Abstract
Objective
To define the long-term outcome and treatment complications for patients undergoing liver resection for multiple, bilobar hepatic metastases from colorectal cancer.
Methods
A retrospective analysis of 165 consecutive patients undergoing liver resection for metastatic colorectal cancer was performed. Patients were divided into a simple hepatic metastasis group, consisting of patients with three or fewer metastases in a unilobar distribution, and a complex hepatic metastases group, consisting of patients with four or more unilobar metastases or at least two bilobar metastases.
Results
The 5-year survival rate was 36% for the simple group and 37% for the complex group. Multivariate analysis revealed that the number of hepatic segments involved by tumor and the maximum diameter of the largest metastasis correlated significantly with the 5-year survival rate. The surgical death rate was 4.9% for the simple group and 9.1% for the complex group; this difference was not significant. Multivariate analysis revealed that extended lobar resection and concomitant colon and hepatic resection were significant and independent predictors of surgical death. The combination of extended lobar resection and concomitant colon resection was used significantly more frequently in the complex group than in the simple group.
Conclusions
Resection of complex hepatic metastases, as defined in this study, results in a 5-year survival rate of 37% and confers the same survival benefit as does resection of limited hepatic metastases. The surgical death rate for this aggressive approach is significantly higher if extended lobar resections are necessary and if concomitant colorectal resection is performed. Patients who have complex hepatic metastases at the time of diagnosis of the primary colorectal cancer and who would require extended hepatic lobectomy should have hepatic resection delayed for at least 3 months after colon resection.
Substantial surgical experience, reported from multiple institutions, 1–5 supports the surgical resection of limited, isolated hepatic metastases from colorectal carcinoma, with 5-year survival rates of 30% or more reported routinely. The majority of the surgical experience reported to date has been in patients with one, two, or three metastatic lesions, usually confined to one lobe of the liver. The suitability for resection of patients with more complex hepatic metastatic disease has not been well defined. The multiinstitutional review reported by Hughes et al 6 of 100 patients surviving 5 years or more after resection of hepatic metastases from colorectal cancer included only three patients with four or more metastatic lesions. Scheele et al, 2 in one of the largest single-institution reports to date, reported experience with only 32 patients undergoing resection of four or more metastatic lesions. Further, reports of resection of multiple metastases are confounded by the classification as multiple lesions of clusters of small lesions in close proximity or of satellite lesions around a large metastasis.
Because surgical guidelines regarding the upper limit of surgical aggressiveness are not well defined, the present study was undertaken to compare the treatment-related complications and long-term outcome between patients with complex metastatic disease and those with more conventional indications for hepatic resection in a single institution that has taken a more aggressive approach toward complex hepatic metastatic disease during the past decade.
METHODS
Patient Population
This retrospective review included all patients who underwent hepatic resection for metastatic colorectal cancer at the Ochsner Medical Institutions before June 30, 1999. Only those in whom all known metastatic disease was resected with curative intent were included. To be considered for hepatic resection, a patient had to be medically fit, with no contraindications to major hepatic surgery; had to have hepatic metastases that were resectable such that an adequately sized, well-vascularized hepatic remnant would remain after resection; and could have no evidence of extrahepatic recurrence on the preoperative workup, which included chest x-ray or computed tomography of the chest, computed tomography of the abdomen and pelvis, and colonoscopy.
The patient population was divided into two groups based on the number and distribution of hepatic metastases. The simple hepatic metastasis group (SHM) was defined as patients with one, two, or three metastatic lesions in a unilobar distribution. The complex hepatic metastasis group (CHM) was defined as patients with four or more distinct and separate lesions within one lobe or at least two distinct and separate lesions in opposite lobes. These definitions were based on the number and distribution of metastatic lesions, not the extent or complexity of the hepatic resection. The definitions were chosen because they correspond to the most widely used criteria for surgical resectability throughout the past decade: that is, patients with four or more metastatic lesions or bilobar disease are considered marginal candidates for resection at best; at many institutions, they are considered to have inoperable disease. Bilobar disease in this study was defined as at least two distinct and separate lesions, one of which has an epicenter to the right of the middle hepatic vein and the other has an epicenter to the left of the middle hepatic vein. Satellite lesions (small lesions separate from but within 2 cm of a larger lesion) were not included when counting the number of metastatic lesions.
Hepatic resection volume was calculated by multiplying the three perpendicular sections of the surgical specimen, as measured by the pathologist. This provided a semiquantitative measurement of specimen volume for comparison between the two groups. It is understood that this method overestimates the actual volume of the resection.
Surgical Management
Before hepatic resection, patients underwent full abdominal exploration to exclude extrahepatic recurrence of colorectal cancer. Porta hepatis lymph nodes were examined but were not resected routinely. From 1988 onward, intraoperative hepatic ultrasound was used routinely. Resections were classified as nonanatomical wedge resections, segmentectomies, lobectomies, or extended lobectomies. Right lobectomy included Couinand 7 segments 5 to 8, and left lobectomy included segments 2 to 4. Extended right lobectomy consisted either of resection of segments 4 to 8 in continuity, or segments 5 to 8 plus a noncontinuous resection of segment 2 or 3. Extended left lobectomy consisted either of resection of segments 2 to 5 and 8 in continuity or segments 2 to 4 plus a noncontinuous resection of two right lobe segments.
Resections were done by the finger fracture technique or by use of the Cavitron ultrasonic aspirator (Valleylab, Boulder, CO), with isolation and division of hilar inflow and hepatic vein outflow before parenchymal transection for lobar resections. During the study, portal inflow occlusion was used with increasing frequency during parenchymal transection. Cryoablation was used in a few procedures as an adjunct to resection. An hepatic artery infusion catheter for postoperative infusion chemotherapy was inserted on a selective basis, in most instances as part of a multiinstitutional protocol evaluating the use of multimodality therapy for patients with CHM.
The policy toward synchronous liver metastases evolved during the study. Early in our experience, hepatic resection was not combined with colorectal resection except if a small, peripherally located hepatic metastasis could be removed by nonanatomical wedge or segmental resection. For larger or more central hepatic metastases during our early experience, our practice was to wait at least 3 months and have the patient undergo a detailed metastatic workup before proceeding with hepatic resection. More recently, complex and major hepatic resections have been combined with colorectal resection with increasing frequency, either immediately (if the colorectal resection was performed at our institution) or within a short interval of 1 or 2 months (if the patient underwent colorectal resection at another institution and was referred to our institution for hepatic resection). For the purpose of this study, concomitant hepatic resection is defined as hepatic resection carried out simultaneously with or within 3 months of colorectal resection.
Patient Follow-Up
Data were abstracted from each patient’s clinical record and included demographic information, details of patient presentation and preoperative workup, surgical information, pathologic findings, and hospital course. Long-term outcome was obtained through clinic follow-up, tumor registry follow-up, and contact with the patient, family, or referring physician when necessary.
Statistical Analysis
Chi-square analysis or the Fisher exact test was used for univariate analysis. 8 The t test was used to analyze continuous variables, and Cox regression analyses 9 were performed using the BMDP software package (BMDP Statistical Software Inc., Los Angeles, CA). P < .05 was considered significant. Deaths within 30 days of hepatic resection or at any time after 30 days if occurring during the same hospital stay as the hepatic resection were considered to be surgical deaths.
RESULTS
Patient Characteristics and Follow-Up
One hundred sixty-five patients underwent curative hepatic resection for metastatic colorectal cancer during this study. The median age was 63 (range 25–90) years. Forty-two percent had synchronous hepatic metastases. Forty-seven percent of the study group had received chemotherapy before hepatic resection. Ninety-four patients (57% of the study group) died of metastatic colorectal cancer or treatment complications. The median follow-up of surviving patients was 30 months.
Complex Versus Simple Hepatic Metastases
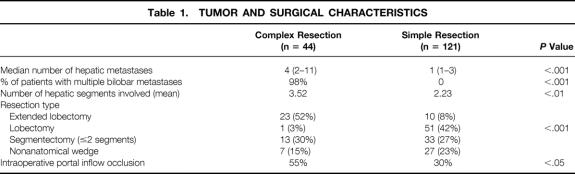
Forty-four patients underwent resection of CHM; 121 patients underwent resection of SHM. There was no significant difference between the two groups in terms of age, American Society of Anesthesiology risk status, percentage of patients receiving chemotherapy before hepatic resection, length of surgery, frequency with which concomitant colon and hepatic resection was carried out, blood transfusion requirement (median, 2 units), or hepatic resection volume. There were significant differences between the two groups with respect to the number of hepatic metastases, bilobar distribution of hepatic metastases, number of hepatic segments involved by metastatic disease, type of hepatic resection, and use of portal inflow occlusion during surgery (Table 1). Cryoablation was used as an adjunct to surgical resection in 7% of the CHM group and in 6% of the SHM group.
Table 1. TUMOR AND SURGICAL CHARACTERISTICS
Changes in Practice Pattern
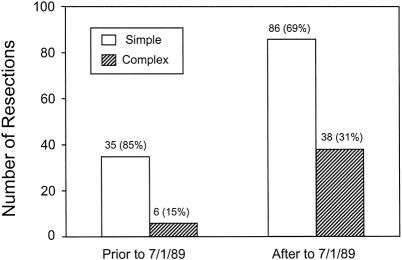
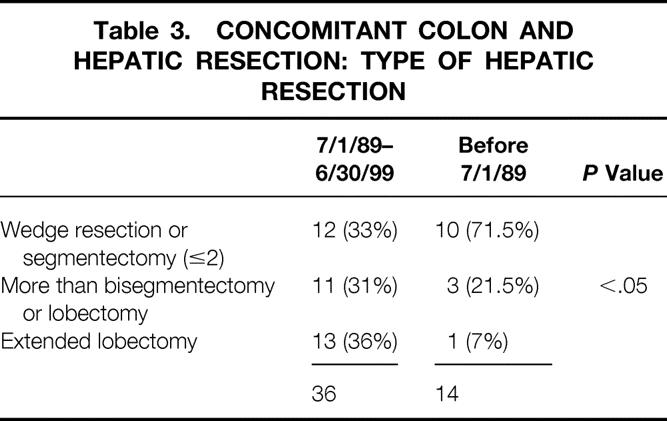
During the past 10 years of this study (July 1, 1989, to June 30, 1999), a significantly increased number and proportion of CHM patients have undergone resection compared with the earlier part of the study (before July 1, 1989;Fig. 1). As shown in Table 1, the proportion of patients with CHM having extended lobectomies was significantly higher than for patients with SHM, and the use and intensity of posthepatic resection chemotherapy have also increased (Table 2). Although the frequency of concomitant colon and hepatic resection did not change between the early and late periods, the use of extended hepatic resection for complex metastatic disease in conjunction with concomitant colon resection increased significantly (Table 3).
Figure 1. Total number of resections and proportion of patients in the simple and complex hepatic metastasis groups as a function of the time period in this study.
Table 2. USE OF CHEMOTHERAPY AFTER HEPATIC RESECTION
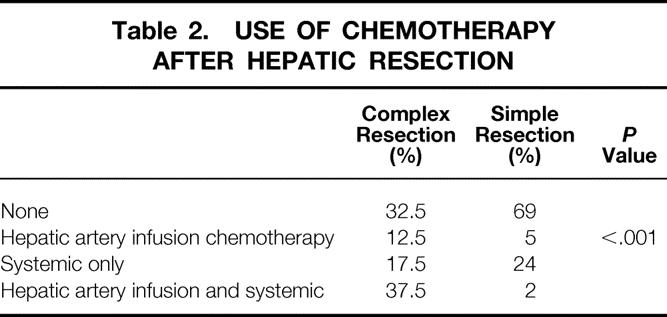
Table 3. CONCOMITANT COLON AND HEPATIC RESECTION: TYPE OF HEPATIC RESECTION
Surgical Death and Complication Rates
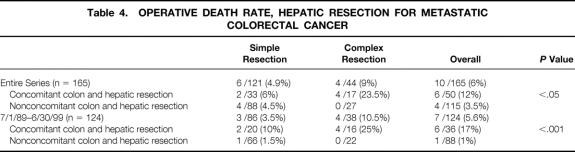
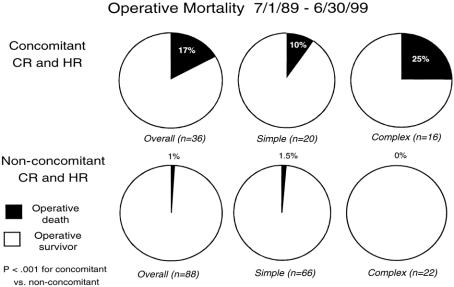
The incidence of postoperative bile leak (SHM 6.8%, CHM 8.2%) and delayed hepatic function (SHM 6.6%, CHM 13.6%) and the median hospital stay (SHM 8 days, CHM 8 days) were similar for the two groups. The overall surgical death rate for the entire series was 6%; it did not differ significantly between the groups (SHM 5%, CHM 9%). The surgical death rate for the most recent 10 years of the study remained constant at 5.6% (7/124, Table 4). The surgical deaths have been concentrated in patients who underwent concomitant hepatic and colon resection: in the past 10 years, the surgical death rate for patients undergoing concomitant resection was 17% (6/36) versus 1% (1/88) in those undergoing hepatic resection alone (P < .001;Fig. 2). Moreover, the surgical death rate has been 25% for patients undergoing hepatic resection for CHM and concomitant colon resection during the past decade, during which time 11 patients with CHM underwent extended lobar resection concomitant with colon resection.
Table 4. OPERATIVE DEATH RATE, HEPATIC RESECTION FOR METASTATIC COLORECTAL CANCER
Figure 2. Surgical death rate for the most recent 10 years of the study for the simple and complex hepatic metastasis groups as a function of whether the patient underwent concomitant colon resection.
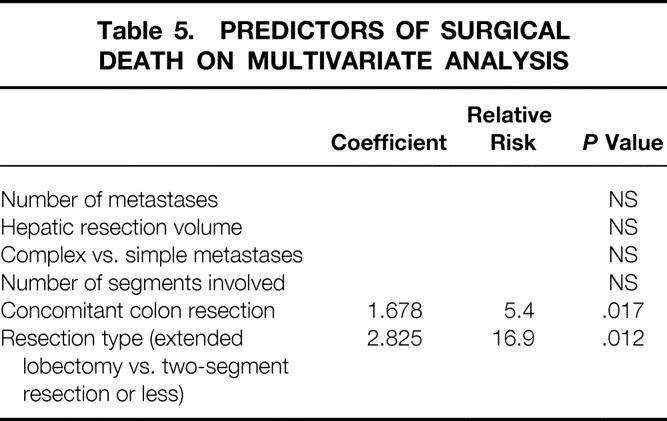
In univariate analysis, the surgical death rate was significantly associated with concomitant colon resection (P = .04), resection type (P = .007), the number of hepatic segments involved (P = .03), and delayed hepatic function after surgery (P = .0001). It was not associated with patient age, administration of chemotherapy before hepatic resection, duration of surgery, number of metastatic lesions, maximal tumor diameter, hepatic resection volume, transfusion requirement, or postoperative bile leak.
Multivariate analysis was performed to test for independent variables predicting for surgical death; delayed hepatic function was not included in the model because the clinical value of this sign as a predictor of surgical death is limited. In multivariate analysis, concomitant colon resection and hepatic resection type (extended lobar resection vs. ≤2 segments) were significant independent variables predicting for surgical death (Table 5).
Table 5. PREDICTORS OF SURGICAL DEATH ON MULTIVARIATE ANALYSIS
Survival
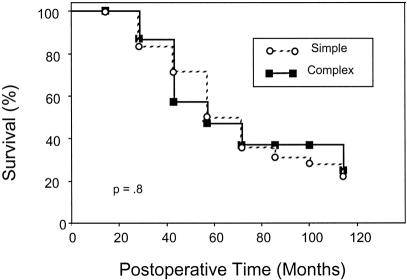
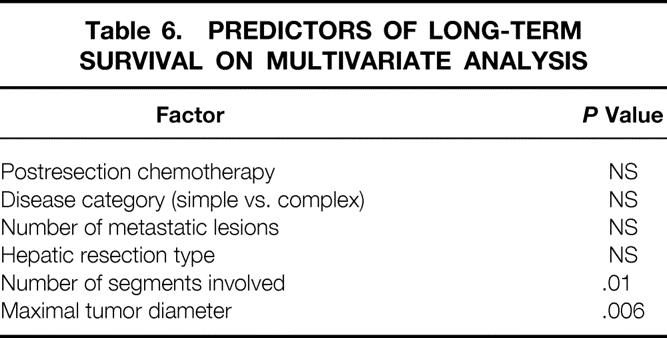
Excluding surgical deaths, the actuarial 5-year survival rate for all patients was 36%. It was the same for the SHM group (5-year survival rate 36%, median survival 43 ± 4 months) and the CHM group (5-year survival rate 37%, median survival 39 ± 11 months;Fig. 3). Multivariate analysis revealed a significant association between long-term survival and the number of hepatic segments involved by metastatic disease, as well as the maximal diameter of the largest hepatic metastasis (Table 6). The number of hepatic metastases, the presence of complex versus simple disease, the use of postresection chemotherapy, and the type of hepatic resection were not predictive of long-term survival in this model.
Figure 3. Survival after hepatic resection for metastatic colorectal cancer for the simple and complex hepatic metastasis groups.
Table 6. PREDICTORS OF LONG-TERM SURVIVAL ON MULTIVARIATE ANALYSIS
DISCUSSION
The efficacy of surgical resection of one to three hepatic metastases from primary carcinoma of the colon and rectum is clear from several reports. 1–5 However, the literature regarding resection of four or more metastatic lesions is conflicting. Cady et al 10 reported that no patient with three or more metastatic lesions survived disease-free for more than 48 months; however, patients with three or four metastatic lesions continued to be considered for resection at their institution because “the issue of the total number of metastatic nodules and long-term outcome is still incompletely resolved.” Hughes et al, 3 in a multicenter survey, noted a 5-year survival rate of 18% in 67 patients with four or more separate metastases but added, “some of these patients may, in fact, have had solitary metastases with well-developed satellites.” Fong et al 1 recently reported a prognostic scoring scale based on outcomes for 1,001 patients undergoing hepatic resection at a single institution that included the presence of multiple metastases as an adverse prognostic factor. In contrast, Scheele et al, 2 in a large single-institution experience, reported that the number of metastatic lesions did not predict outcome, provided that a margin-negative resection was achieved.
The surgical literature also contains conflicting reports about the prognostic significance of bilateral hepatic involvement. In the report by Fong et al, 1 bilateral tumor was not predictive of long-term outcome; however, Gayowski et al, 5 in a sizable single-institution experience, reported that bilobar involvement significantly decreased survival. Further, available reports, when reporting their experience with bilobar tumors, do not always distinguish between a single large tumor that has extended across the middle hepatic vein and multiple separate metastatic lesions on either side of the middle hepatic vein.
In our study, the presence of four or more distinct, separate metastatic lesions was chosen as the breakpoint between simple and complex disease because, in our experience, it is unusual for a patient with four or more separate lesions to have all of the disease confined to one lobe, and the presence of multiple, bilobar metastatic disease confronts the surgeon with the difficult interplay between resectability, adequacy of margin clearance, and adequacy of hepatic remnant function (and therefore surgical risk). Of course, a patient with a single metastatic lesion may present difficult issues with regard to resectability, margin clearance, and remnant function depending on the size and location of the lesion, and an occasional patient with four or more metastatic lesions can undergo resection using a straightforward hepatic resection, but in general patients with multiple bilobar metastases present surgical challenges that are more complex than those in patients with solitary or limited unilobar disease.
In our series, the patients with complex disease received clear therapeutic benefit from surgical resection, with a long-term outcome equivalent to that of patients with simple disease. The influence of multimodality therapy on this survival benefit is not clear from our study. Although the complex group received postresection chemotherapy significantly more frequently and at greater intensity than the simple group (see Table 3), the nonrandomized nature of our study precludes us from drawing conclusions in this regard. The recently reported randomized prospective study by Kemeny et al 11 demonstrated short-term survival benefit (at 2 years) for patients receiving combined hepatic artery infusion and systemic chemotherapy after hepatic resection compared with patients receiving systemic chemotherapy only; the benefit in this study was most pronounced for patients with two to four metastatic lesions. However, a second randomized prospective study of postresection hepatic artery infusion chemotherapy only has not shown survival benefit. 12
Although the survival benefit for resection of complex disease seemed clear in our study, the complications of such treatment were high, with a postoperative death rate of 9% in patients with complex disease undergoing hepatic resection. The surgical deaths were concentrated among patients with synchronous hepatic metastases who underwent concomitant colon and hepatic resection. The definition of concomitant colon and hepatic resection used in this study (both simultaneous resections and those performed within 3 months) may seem arbitrary, but the use of both simultaneous and short-interval hepatic resection for patients with complex hepatic disease requiring extended resection represented a clear change in our surgical practice during the past 10 years. Conventional surgical practice 13,14 has been to perform simultaneous colon and hepatic resection only for patients who had minimal, peripherally located hepatic disease that could be resected by simple wedge resection or segmental resection. Patients with more extensive metastatic disease usually had their hepatic resection deferred by at least 3 months, and often more. The reasons for this delay were threefold:
1. In many instances, the metastatic disease was discovered incidentally at the time of colonic resection, and three-dimensional imaging of the liver was not available to exclude other metastatic foci within the liver.
2. Many patients had not had a complete metastatic workup to exclude the presence of extraabdominal metastatic disease.
3. Concern existed about the increased surgical complication rate with a combined colon resection and extensive hepatic resection.
The view that major hepatic resection should be routinely delayed more than 3 months after resection of primary colorectal cancer has been challenged in recent years. 15–18 Our reasons for adopting a more aggressive stance included the availability in most patients of preoperative three-dimensional imaging of the liver or the availability during surgery of hepatic ultrasound, and our concern that a delay of 3 months or more for patients who had multiple bilateral metastatic lesions might preclude the possibility of resection. Further, the inclusion of many of the patients with complex disease in this study in an investigational protocol that did not allow systemic chemotherapy before resection of metastatic disease probably pushed us to be more aggressive surgically, as indicated by the significantly more frequent use of extended lobar resection in the complex group undergoing concomitant colon and hepatic resection. In retrospect, the increased rates of death and complications of this early aggressive surgical approach almost certainly outweighed any benefit.
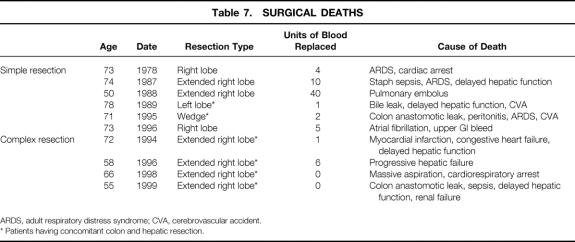
The cause of increased death and complications among patients undergoing concomitant hepatic resection for complex disease and colon resection in our study is probably multifactoral. In two instances, colon anastomotic leak and sepsis were clearly the determining factors in the patient’s postoperative death. Of the remaining four patients undergoing concomitant resection who did not survive the postoperative period, three underwent extended right hepatic resection and one underwent standard left hepatic resection, and delayed hepatic function played a major role in three of the four deaths (Table 7). In this regard, an experimental study reported by Miyazaki et al 19 demonstrated delayed hepatic regeneration and an increased risk of colon anastomotic leak when ileocolectomy or transverse colectomy was combined with 70% hepatic resection, compared with hepatic or colon resection alone.
Table 7. SURGICAL DEATHS
ARDS, adult respiratory distress syndrome; CVA, cerebrovascular accident.
* Patients having concomitant colon and hepatic resection.
In reassessing our strategy for patients with complex but potentially resectable hepatic metastases presenting synchronously with the colon primary, an alternative approach might be to resect the primary colorectal carcinoma as the initial step, followed by 3 to 6 months of systemic chemotherapy. After chemotherapy, if the metastatic workup reveals no extrahepatic metastatic disease, surgical resection of hepatic metastases could be carried out with implantation of an hepatic artery catheter for postoperative hepatic artery infusion therapy to complete the treatment course. We believe that such a treatment plan might allow patients with synchronous primary colorectal cancer and complex but potentially resectable hepatic metastases to undergo aggressive, potentially curative therapy with a lower surgical risk and with the possible survival benefit derived from systemic and hepatic artery infusion chemotherapy.
Discussion
Dr. Michael J. Edwards (Louisville, Kentucky): I think it is clear from this report, and certainly from others, that the morbidity and mortality of hepatic resection has dropped over time. The operation has become safer with the passage of time and with refinement of the technique. And as with any therapy, anytime we get better at it, we need to constantly go back and reevaluate the original indication for the procedure.
In this case, I would like to commend the authors on what I believe to be a very thoughtful reanalysis of our old rules, if you will, regarding who we resect and who we don’t. I think they have made two primary, important observations, one of certainly confirms our bias in Louisville, that the classic indications for hepatic resection should be reevaluated and that there is a significant impact in tackling patients with more extensive disease, defined by those patients having more than three isolated metastases, and also the patients who have bilobar disease. So I compliment them on that observation.
Another observation they point out, about colon resection at the time—and they defined it as concomitant, occurring within 3 months—I’d certainly like to know a little bit more about that. How many were actually done at the time of the hepatic lobectomy?
I think that’s important, because when John showed those graphs, you can see that when he does not do a colon resection, you are looking at about a 1% mortality. When you do a colon resection, even with a simple hepatic resection, you are looking at a tenfold increase in mortality, at least a 10% mortality rate. And I would submit that maybe we don’t need to go back and study that anytime soon, or at least in the next several years, until maybe another generation of technique has evolved, since it seems to have proven that a colon resection in the face of hepatic resection, when it’s a major resection, is clearly something we’d like to avoid.
I’d also like to point out that when we do think about treating the liver synchronously with a colon resection, we now have other techniques to do just that. We have tended toward using radiofrequency ablation in Louisville for what we believe to be—and I hope to hear later—a less morbid approach. We think that RFA is less morbid than cryoablation, and we are looking forward to learning more about that. Did you use radiofrequency ablation in your series of patients?
You talked about chemotherapy. I noted that over 40% of your patients were treated with chemotherapy before the operation, most of them in the adjuvant setting, and we think that’s appropriate if they were node-positive. But in Louisville, we have a problem with our medical oncologists not recognizing the therapeutic impact of hepatic resection and not referring these patients. Did you prescribe this preoperative chemotherapy, or was it, as I suspect, an erroneous management program set up by a medical oncologist?
Finally, you noted that there was increasing use of chemotherapy, or at least it was variable over time. In node-positive patients with colon cancer, you reduce the death rate by a third by giving systemic chemotherapy after resection; should there not be a similar impact—albeit less well-defined—when we resect distant disease and give systemic chemotherapy? What are your current indications for so-called adjuvant chemotherapy after resection of hepatic disease?
Dr. Yuman Fong (New York, New York): It was less than 20 years ago that there was controversy surrounding any resection of hepatic metastatic disease. But over the last two decades, the data clearly shows that for limited disease, the operations are not only safe but supported by the biology of the disease.
In this field, the relevant questions are no longer could and should we do the operations, the questions now are, what are the limits of the disease that is reasonable to resect? How can we select these patients? And what are the adjuvant therapies after resection? This study presented by Dr. Bolton is part of a growing literature that advances the field by answering some of these questions.
I have three questions that I would like to ask:
The first relates to the selection of patients. The authors have concentrated mainly on the number of tumors. Can they tell us a little bit more about the other characteristics? How many of these patients actually have more than three of those criteria that we use for selecting patients at Memorial Sloan-Kettering? More importantly, can the authors tell us how they now select patients in terms of biological limits of resection for this disease?
The second question relates to the combined colon and liver resection because we have a slightly different approach at Sloan-Kettering. We don’t believe that there is an increase in complications by doing a combined liver/colon resection. How many of the colon resections in this paper were actually sigmoid or rectal resections, where the resection may be compromised technically by the incision? In our institution, if the patient has a right, transverse, or high left colon lesion, we have no hesitation about doing a combined resection, because technically it can be done.
Lastly, with regard to chemotherapy, a large number of these patients had intraarterial chemotherapy. Did the placement of the pumps or the ports compromise the resections in any way or cause any other complications? This is data that people are going to want to know in terms of planning adjuvant intraarterial chemotherapy. How do the authors think that giving the adjuvant chemotherapy affected their outcome in terms of the complex group? What is their standard adjuvant chemotherapy now at the Ochsner Clinic?
Dr. George M. Fuhrman (Closing Discussion): Dr. Edwards started asking us about what percentage of these patients were truly concomitant resections. The answer to that really reflects the Ochsner Clinic’s role as a referral center for treating this disease. In our early experience, virtually 100% of the patients we defined as concomitant had their colon resection at the time of their liver surgery. In the last 10 years we have seen a dramatic increase in the volume of patients referred after their colectomy in their own community and, therefore, only about 50% of the patients in that latter group in the past 10 years actually truly had simultaneous colectomy with their hepatic resection.
Regarding the use of other therapies beside resection to treat colorectal metastases, we have not utilized radiofrequency ablation in any of these patients. We are in our infancy right now in exploring this new modality. We do have experience with cryotherapy in about three dozen patients, and as mentioned by Dr. Bolton, it was used as an adjunct in 5% of the patients in both groups.
Regarding the issue of chemotherapy, we also recognize that there is a problem in the medical oncology community of a failure to recognize the value that surgeons have in treating these patients with metastatic disease. The bulk of these patients, about two thirds, who received chemotherapy prior to surgery did have adjuvant chemotherapy after their original colectomy. But about a third of the patients who had chemotherapy did receive chemotherapy for their liver disease prior to their referral.
Your last question regarding our standard recommendations for chemotherapy following hepatic resection: the patients who have complex disease are being treated as part of a North Central Cancer Treatment Group trial, which is exploring the use of combined hepatic artery chemotherapy with systemic therapy. I think that the enthusiasm for that trial, if anything, has been increased based on the preliminary data from Sloan-Kettering and Dr. Kemeny’s group, demonstrating its efficacy based on 2-year follow-up.
Off protocol, our current recommendation is to treat patients with systemic therapy only. I think that the potential added morbidity of the regional chemotherapy catheter device as well as the added expense is not justified as standard therapy at this time.
Regarding Dr. Fong’s questions about our current selection of patients for surgical therapy, is generally based on our belief preoperatively that we can accomplish complete resection of the disease, achieve negative margins, and leave a viable and functional hepatic remnant. So we don’t use number of metastases in making that selection.
We have employed Dr. Fong’s five selection criteria to our group of patients. We only identified a single patient who had all five of the criteria that we had operated on. Everyone else had four criteria or less, and based on your data, I think that we have not identified any combination of four factors or less that would prohibit the use of surgery for these patients with metastatic colorectal carcinoma to the liver—that even with four of the five selection criteria, you still demonstrate about a 20% long-term survival, and that’s certainly better than the alternative.
Regarding the distribution of the primary colon and rectal tumor, I do not have that data available, so I can’t answer that question. And we have, I think, pretty significant experience now in placing hepatic artery catheters after or at the time of resection because of the North Central trial, and it has not inhibited or impacted our ability to perform liver resection.
Footnotes
Correspondence: John S. Bolton, MD, Ochsner Clinic, 1514 Jefferson Highway, New Orleans, LA 70121.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
E-mail: Jbolton@ochsner.org
Accepted for publication December 1999.
References
- 1.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19:59–71. [DOI] [PubMed] [Google Scholar]
- 3.Hughes K, Scheele J, Sugarbaker PH. Surgery for colorectal cancer metastatic to the liver. Optimizing the results of treatment. Surg Clin North Am 1989; 69:339–359. [DOI] [PubMed] [Google Scholar]
- 4.Harmon KE, Ryan JA Jr, Biehl TR, Lee FT. Benefits and safety of hepatic resection for colorectal metastases. Am J Surg 1999; 177:402–404. [DOI] [PubMed] [Google Scholar]
- 5.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery 1994; 116:703–710. [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes K, Rosenstein R, Songhorabodi S. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of long-term survivors. Dis Colon Rectum 1988; 31:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couinaud C. Bases anatomiques des hepatectomies gaucle et droite reglees. J Chirugie 1954; 70:933–966. [PubMed] [Google Scholar]
- 8.Colton T. Statistics in Medicine. Boston: Little, Brown and Co.; 1974.
- 9.Cox DR. Regression models and life tables (with discussion). J R Stat Soc B 1972;:187–220. [Google Scholar]
- 10.Cady B, Stone M, McDermott W. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg 1992; 127:561–569. [DOI] [PubMed] [Google Scholar]
- 11.Kemeny N, Cohen A, Huang W, et al. Randomized study of hepatic arterial infusion (HAI) and systemic chemotherapy (SYS) versus SYS alone as adjuvant therapy after resection of hepatic metastases from colorectal cancer. Proceedings of the 35th Annual Meeting of the American Society of Clinical Oncology, Atlanta, GA, May 15–18, 1999; 18:263.
- 12.Lorenz M, Muller HH, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann Surg 1998; 228:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bismuth H, Castaing D, Traynor O. Surgery for synchronous hepatic metastases of colorectal cancer. Scand J Gastroenterol 1988; 149(suppl):144–149. [DOI] [PubMed] [Google Scholar]
- 14.Adson M, Van Heerden J, Adson M. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984; 119:647–651. [DOI] [PubMed] [Google Scholar]
- 15.Vogt P, Raab R, Ringe B. Resection of synchronous liver metastases from colorectal cancer. World J Surg 1991; 15:62–67. [DOI] [PubMed] [Google Scholar]
- 16.Jaeck D, Bachellier P, Weber J. Strategie chirurgicale dans le traitement des metastases hepatiques synchrones des cancers colorectaux. Analyse d’une sere de 59 malades operes. Chirurgie 1999; 124:258–263. [DOI] [PubMed] [Google Scholar]
- 17.Belghiti J. Metastases hepatiques synchrones et resectables d’un cancer colo-rectal: y a-t-il un delai mimimum a respector avant de faire la resection hepatique? Ann Chir 1990; 44:427–432. [PubMed] [Google Scholar]
- 18.Jatzko G, Wette V, Muller M, et al. Simultaneous resection of colorectal carcinoma and synchronous liver metastases in a district hospital. Int J Colorectal Dis 1991; 6:111–114. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki M, Kohda S, Itoh H, et al. Inhibition of hepatic regeneration after 70% partial hepatectomy by simultaneous resection of the bowel in rats. Eur Surg Res 1995; 27:396–405. [DOI] [PubMed] [Google Scholar]