clinical literature that shows that early tracheostomy probably does benefit these critically ill patients. Dr. Luchette, who is present at this meeting, and Dr. Rodriguez published a very nice paper on early tracheostomy and showed that intensive care unit days and associated nosocomial pneumonia were lower in those patients who had early tracheostomy. However, if you look at our data, our average days of translaryngeal intubation prior to tracheostomy is about 10 days. If you look at the older tracheostomy literature, that’s about the break-even point for translaryngeal complications beginning to become significant—in other words, increasing dramatically. So we do feel, in terms of timing, that around 10 or 11 days is when you ought to consider putting a tracheostomy tube in. And if you can predict ahead of time which patients are going to be on the ventilator past that time, then it’s reasonable to put a tracheostomy tube in those patients early.
Some of our other early tracheostomy tubes were really directly related to a tenuous airway. The patients had facial fractures, maybe a marginal airway, and it was just easy for us to go ahead and do the tracheostomy early and establish a secure airway in these patients.
In terms of cost, we did not particularly look at cost. My colleague who is here, Dan Kenady, has thrown some rocks at us and thinks that the cost of open bedside tracheostomy are actually less than they are for percutaneous tracheostomy. I can’t argue with him too much. I think that there is data both ways about costs, and some of the problems with the literature regarding cost analyses is that some of it is charge data, some is cost data, and the other problem is that some people are comparing bedside percutaneous tracheostomy with open tracheostomy performed in the operating room, which is not really an adequate or fair comparison because of the incremental cost associated with the anesthesia and operating room use that don’t come into play for patients who get done in the intensive care unit.
Regarding tracheal stenosis, we had nine tracheal stenoses in this series. Five of the patients had a repair of their tracheal stenoses. Four did not, because they were debilitated patients who were going to chronic ventilator facilities; they had a limited lifespan, and we did not proceed with repair, although they clearly had stenoses.
We only evaluated patients who had symptoms. Every patient who has a tracheostomy will have some element of stenosis. It’s pretty clear from the older literature that you don’t need to evaluate everybody; you only need to evaluate the patients who have symptoms, and most patients will develop symptoms within 12 weeks of their decannulation.
Regarding the training that Dr. Flint asked about, we feel very strongly—and I have done about 300 of these 800 or so tracheostomies—that the residents need to have about 10 to 15 supervised procedures, and you should use bronchoscopy for some of them just so they get an idea of what the anterior tracheal wall looks like, and putting the needle in place and putting the guidewire down. This does help them understand the mechanics of the dilational procedure. Once they become comfortable and get the feel of this procedure, there is certainly no need to continue bronchoscopic guidance, and it can be reserved for when you have problems, such as difficult anatomy. That’s when we use it—we don’t use it routinely because it becomes quite expensive to use it routinely. In our institution, the cost alone is about $250 for the cart, having a technician come, and using the bronchoscopic equipment. It is true, however, that some of our complications could have been prevented if we had bronchoscopic guidance early on. But these complications have dissipated over time as we have gained experience and technical expertise with the procedure.
Dr. Carrico, I would agree that one large weakness of this particular case series, we do have a large number of patients. We did not compare it directly with open bedside tracheostomy. I am not sure I could talk my colleagues into doing it any longer. We all had experience with open tracheostomy and feel, based on our experience, that this is a faster, safer procedure. But it would be worthwhile to try and to a direct comparison between open and percutaneous tracheostomy.
Dr. Watkins, I don’t think that we should add percutaneous tracheostomy to the ATLS protocols. The main reason for this is that this procedure really is for the intubated patient on a mechanical ventilator. This is not meant for nonintubated patients. And the issue of percutaneous cricothyroidotomy or tracheostomy in the form of emergency airway management was not the specific topic of this. That’s a whole different discussion, and I would like to continue that at some other time.
We do, again, use bronchoscopy, which keeps the level of angina down in the faculty. And I do think that you need to have tight faculty supervision for this until you feel comfortable that the residents are adequately trained. We then do allow our senior residents to supervise more junior-level residents in this procedure, but there is always a faculty member present. I think it is very, very important. We are talking about somebody’s airway. The consequences of airway loss in the ICU are significant, and this is not something we hand down to the junior-level residents to handle after hours.
We do not keep tracheostomy trays at the bedside any longer. We did early on in the series, but we stopped doing it because the number of times where we had a problem was so few that, just having one in the ICU available, we could dredge it out and bring it over if we needed it. It was just a very infrequent problem.
Lastly, Dr. Spencer’s comments are appreciated. We do firmly believe—and I’m going to invoke Dr. Luchette’s name again, I think he firmly believes, as do many of us—that early tracheostomy does prevent some of the pulmonary infections, and their very nice prospective study showed that, and it shortens the ICU days and ventilator days, which are responsible for a lot of excessive costs in the care of these very difficult patients.
Footnotes
Correspondence: Paul A. Kearney, MD, C223 Dept. of Surgery, University of Kentucky Medical Center, 800 Rose St., Lexington, KY 40536.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Reprints will not be available from the authors.
E-mail: Pakear0@pop.uky.edu
Accepted for publication December 1999.
References
- 1.Kastanos N, Eatopa R, Perez AM, et al. Laryngotracheal injury due to endotracheal intubation: incidence, evolution, and predisposing factors. A prospective long-term study. Crit Care Med 1983; 11:362–367. [DOI] [PubMed] [Google Scholar]
- 2.Whited RE. A prospective study of laryngotracheal sequelae in long-term intubation. Laryngoscope 1984; 94:367–377. [DOI] [PubMed] [Google Scholar]
- 3.Santos P, Afrassiabi A, Weymuller E. Risk factors associated with prolonged intubation and laryngeal injury. Otolaryngology 1994; 111:453–459. [DOI] [PubMed] [Google Scholar]
- 4.Dayal VS, el Masri W. Tracheostomy in the intensive care setting. Laryngoscope 1986; 96:58–60. [DOI] [PubMed] [Google Scholar]
- 5.Indeck M, Peterson S, Smith J, et al. Risk, cost and benefit of transporting ICU patients for special studies. J Trauma 1988; 28:1020–1025. [DOI] [PubMed] [Google Scholar]
- 6.Smith I, Fleming S, Cernaianu A. Mishaps during transport from the intensive care unit. Crit Care Med 1990; 18:278–281. [DOI] [PubMed] [Google Scholar]
- 7.Waddell G. Movement of critically ill patients within hospital. Br Med J 1975; 2:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelden CH, Pudenz R, Freshwater D, et al. A new method for tracheotomy. J Neurosurg 1955; 12:428–431. [DOI] [PubMed] [Google Scholar]
- 9.Toy F, Weinstein J. A percutaneous tracheostomy device. Surgery 1969; 65:384–389. [PubMed] [Google Scholar]
- 10.Griggs W, Worthley L, Gilligan J, et al. A simple percutaneous tracheostomy technique. Surg Gynecol Obstet 1990; 170:543–545. [PubMed] [Google Scholar]
- 11.Schachner A, Ovil Y, Sidi J, et al. Percutaneous tracheostomy: a new method. Crit Care Med 1989; 17:1052–1056. [DOI] [PubMed] [Google Scholar]
- 12.Fantoni A, Ripamonti D. A non-derivative, non-surgical tracheostomy: the translaryngeal method. Intens Care Med 1997; 23:386–392. [DOI] [PubMed] [Google Scholar]
- 13.Seldinger S. Catheter replacement of the needle in percutaneous arteriography: a new technique. Acta Radiol 1953; 39:368. [DOI] [PubMed] [Google Scholar]
- 14.Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy—a simple bedside procedure; a preliminary report. Chest 1985; 87:715–719. [DOI] [PubMed] [Google Scholar]
- 15.Ciaglia P, Graniero K. Percutaneous dilatational tracheostomy results and long-term follow-up. Chest 1992; 101:464–467. [DOI] [PubMed] [Google Scholar]
- 16.Hazard P, Jones C, Benitone J. Comparative clinical trial of standard operative tracheostomy with percutaneous tracheostomy. Crit Care Med 1991; 19:1018–1024. [DOI] [PubMed] [Google Scholar]
- 17.Crofts S, McGuire G, Charles D. A comparison of percutaneous and operative tracheostomy in intensive care patients. Can J Anaesth 1995; 42:775–779. [DOI] [PubMed] [Google Scholar]
- 18.Friedman Y, Fildes J, Mizock B, et al. Comparison of percutaneous and surgical tracheostomies. Chest 1996; 110:480–485. [DOI] [PubMed] [Google Scholar]
- 19.Holdgaard HO, Pedersen J, Jensen R, et al. Percutaneous dilatational tracheostomy versus conventional surgical tracheostomy. Acta Anaesth Scand 1998; 42:545–550. [DOI] [PubMed] [Google Scholar]
- 20.Toursarkissian B, Zweng T, Kearney P, et al. Percutaneous dilational tracheostomy: report of 141 cases. Ann Thorac Surg 1994; 57:862–867. [DOI] [PubMed] [Google Scholar]
- 21.Hill B, Zweng T, Maley R, et al. Percutaneous dilational tracheostomy: report of 356 cases. J Trauma 1996; 40:238–244. [DOI] [PubMed] [Google Scholar]
- 22.Roe B. Bedside tracheostomy. Surg Gynecol Obstet 1962; 115:239–241. [PubMed] [Google Scholar]
- 23.Hawkins M, Burrus E, Treat R, et al. Tracheostomy in the intensive care unit: a safe alternative to the operating room. South Med J 1989; 82:1096–1097. [DOI] [PubMed] [Google Scholar]
- 24.Westpahl K, Byhahn C, Rinne T, et al. Tracheostomy in cardiosurgical patients: surgical tracheostomy versus Ciaglia and Fantoni method. Ann Thorac Surg 1999; 68:486–492. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez J, Steinberg S, Luchetti F, et al. Early tracheostomy for primary airway management in the surgical critical care setting. Surgery 1990; 108:655–659. [PubMed] [Google Scholar]
- 26.Kearney P, Johnson S. Tracheostomy for the critically ill. Curr Surg 1993; 50:558–563. [Google Scholar]
- 27.Wang M, Berke G, Ward P, et al. Early experience with percutaneous tracheotomy. Laryngoscope 1992; 102:157–162. [DOI] [PubMed] [Google Scholar]
- 28.Leinhart D, Mughal M, Bowles B, et al. Appraisal of percutaneous tracheostomy. Br J Surg 1992; 79:255–258. [DOI] [PubMed] [Google Scholar]
- 29.Cobean R, Beals M, Moss C, et al. Percutaneous dilatational tracheostomy. Arch Surg 1996; 131:265–271. [DOI] [PubMed] [Google Scholar]
- 30.Winkler W, Karnik R, Seelman O, et al. Bedside percutaneous dilational tracheostomy with endoscopic guidance: experience with 71 ICU patients. Intens Care Med 1994; 20:476–479. [DOI] [PubMed] [Google Scholar]
- 31.Van Heurn L, van Geffen G, Brink P. Clinical experience with percutaneous dilatational tracheostomy: report of 150 cases. Eur J Surg 1996; 162:531–535. [PubMed] [Google Scholar]
- 32.Billy M, Bradrick J. Percutaneous dilatational subcricoid tracheostomy. J Oral Maxillofac Surg 1997; 55:981–986. [DOI] [PubMed] [Google Scholar]
- 33.Powell D, Price P, Forrest L. Review of percutaneous tracheostomy. Laryngoscope 1998; 108:170–177. [DOI] [PubMed] [Google Scholar]
- 34.McHenry C, Raeburn C, Lange R, et al. Percutaneous tracheostomy: a cost-effective alternative to standard open tracheostomy. Am Surg 1997; 63:646–651. [PubMed] [Google Scholar]
- 35.Van Natta T, Morris J, Eddy V, et al. Elective bedside surgery in critically injured patients is safe and cost-effective. Ann Surg 1998; 227:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernard A, Kenady D. Conventional surgical tracheostomy as the preferred method of airway management. J Oral Maxillofac Surg 1999; 57:310–315. [DOI] [PubMed] [Google Scholar]


