Abstract
Objective
To evaluate the feasibility and potential benefits of hand-assisted laparoscopic surgery with the HandPort System, a new device.
Summary Background Data
In hand-assisted laparoscopic surgery, the surgeon inserts a hand into the abdomen while pneumoperitoneum is maintained. The hand assists laparoscopic instruments and is helpful in complex laparoscopic cases.
Methods
A prospective nonrandomized study was initiated with the participation of 10 laparoscopic surgical centers. Surgeons were free to test the device in any situation where they expected a potential advantage over conventional laparoscopy.
Results
Sixty-eight patients were entered in the study. Operations included colorectal procedures (sigmoidectomy, right colectomy, resection rectopexy), splenectomy for splenomegaly, living-related donor nephrectomy, gastric banding for morbid obesity, partial gastrectomy, and various other procedures. Mean incision size for the HandPort was 7.4 cm. Most surgeons (78%) preferred to insert their nondominant hand into the abdomen. Pneumoperitoneum was generally maintained at 14 mmHg, and only one patient required conversion to open surgery as a result of an unmanageable air leak. Hand fatigue during surgery was noted in 20.6%.
Conclusions
The hand-assisted technique appeared to be useful in minimally invasive colorectal surgery, splenectomy for splenomegaly, living-related donor nephrectomy, and procedures considered too complex for a laparoscopic approach. This approach provides excellent means to explore, to retract safely, and to apply immediate hemostasis when needed. Although the data presented here reflect the authors’ initial experience, they compare favorably with series of similar procedures performed purely laparoscopically.
In hand-assisted laparoscopic surgery (HALS), a recent development, the surgeon inserts a hand into the abdomen while pneumoperitoneum is maintained and uses the hand to assist the laparoscopic instruments directly. The approach of using an incision from the very beginning of the operation, which would be created to assist in the extraction of a resected specimen at some point during the procedure, seems logical, especially in the context of advanced laparoscopic procedures. It has been demonstrated that most major surgical procedures are amenable to the laparoscopic approach. However, advanced laparoscopic procedures have not been adopted for a number of reasons. They often take much longer then conventional surgery, rendering the cost/benefit ratio questionable. 1–4 This partly reflects the technical difficulties, lack of training and experience, and limited instrumentation available for advanced laparoscopic surgery, as well as the lack of tactile feedback and the absence of depth perception on the two-dimensional video monitor.
The appearance of hand-assist devices in the mid-1990s raised great expectations in the laparoscopic community. The presence of an assisting hand can provide tactile feedback. Gentle blunt dissection and hand-assisted retraction during advanced laparoscopic procedures may help solve these problems. However, because a 7- to 8-cm incision is required to accommodate the surgeon’s hand, some of the benefits of minimal access surgery (reduced postoperative pain, faster recovery, shorter hospital stay) are expected to be lost. Although initial reports of HALS suggested potential usefulness in certain applications, 5–10 many laparoscopic surgeons were discouraged by their initial experience with early prototypes of hand-assist devices. 11
The HandPort System (Smith and Nephew Inc., Endoscopy Division, Andover, MA), a new hand-assist device developed in collaboration with the Department of Surgery at the University of Massachusetts Medical School, was designed with special attention to ease of use, reliable maintenance of pneumoperitoneum, and hand comfort. The purpose of this study was to test the HandPort System and the potential applications of HALS in a feasibility study by a group of expert laparoscopic surgeons, The HALS Study Group.
METHODS
A group of expert laparoscopic surgeons from four U.S. and six international academic and community medical centers were invited to participate in a nonrandomized feasibility trial to test potential applications of HALS using the HandPort System. Any advanced laparoscopic procedure was entered in the study if the surgeon believed that the use of the hand would be of benefit. Patient enrollment in the study was initiated in February 1998 and ended in April 1999.
Patients scheduled for abdominal surgery were eligible to be entered in the study if they were older than 18 years, there was no contraindication to general anesthesia, there was no contraindication to pneumoperitoneum, and they had not participated in any other investigational study in the past 30 days. A negative pregnancy test was mandatory for female patients of childbearing age. Patients were enrolled only if they fully understood the nature of the study and gave written consent in advance. Approval by the institutional review board at each participating institution was mandatory. All data were collected by research nurses.
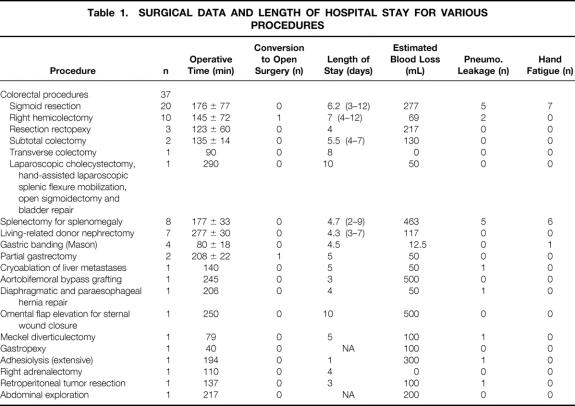
The HandPort System consists of a base retractor, a bracelet, and a sleeve (Fig. 1). The base retractor has an inflatable outer rim, an internal skirt, an internal ring, and an inflation pump with a valve. The bracelet should be placed under sterile conditions to the wrist of the surgical gown over a surgical glove. A second pair of brown gloves to prevent glaze is placed over the bracelet. The sleeve of the device is then placed over the forearm and its tapered rigid end secured to the bracelet.
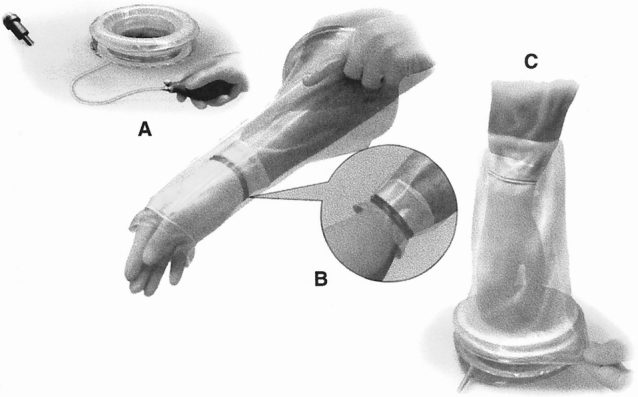
Figure 1. Deploying the HandPort System. (A) The base retractor is placed, and the external ring is inflated. (B) The sleeve is pulled on the arm, and the smaller ring is secured onto the bracelet. (C) The hand is inserted into the abdomen through the base retractor, and the wider ring of the sleeve is attached to the inflated external rim.
The hand-assist device should be placed after the abdomen has been insufflated. The distortion of the abdominal wall under pneumoperitoneum changes the ultimate position and length of the incision. The size of the incision corresponds to the width of the surgeon’s hand.
After loss of pneumoperitoneum, the inner ring and the skirt of the base retractor are placed in the abdominal cavity and flattened on the internal surface of the abdominal wall. The base retractor is then inflated to seal the inner ring and a part of the skirt against the abdominal wall. The valve is then closed. The surgeon’s hand is inserted into the abdomen through the base retractor, the sleeve is everted, and its wider end is snapped onto the inflated outer rim; pneumoperitoneum is then reintroduced. An insufflation cap, another part of the system, may seal the base retractor and maintain pneumoperitoneum, which allows the surgeon to continue with standard laparoscopy if needed.
For the purpose of this study, conversion to open surgery was defined as removal of the base retractor and extension of the incision to complete the procedure safely for reasons other than specimen extraction. All values are expressed as mean ± standard error of the mean.
RESULTS
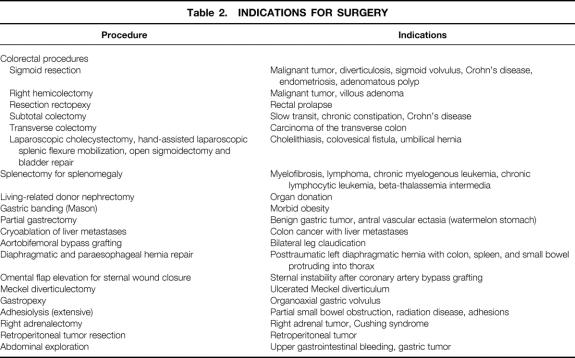
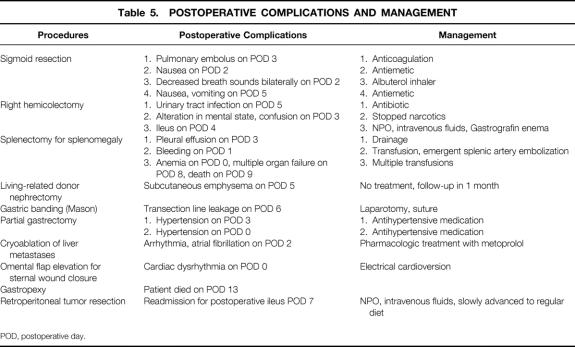
Sixty-eight patients were entered in the study. There were 39 women and 29 men; mean age was 54.4 ± 17.7 years, mean weight was 75.4 ± 17.6 kg, and mean height was 165.6 ± 10.6 cm. Thirty-seven colorectal procedures were performed: 20 sigmoid resections, 10 right hemicolectomies, 2 subtotal colectomies with ileorectal anastomosis, 3 resection rectopexies, 1 transverse colectomy, and 1 hand-assisted laparoscopic splenic flexure mobilization as part of a combined laparoscopic cholecystectomy and open sigmoidectomy with bladder repair. Splenectomy for splenomegaly was performed in eight patients, living-related donor nephrectomy in seven, Mason-type gastric banding for morbid obesity in four, and partial gastrectomy in two. In addition, isolated cases of the following procedures were included: cryoablation of liver metastases, aortobifemoral bypass grafting, diaphragmatic and paraesophageal hernia repair, sternal wound débridement and omental flap elevation for sternal wound closure, Meckel’s diverticulectomy, gastropexy for gastric volvulus, extensive adhesiolysis, right adrenalectomy, retroperitoneal tumor resection, and abdominal exploration for metastatic gastric cancer.
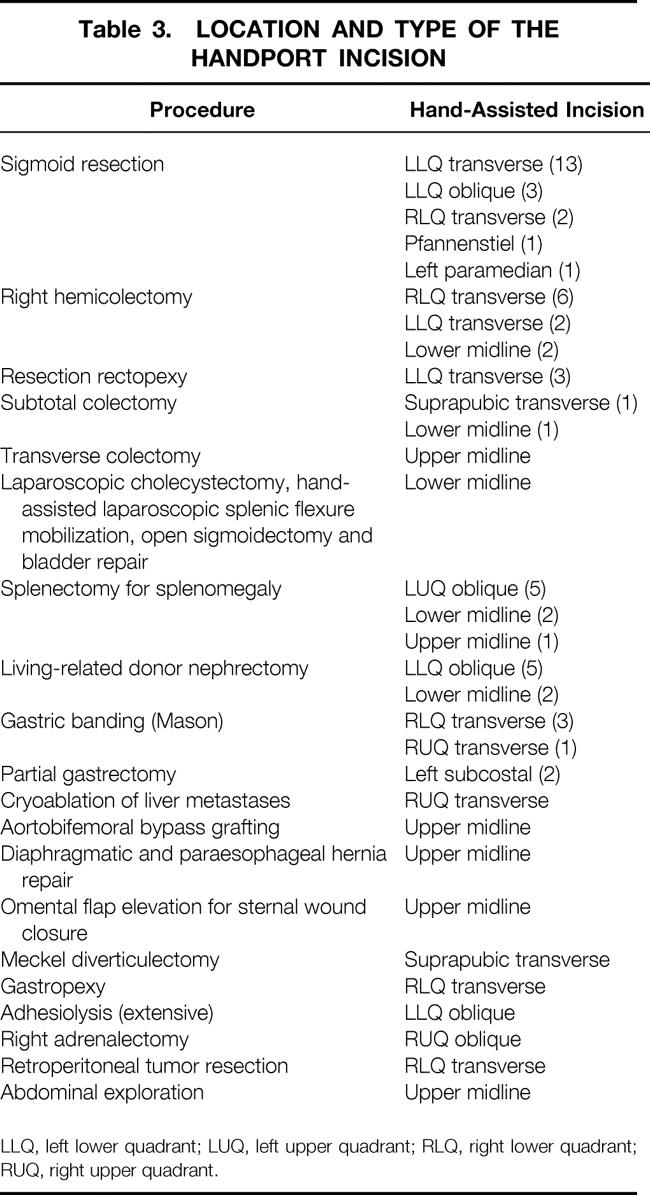
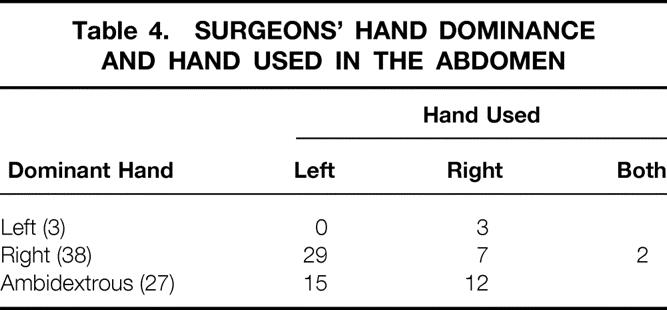
Surgical data with length of procedure and hospital stay for the various procedures are summarized in Table 1. Indications for surgery are listed on Table 2. The location and type of incisions used are listed in Table 3. Three fourths of surgeons preferred to use their nondominant hand in the abdomen (Table 4). The mean incision size for hand insertion was 7.45 ± 0.9 cm, and the mean glove size was 7.44 ± 0.47. In total, 51 resected specimens were delivered through the HandPort, 3 of which required extension of the incision (5.9%). Warm ischemia time for the living-related donor nephrectomies was less than 1 minute in all procedures.
Table 1. SURGICAL DATA AND LENGTH OF HOSPITAL STAY FOR VARIOUS PROCEDURES
Table 2. INDICATIONS FOR SURGERY
Table 3. LOCATION AND TYPE OF THE HANDPORT INCISION
LLQ, left lower quadrant; LUQ, left upper quadrant; RLQ, right lower quadrant; RUQ, right upper quadrant.
Table 4. SURGEONS’ HAND DOMINANCE AND HAND USED IN THE ABDOMEN
The mean pressure of pneumoperitoneum was 14 ± 1.4 mmHg. Some leakage of pneumoperitoneum through the HandPort device was encountered in 17 procedures (25%), but in only one was conversion to open technique required as a result of this problem. Hand fatigue during some part of the procedure, ranging in severity from mild fatigue to severe cramping, was noted in 14 procedures (20.6%). In two procedures (2.9%), the hand-assisted laparoscopic technique required conversion to conventional open surgery, with removal of the HandPort and extension of the incision: a right hemicolectomy in an obese patient, where the internal skirt of the base retractor proved to be too short and the device kept slipping out of the incision, and a gastrectomy for a benign gastric tumor, where the size and the location of the lesion necessitated the conversion.
Postoperative complications were recorded in 18 patients (Table 5). No postoperative complication was directly related to the HandPort device. Bleeding developed in one patient on postoperative day 1 after a hand-assisted laparoscopic splenectomy for splenomegaly; he was eventually managed by fluid resuscitation, transfusion, and splenic artery embolization. A transection line leak developed in another patient as a result of stapler malfunction on postoperative day 6 after Mason-type vertical gastric banding for morbid obesity. This patient underwent laparotomy and the leak site was oversewn.
Table 5. POSTOPERATIVE COMPLICATIONS AND MANAGEMENT
POD, postoperative day.
Two patients died: an 88-year-old woman who underwent gastropexy for organoaxial gastric volvulus died on postoperative day 13, and a 65-year-old man with chronic myelogenous leukemia, anemia, leukocytosis, weight loss, and chronic obstructive pulmonary disease, who underwent splenectomy for splenomegaly, died on postoperative day 9.
DISCUSSION
Some advanced minimal access surgical procedures have been criticized for being technically too demanding, too long, too expensive, or unsafe. Although instrumentation has dramatically improved over the past 10 years, the lack of three-dimensional visualization, the absence of gentle and safe laparoscopic retracting devices, and the lack of tactile feedback are still occasional sources of frustration in the operating room.
Hand-assisted laparoscopic surgery is a new concept for surgeons. It offers the ability to perform more complex operations more safely by allowing tactile feedback, gentle traction and countertraction on tissues, and digital blunt dissection. However, the 7- to 8-cm incision required may result in increased postoperative pain and wound complications.
Perhaps the most suitable operations for HALS are those that require extraction of a specimen and therefore necessitate an incision anyway. These procedures might be the most suitable for surgeons to consider for HALS. Participants in this study group were free to test the device in any complex advanced laparoscopic procedure if they believed that the use of the hand would be of benefit or if specimen removal was necessary. In addition, the participants attempted to replace conversion to open surgery with conversion to hand-assisted laparoscopic surgery if temporary tactile feedback or manual assistance was thought to be helpful. Finally, procedures were considered for HALS if they were too difficult or time-consuming for purely laparoscopic surgery.
We found that the vast majority of indications for HALS were colorectal disease. This reflects the fact that colorectal operations are complex and difficult to perform purely laparoscopically, which also explains why the penetration of laparoscopic colorectal surgery has been low among colorectal surgeons. But because the prevalence of colorectal disease in Western societies is high, 12,13 an increasing number of procedures can be performed laparoscopically. 4,14,15 In addition, in colorectal procedures an incision is often required for extraction of the specimen, so it is a natural fit for HALS. 16
The base retractor component of the device served as an excellent retractor in itself, allowing surgeons to perform extracorporeal anastomoses. If direct visualization of the surgical field was optimal through the device, then part of the procedure could be performed with conventional open techniques. Participants in the study group did not consider such procedures to have been converted to open surgery unless the base retractor was removed and the incision extended to complete the procedure.
The most important characteristic of a HALS device is reliability. When we developed the HandPort device, we believed that it had to adhere to three principles: ease of use, reliable maintenance of pneumoperitoneum, and the ability to switch rapidly between open, laparoscopic, and hand-assisted laparoscopic techniques. Previous devices were not reliable. The first reports of HALS described makeshift attempts to maintain pneumoperitoneum, and initial hand-assist devices had problems maintaining pneumoperitoneum and produced arm discomfort. 5,10,11 However, because of the inherent appeal of placing the hand in the abdominal cavity, we believed that a reliable device would allow the widespread development of HALS. 9,17,18
We found several surgical procedures in which use of the hand was considered beneficial. One of the most significant was laparoscopic living-related donor nephrectomy. Living-related donor nephrectomy effectively addresses the increasing shortage of donor kidneys. The availability of minimal access techniques for safe removal of a kidney for organ donation appeals to many potential donors. Excellent results have been published with the conventional laparoscopic technique, but an extraction incision is always required to remove the resected kidney. 19 The critical moments of this procedure are the minutes that pass between the transection of the renal artery and the placement of the kidney in iced saline solution (warm ischemia time). In this subgroup of patients, we found a definite advantage in terms of minimizing warm ischemia time. In addition, we believe that with the hand in the abdomen, we can gain more vessel length at the time of renal artery and vein transection. 20,21 Therefore, this has become the standard of care in our institution.
Another subgroup of procedures requiring special attention is laparoscopic splenectomy, which has become the gold standard in spleen removal. 22,23 However, massive spleens can be challenging, because current laparoscopic devices are often inadequate for safe retraction of the spleen; they can easily lacerate the splenic surface, especially when the spleen is bulky. The probability of bleeding is therefore high, and it is usually impossible to provide hemostasis once such a lesion occurs. In an analysis of 13 laparoscopic splenectomies for spleens more than 1000 g, Targarona et al 24 noted that splenomegaly should not be considered a contraindication for the laparoscopic approach. However, most authors still warn against attempting laparoscopic surgical management in such instances, or recommend preoperative splenic artery embolization. 22,25 We believe that the hand allows improved manipulation of the spleen and easier bagging for extraction and ensures immediate hemostasis. At this stage, we do not consider the use of HALS necessary for normal-sized spleens.
Other procedures that may be of interest in specialized practices have been described, including hand-assisted laparoscopic aortic surgery, cryoablation of liver metastases, diaphragmatic and paraesophageal hernia repair, and the development of an omental flap for closure of a sternal wound.
There are some caveats to HALS and the HandPort device. In massive obesity, the device is less likely to be reliable because of inadequate skirt length. This issue has been addressed by creating a longer (and adjustable) skirt length. If the device is placed too close (<2 cm) to bony prominences (e.g., inherent or rib margin), the falciform ligament, or adhesions such that the internal rim is deformed, there can be air leakage. In all other circumstances, we found the device to be suitable for our purposes.
Most surgeons (78%) preferred to place their nondominant hand in the abdomen. Hand fatigue occurred in 14 procedures, the majority being left-sided abdominal procedures (splenectomy, sigmoid resection). The surgeon sometimes had to remove and rest the hand for a few minutes. The best way to prevent hand fatigue is careful choreography and planning of trocar and HandPort placement. The location of the incision depends on the target organ and on whether the surgeon is left- or right-handed. The basic principle of instrument triangulation is valid for HALS, with the hand being considered as an instrument (Fig. 2). For precise planning of the procedure, laparoscopy should always precede placement of the HandPort incision. The HandPort incision should never be placed directly over the target area: some distance should be allowed for forward access. Finally, the HandPort incision should be planned so that turning it into a full conversion incision can be done with a simple extension, if necessary.
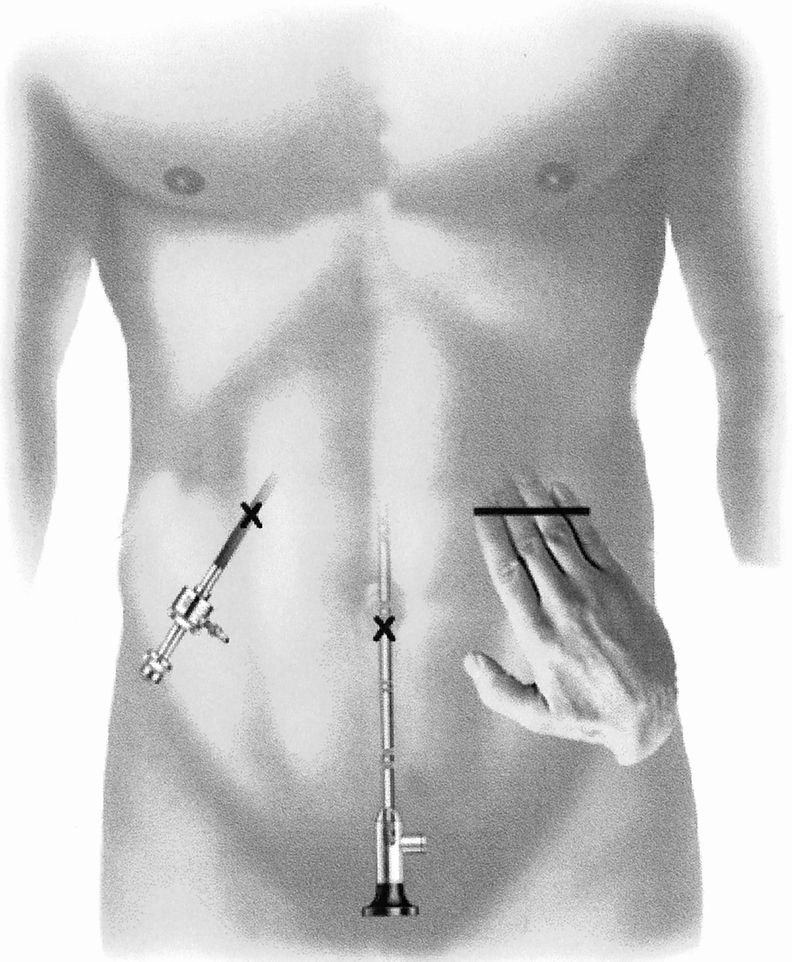
Figure 2. Strategy of port placement in hand-assisted laparoscopic surgery. Basic principles of instrument triangulation should be followed, with the hand considered as an instrument. This technique minimizes intraoperative hand fatigue.
In summary, HALS using the HandPort device appears to be a feasible approach to advanced laparoscopic surgery. It may allow surgeons to perform more complex operations by offering the surgeon the advantages of tactile feedback, safe retraction, and the ability to perform blunt dissection. We believe this will become a part of the surgeon’s armamentarium and will be incorporated into the modern paradigm of laparoscopic surgery.
Discussion
Dr. R. Scott Jones (Charlottesville, Virginia): During the last 10 years, with the development and advancement of laparoscopic and thoracoscopic surgery, we have really seen this great advance which has improved care and decreased hospitalization and stimulated a lot of technological development and real skill development in the profession, and that’s going to continue, clearly, for the foreseeable future. The development of this HandPort is just another important step in this process that I think we have to continue to encourage.
I basically had two questions. First, an observation. It seems to be a matter of judgment about when to go from laparoscopic to HandPort to open procedure. In other words, is there some point at which an open procedure might be just as well or better in terms of recovery and exposure, and so forth, as HandPort? Obviously, you will only answer that question with additional experience. But I would appreciate it if in closing you would comment a little bit about the judgment in this gray zone between purely laparoscopic and an open procedure, which is where you are working. And how are you planning to address that and develop it in the future?
My second question is to ask whether this device is readily available to surgeons at the moment in the marketplace. If you could tell us whether we could get this and use it without being in your protocol, I think the audience would be interested to know that. And that did remind me of one other question that, inevitably, will come up, and I might as well go ahead and get it in now—if you would comment on what does this do to the relative cost of the procedure when you use your HandPort.
Dr. Theodore N. Pappas (Durham, North Carolina): I can remember when I was a resident doing a rotation at the Boston Children’s Hospital, and I had started an appendectomy with an incision that was too small. Harvey Hendron came in, and in a very kind and supportive way, told me I had made the incision way too small and that incisions heal side-to-side, not end-to-end.
We then all learned through lap/chole that that wasn’t true, that size is important with incisions, and then we became smaller and smaller with our incisions, to the point of microinstruments. And now we are hearing that a 7-cm incision really doesn’t make a difference, that it is okay to make a bigger incision. My question is, what is the importance of the size of the incision? Are there other factors that are, in fact, more important in the recovery of the patient? Does the placement of a retractor have greater importance? The temperature of the bowel? Are there other factors that need to be discussed?
Why is it okay to make a 7-cm incision, and your implication being that the patients recover in a similar fashion?
The second question is what are the costs involved with this technique and is the presumed time savings paid for—do the time savings pay for the instrument itself or does it exceed the cost of a conventional laparoscopic procedure?
The third question: you have assembled an array of experts in this study. But did you have a sense that these experts would have been able to do all these procedures laparoscopically if this port was not available?
Dr. C. Randle Voyles (Jackson, Mississippi): I, too, congratulate Dr. Meyers on orchestrating an international study, 10 different centers, six countries, and 20 surgeons. And perhaps it also leads to what is potentially one of the weaknesses, in that there were 68 patients spread among these different places.
Intuitively, the hand-assisted laparoscopic technique is a good idea. Let’s simply retain the ability of the surgeon’s finger to touch, feel, and manipulate tissues as we would with an open procedure. And this HandPort truly does allow that.
But I think we also have to be a little bit cynical and critical and say, well, did the experiment really work? What happened with the real outcomes in this study? Because I know there are some cynics in the audience who would say “Colon resections, going home on the sixth or seventh day, it’s the same that you get with an open procedure with full-time clinical surgeons.” This procedure clearly took longer than an open procedure, and there is additional cost—not just with the HandPort device but also with the additional OR time.
And, finally, there might even be one or two of you that might say, “Well, one or two of those complications might have been avoided with an open procedure.” Without question, I think there are clearly patients that benefit from the hand-assisted laparoscopy, but it still remains unclear exactly who those people are.
Dr. Meyers, I wonder would your data have been stronger if you had concentrated all your work in one center where you can control for the expertise of surgeons? If the answer is yes, and I suspect it is, then there is implication of a fairly steep learning curve. So the next question is, can this technology be incorporated into the practice of a broad range of surgeons across the country?
Finally, as a surgeon who has really enjoyed the growth of laparoscopy in the last 10 years, I do have to ask, are we nearing the point where we are having full maturation of laparoscopic benefits with a diminishing yield with all of our new technologies?
Dr. George C. Hoffman (Norfolk, Virginia): I very much enjoyed hearing this paper, which addresses one of the fundamental inherent weaknesses of laparoscopic surgery—our ability to feel and palpate the bowel. We lose that sense of touch.
A number of years ago, we presented a paper at this meeting about our experience with laparoscopic colectomy. In the discussion, Dr. Jonathan Rhoads told a story about a patient upon whom he had done an open appendectomy many years ago and who came back in recent years with nondescript abdominal pain and some right-sided tenderness. Eventually, the patient had a laparoscopic cholecystectomy and it didn’t help; ultimately the patient was found to have a cancer of the cecum, which was hidden from the laparoscopic eye behind the adhesions of the old appendectomy from many years ago. He pointed out, I think correctly so, that the loss of the sense of touch and palpation probably cost this patient an earlier diagnosis. So it is a concern, and it leads to my first question.
Will this technique ever become inexpensive enough and efficient enough for us to use it in routine laparoscopic cases, or will it always be reserved for the more complex procedures?
My second question comes from the point of view of the patient: is this a step forward or a step back? In looking at the data, patients stayed in the hospital 6 to 7 days after a right or left colectomy. Those are numbers that I associate more with an open procedure. Operative times were between 2 and 3 hours for a colon resection—again, more like the learning curve of a laparoscopic procedure. Do we expect the clinical course of these patients to be like that of a laparoscopic case or an open procedure?
Once you get your hand in, there must be a great temptation to put in pads, packs, or retractors to keep the bowel out of the way so you can see what you are doing. Do you do that? Do you avoid it? Does it make any difference?
Dr. John G. Hunter (Atlanta, Georgia): Laparoscopy has been limited for the last 10 years by the inability to remove large specimens without morselating them beyond recognition, but this technology really allows us to do that. I have two questions, one of them very specific, one general.
The general question amplifies upon what the other discussants have said. What are the data that hand-assisted laparoscopy is superior to conventional laparoscopy? I think many discussants have concluded that it enables procedures to be done that could not otherwise be accomplished with laparoscopy, but are there data available proving this?
The second question is technical. How does one choose the site for the HandPort? Do you put it in the central abdomen? If so, does it get in the way of the laparoscope? Do you put it in the peripheral abdomen? Specific to that, how do you use it for a massive spleen? I have been trying to determine whether it is worthwhile to use this device for massive splenomegaly. Do you have any experience with this application?
Dr. Demetrius E. M. Litwin (Closing Discussion): Starting from the top, with Dr. Jones’ comments, I think that the purpose of this trial was not to determine whether this was better than pure laparoscopy or open surgery, because it was not comparative in nature. But we have completed a trial of hand-assisted laparoscopic surgery versus pure laparoscopic colorectal surgery utilizing the same groups of surgeons. We have 75 cases accrued; we have closed the trial, and we are on the brink of presenting that. The results of that trial lead me to believe that there is a benefit in some cases of colorectal surgery with the hand-assisted approach. Our next trial will be a pure hand-assisted laparoscopic surgery trial versus open colorectal surgery utilizing the same group of surgeons to try and answer some of the questions that have been asked here.
The device is readily available. It was FDA-approved about 6 months ago, and we ran these two trials concurrently in order to facilitate FDA approval. It’s now available from Smith and Nephew Endoscopy for around $400. I think, as competitive products arise, the costs will go down significantly.
Regarding Dr. Pappas’s concerns about incision size, we have been intrigued by the same issues. The question for hand-assisted laparoscopy is, if we go a little bit larger with the hand, how detrimental is that? I think the only way to answer that is with comparative trials, which we are trying to do to really be able to put numbers on what the benefit is or whether or not there is a benefit.
In response to other questions, I think at this point there is an increased cost. Certainly from the colon trial, we found that the operating times were similar to pure laparoscopic surgery, so this will be an added-on cost at this point in time. I think there was a component of learning curve with respect to our first uses of the device, and I actually think that those numbers—i.e., the operating time—will come down.
In response to the comment that the operative times in general were long, I must say that one has to remember that the cases selected for HandPort usage were, by and large, the more difficult cases. Easy cases among this group of advanced laparoscopic surgeons were handled purely laparoscopically. It was those cases in which there was a phlegmonous mass or some other factor that made the surgeon believe that the introduction of the hand would be beneficial that were tackled using the HandPort device.
The other question that was asked was what are the factors that may be responsible for improving patient outcome in laparoscopic surgery. The simple answer is that we are not sure. The early answer that appealed to most of us was that incision size had a role. But I think that there are other issues, for example, bowel manipulation, desiccation of the bowel, or temperature differences that occur with an open incision that may cause ileus or other problems. So I don’t think we know what the real factors are.
There were also some questions about whether or not it is useful for routine or complex operations. I think for the experienced laparoscopic surgeon, it will be very useful for complex operations. They will probably continue to do routine operations purely laparoscopically. I think, however, it will allow neophyte laparoscopic surgeons to do more complex operations than they would normally do laparoscopically.
Is it a step forward or a step back? I think it is a step forward, because I do think that more patients will be operated on laparoscopically and some of these patients will do better than with conventional open surgery.
Finally, how do you use the device? Determining the appropriate strategy for this device is critical, because if you use your hand ineffectively, it gets in the way. Making an incision over the structure is a mistake; you can’t see around your hand. So typically we use the hand like a triangulation port so that your hand and the dissecting instrument approach the structure at about a 60- to 90-degree angle, and in that way, the hand is most efficiently used. Typically for a splenectomy, I use my right hand through a left upper quadrant incision and my left hand is the dissecting hand through the midline, and I am able to approach the hilum of the spleen, which is the important area, at about at 60- to 90-degree angle to be able to facilitate the dissection of the hilum, which is the most important aspect of that operation.
Footnotes
Correspondence: William C. Meyers, MD, Dept. of Surgery, University of Massachusetts Medical School, 55 Lake Ave., North Worcester, MA 01655.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Supported by an educational grant from Smith and Nephew Endoscopy, Inc., Andover, Massachusetts.
E-mail: MeyersW@ummhc.org
Accepted for publication December 1999.
References
- 1.Gagner M, Pomp A. Laparoscopic pancreatic resection: is it worthwhile? J Gastrointest Surg 1997; 1:20–26. [DOI] [PubMed] [Google Scholar]
- 2.Goh PM, Alponat A, Mak K, Kum CK. Early international results of laparoscopic gastrectomies. Surg Endosc 1997; 11:650–652. [DOI] [PubMed] [Google Scholar]
- 3.Rau HG, Buttler E, Meyer G, et al. Laparoscopic liver resection compared with conventional partial hepatectomy—a prospective analysis. Hepato-Gastroenterology 1998; 45:2333–2338. [PubMed] [Google Scholar]
- 4.Swanstrom LL, Hansen P. Laparoscopic total esophagectomy. Arch Surg 1997; 132:943–949. [DOI] [PubMed] [Google Scholar]
- 5.Ou H. Laparoscopic-assisted mini-laparotomy with colectomy. Dis Colon Rectum 1995; 38:324–326. [DOI] [PubMed] [Google Scholar]
- 6.Kusminsky RE, Boland JP, Tiley EH, Deluca JA. Hand-assisted laparoscopic splenectomy. Surg Laparosc Endosc 1995; 5:463–467. [PubMed] [Google Scholar]
- 7.Kusminsky RE, Boland JP, Tiley EH. Hand-assisted laparoscopic surgery [letter; comment]. Dis Colon Rectum 1996; 39:111. [DOI] [PubMed] [Google Scholar]
- 8.Bemelman WA, Ringers J, Meijer DW, et al. Laparoscopic-assisted colectomy with the dexterity pneumo-sleeve. Dis Colon Rectum 1996; 39:S59–S61. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly MJ, Saye WB, Mullins SG, et al. Technique of hand-assisted laparoscopic surgery. J Laparoendosc Surg 1996; 6:239–244. [DOI] [PubMed] [Google Scholar]
- 10.Gorey TF, Bonadio F. Laparoscopic-assisted surgery. Semin Laparosc Surg 1997; 4:102–109. [DOI] [PubMed] [Google Scholar]
- 11.Southern Surgeons’ Club Study Group. Handoscopic surgery: a prospective multicenter trial of a minimally invasive technique for complex abdominal surgery. Arch Surg 1999; 134:477–486. [PubMed] [Google Scholar]
- 12.Van Cutsem E, Peeters M, Verslype C, et al. The medical treatment of colorectal cancer: actual status and new developments. Hepato-Gastroenterology 1999; 46:709–716. [PubMed] [Google Scholar]
- 13.Sandler RS. Epidemiology and risk factors for colorectal cancer. Gastroenterol Clin North Am 1996; 25:717–735. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson AR, Stitz RW, Lumley JW, Fielding GA. Laparoscopically assisted anterior resection for diverticular disease: follow-up of 100 consecutive patients. Ann Surg 1998; 227:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fielding GA, Lumley J, Nathanson L, et al. Laparoscopic colectomy. Surg Endosc 1997; 11:745–749. [DOI] [PubMed] [Google Scholar]
- 16.Mooney MJ, Elliott PL, Galapon DB, et al. Hand-assisted laparoscopic sigmoidectomy for diverticulitis. Dis Colon Rectum 1998; 41:630–635. [DOI] [PubMed] [Google Scholar]
- 17.Wolf JS Jr, Moon TD, Nakada SY. Hand-assisted laparoscopic nephrectomy: technical considerations. Tech Urol 1997; 3:123–128. [PubMed] [Google Scholar]
- 18.Nakada SY, Moon TD, Gist M, Mahvi D. Use of the pneumo-sleeve as an adjunct in laparoscopic nephrectomy. Urology 1997; 49:612–613. [DOI] [PubMed] [Google Scholar]
- 19.Flowers JL, Jacobs S, Cho E, et al. Comparison of open and laparoscopic live donor nephrectomy. Ann Surg 1997; 226:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf JS Jr, Moon TD, Nakada SY. Hand-assisted laparoscopic nephrectomy: comparison to standard laparoscopic nephrectomy. J Urol 1998; 160:22–27. [PubMed] [Google Scholar]
- 21.Slakey DP, Wood JC, Hender D, et al. Laparoscopic living donor nephrectomy: advantages of the hand-assisted method. Transplantation 1999; 68:581–583. [DOI] [PubMed] [Google Scholar]
- 22.Poulin EC, Mamazza J. Laparoscopic splenectomy: lessons from the learning curve. Can J Surg 1998; 41:28–36. [PMC free article] [PubMed] [Google Scholar]
- 23.Gossot D, Fritsch S, Celerier M. Laparoscopic splenectomy: optimal vascular control using the lateral approach and ultrasonic dissection. Surg Endosc 1999; 13:21–25. [DOI] [PubMed] [Google Scholar]
- 24.Targarona EM, Espert JJ, Balague C, et al. Splenomegaly should not be considered a contraindication for laparoscopic splenectomy. Ann Surg 1998; 228:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulin EC, Mamazza J, Schlachta CM. Splenic artery embolization before laparoscopic splenectomy. An update. Surg Endosc 1998; 12:870–875. [DOI] [PubMed] [Google Scholar]