Abstract
Objective
To compare the effects of 35% hepatic cryoablation with a similar degree of radiofrequency ablation (RFA) on lung inflammation, nuclear factor κB (NF-κB) activation, and production of NF-κB dependent cytokines.
Summary Background Data
Multisystem injury, including acute lung injury, is a severe complication associated with hepatic cryoablation of 30% to 35% or more of liver parenchyma, but this complication has not been reported with RFA.
Methods
Sprague-Dawley rats underwent 35% hepatic cryoablation or RFA and were killed at 1, 2, and 6 hours. Liver and lung tissue were freeze-clamped for measurement of NF-κB activation, which was detected by electrophoretic mobility shift assay. Serum concentrations of tumor necrosis factor α and macrophage inflammatory protein 2 were measured by enzyme-linked immunosorbent assay. Histologic studies of pulmonary tissue and electron microscopy of ablated liver tissue were compared among treatment groups.
Results
Histologic lung sections after cryoablation showed multiple foci of perivenular inflammation, with activated lymphocytes, foamy macrophages, and neutrophils. In animals undergoing RFA, inflammatory foci were not present. NF-κB activation was detected at 1 hour in both liver and lung tissue samples of animals undergoing cryoablation but not after RFA, and serum cytokine levels were significantly elevated in cryoablation versus RFA animals. Electron microscopy of cryoablation-treated liver tissue demonstrated disruption of the hepatocyte plasma membrane with extension of intact hepatocyte organelles into the space of Disse; RFA-treated liver tissue demonstrated coagulative destruction of hepatocyte organelles within an intact plasma membrane. To determine the stimulus for systemic inflammation, rats treated with cryoablation had either immediate resection of the ablated segment or delayed resection after a 15-minute thawing interval. Immediate resection of the cryoablated liver tissue prevented NF-κB activation and lung injury; however, pulmonary inflammatory changes were present when as little as a 15-minute thaw interval preceded hepatic resection.
Conclusions
Hepatic cryoablation, but not RFA, induces NF-κB activation in the nonablated liver and lung and is associated with acute lung injury. Lung inflammation is associated with the thawing phase of cryoablation and may be related to soluble mediator(s) released from the cryoablated tissue. These findings correlate the clinical observation of an increased incidence of multisystem injury, including adult respiratory distress syndrome (ARDS), after cryoablation but not RFA.
Hepatic resection remains the standard treatment method for primary and metastatic liver tumors in properly selected patients. 1 However, newer surgical modalities, including cryoablation 2–6 and radiofrequency ablation (RFA), 7–9 have been introduced to treat patients with unresectable tumors. Cryoablation involves the circulation of liquid nitrogen through metallic probes placed into the center of the tumor or on the liver surface, inducing cell death by a variety of mechanisms, including internal freezing, solute–solvent shifts, and small vessel obliteration. RFA involves radiofrequency current passed through a 15-gauge needle and deployable electrode arrays placed in the tumor center, generating heat that leads to tumor cell destruction by means of a coagulative-type process.
Although cryoablation of small areas of the liver is usually well tolerated, significant side effects, including thrombocytopenia, disseminated intravascular coagulation, renal failure, hepatic failure, and ARDS, have been noted when 30% to 35% or more of the liver is treated 10–12; this has been described as the “cryoshock phenomenon.”13 Multisystem injury is a recognized complication of large-volume cryoablation, but the mechanisms of distant organ injury have not been defined. Interestingly, similar systemic complications have not been noted when RFA has been used for tumor ablation. 7,8 Because technology limitations of most available RFA systems allow ablation of liver tumor volumes of no more than 3 cm in maximum diameter, it has been unclear whether RFA is a safer method of ablation or whether the minimal reported side effects have been related to the small volume of treatment.
When multisystem injury and the systemic inflammatory response syndrome (SIRS) develop, there is usually overexpression of inflammatory mediators as part of the host response. Cytokines are an important inflammatory mediator in this process and are produced by a number of cells, predominately macrophages, in response to a variety of stimuli. These mediators are important in normal physiologic responses but also may result in pathologic states, including SIRS and ARDS, when they are excessive or dysregulated. Under most circumstances, cytokines are not stored in a preactivated form in macrophages (or other cells). Cytokine production requires active gene transcription of messenger RNA unique to each cytokine, a process closely regulated by the transcription factor complex nuclear factor κB (NF-κB) in the cell. 14 Recent studies suggest that NF-κB activation in alveolar macrophages in the lung occurs as an important early step in the development of ARDS. 15 Because Kupffer cells in the liver represent the largest site of fixed macrophages in the body, the liver is probably an important site for mediator release in response to direct liver injury and possibly in response to systemic injury.
In previous investigations of cryoablation-induced multisystem injury, we have demonstrated activation of NF-κB in liver and lung tissue with elevation of NF-κB dependent cytokines. 16 The current study was undertaken to compare the effects of an equivalent 35% hepatic ablation using either cryoablation or RFA on lung inflammation in a rat model and to determine possible factors responsible for induced lung injury.
METHODS
Surgical Procedures
Sprague-Dawley rats (weight 250–400 g) were anesthetized with intramuscular ketamine hydrochloride (87 mg/kg) and xylazine (13 mg/kg). Animals were placed on a small heated animal operating table, the abdomen was prepared with povidone–iodine using aseptic technique, and a midline laparotomy was performed. Aliquots of blood were aspirated directly from the inferior vena cava with a 25-gauge needle. Avascular attachments to the liver (falciform, gastrohepatic ligaments) were divided, and the liver was mobilized.
Cryoablation (n = 35) was performed with a small disc probe (Cryotech LC System 2000; Cryogenic Technology Ltd., Derbyshire, UK) with careful isolation of adjacent structures, including the gastrointestinal tract, to avoid inadvertent organ injury. RFA (n = 19) was performed by directing a needle guide into the liver (RITA Medical, Inc., Mountain View, CA), with advancement of the thermocouple guides into the parenchyma undergoing ablation. Ablative therapy using either cryoablation or RFA of the left lobe was performed to encompass approximately 35% of the total liver volume, with careful maintenance of normothermia during ablative therapy. After completion of ablation, 10 mL normal saline was instilled into the abdominal cavity, and the fascia and skin were closed in layers. After recovery, animals were killed at predetermined intervals (1, 2, and 6 hours). Serum and tissue samples were collected from the liver and lung and were freeze-clamped and stored at −80°C until subsequent analysis.
To assess the effect of the timing of the cryosurgery injury, animals underwent 35% cryoablation of the left lobe followed by either immediate hepatic resection (n = 18) with minimal or no liver thawing or delayed resection (n = 14) after a 15-minute thaw interval and were killed at 1, 2, and 6 hours. Aliquots of blood and tissue samples were collected as in animals undergoing cryoablation without resection.
Enzyme-Linked Immunosorbent Assay
Levels of tumor necrosis factor α (TNF-α) and macrophage inflammatory protein 2 (MIP-2) were determined in serum at baseline and at 1, 2, and 6 hours after cryoablation or RFA. Assays were performed with specific enzyme-linked immunosorbent assay kits (TNF-α, Genzyme Corp., Cambridge, MA; MIP-2, Biosource International, Camarillo, CA).
Nuclear Protein Extractions
Nuclear protein extracts were prepared from lung and liver tissue by the method of Deryckere and Gannon, 17 as previously outlined. 16 Aliquots of frozen tissue were mixed with liquid nitrogen and ground to powder using a mortar and pestle. Four milliliters of solution A (0.6% NP-40, 150 mmol/L NaCl, 10 mmol/L HEPES [pH 7.9], 1 mmol/L EDTA, 0.5 mmol/L PMSF) was added to the mortar. The contents of the mortar were placed in a Dounce tissue homogenizer, and the cells were lysed with five strokes of the pestle. After transfer to a 15-mL tube, debris was pelleted by centrifuging at 2,000 rpm for 30 seconds. The supernatant containing intact nuclei was transferred to 50-mL Corex tubes, incubated on ice for 5 minutes, and centrifuged for 10 minutes at 5,000 rpm. Nuclear pellets were then resuspended in 300 μL solution B (25% glycerol, 20 mmol/L HEPES [pH 7.9], 420 mmol/L NaCl, 1.2 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.5 mmol/L DTT, 0.5 mmol/L PMSF, 2 mmol/L benzamidine, 0.5 g/mL pepstatin, 0.5 g/mL leupeptin, 0.5 g/mL aprotinin) and incubated on ice for 30 minutes. The mixture was transferred to microcentrifuge tubes, and nuclei were pelleted by centrifugation at 14,000 rpm for 1 minute. Supernatants containing nuclear proteins were saved, aliquoted, and stored at −70°C. Protein quantification was performed using the method of Bradford. 18
Electrophoretic Mobility Shift Assays (EMSAs)
Gel shift assays were performed as previously described. 16 A consensus double-stranded NF-κB oligonucleotide (Stratagene, La Jolla, CA) (5′-GATCGAGGGGACTTTCCCTAGC-3′) was used for EMSA. End labeling was accomplished by treatment with T4 kinase in the presence of 32P-ATP. Labeled oligonucleotides were purified on a Sephadex G-25 mol/L column (Pharmacia Biotech, Inc., Piscataway, NJ). Five grams nuclear protein was added to a binding reaction mixture containing 10 mmol/L Tris HCl (pH 7.5), 100 mmol/L NaCl, 1 mmol/L EDTA, 0.2% Nonidet P-40, and 0.5 mmol/L DTT and a nonspecific blocker, salmon testis DNA (0.1 g/L) and was incubated on ice for 15 minutes. Next, approximately 150,000 cpm labeled double-stranded oligonucleotide was added to each sample. This mixture was incubated at 25°C for 20 minutes and separated by electrophoresis on a 6% polyacrylamide gel (PAGE) in 1 × TGE buffer. Gels were vacuum-dried and subjected to autoradiography. Cold competition was performed by adding 50 ng specific, unlabeled, double-stranded probe to the reaction mixture. Nonspecific competition was done by adding 50 ng unlabeled double-stranded oligonucleotide that does not bind NF-κB. Supershift assays were done with polyclonal antibodies to the NF-κB proteins (RelA; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). These antibodies were added to the above reaction mixtures at a concentration of 1 μg/15 μL. The samples were then incubated at 25°C for 1 hour before gel loading.
Histology and Electron Microscopy Studies
Lung and liver tissue samples obtained when the animal was killed were immediately sectioned and placed in formalin for subsequent light microscopy with conventional hematoxylin and eosin staining. Slide review was performed by a masked pathologist for the following features: margination of leukocytes in pulmonary vasculature, airspace edema, perivascular lymphoid cuffs, airspace edema and hemorrhage, and the presence of macrophages with foamy cytoplasm. In selected cases, tissue samples were placed in glutaraldehyde or for electron microscopy assessment. In these studies, samples were fixed for 48 hours in glutaraldehyde, then washed in phosphate-buffered 7.5% sucrose and postfixed in phosphate-buffered 2% osmium tetroxide for 1 hour. The samples were then stained with 1% uranyl acetate, dehydrated in a graded series of alcohols, cleared in propylene oxide, and embedded in an araldite resin. Thick sections (1.5 μm) stained with toluidine blue were examined by light microscopy, and appropriate areas were chosen for thin (<1-μm) sections.
Statistical Analysis
Data are expressed as mean ± standard error. Comparisons were made between baseline values and postablation results (cryoablation vs. radiofrequency parameters) using unpaired t tests. We assumed significance at P < .05.
RESULTS
Lung Inflammation
Lung sections from animals undergoing hepatic cryoablation demonstrated prominent patchy perivenular inflammation, with discrete lesions measuring up to 2 mm. They were most prominent 2 hours after cryoablation (Fig. 1A) and were present in all assessed animals undergoing cryoablation at 2 hours. The smallest perivenular lesions consisted of a cuff of small lymphocytes admixed with larger, activated-appearing lymphocytes surrounding a small to medium pulmonary vein. In larger lesions, the inflammatory infiltrate expanded to involve the interstitium of the adjacent pulmonary parenchyma. The inflammatory infiltrate in these larger lesions was predominantly mononuclear but also contained neutrophils. Focal spillover of inflammatory cells into alveolar spaces occurred in the animals killed at 1 hour, although the inflammatory process was primarily interstitial. Clusters of foamy macrophages were present in the larger lesions.
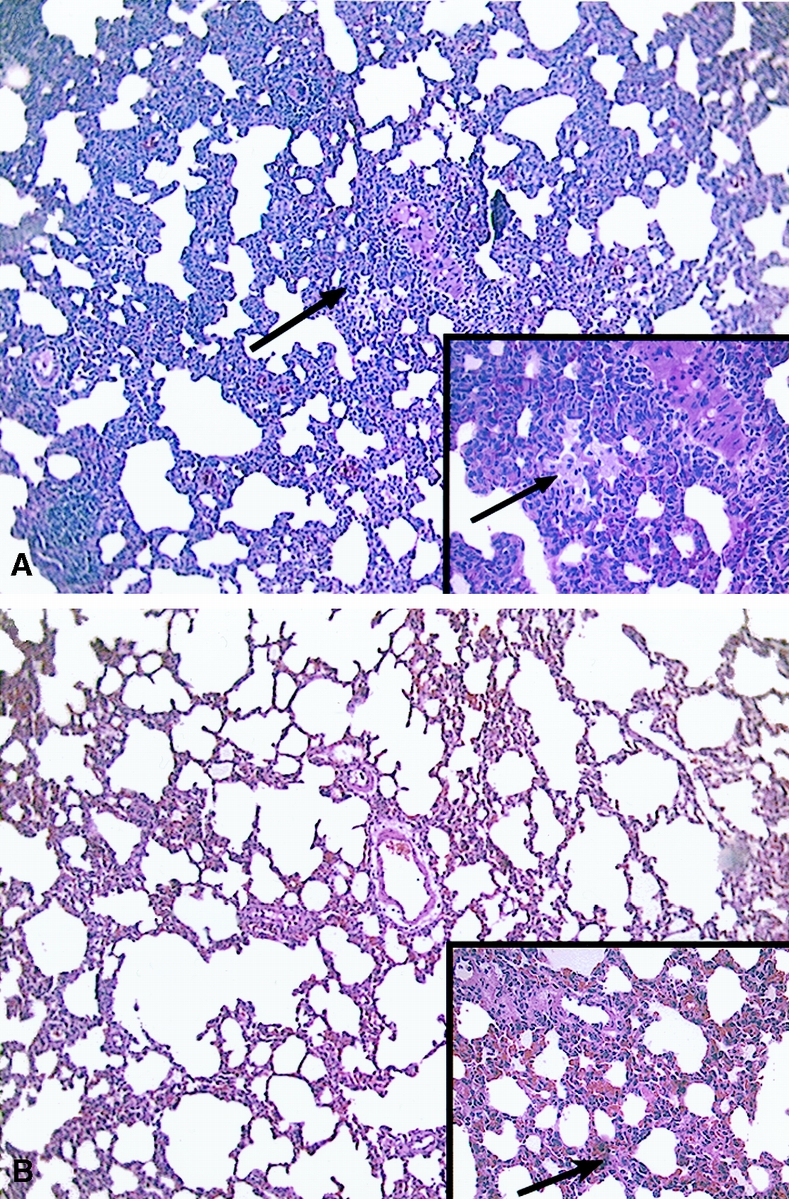
Figure 1. Photomicrographs of lung sections 2 hours after 35% hepatic cryoablation (A) or 35% radiofrequency ablation (B). In animals undergoing cryoablation treatment, there was a characteristic pattern of marked perivenular infiltration of activated-appearing lymphocytes with admixed neutrophils and clusters of foamy macrophages (arrows). These changes were not present in lung sections from animals undergoing radiofrequency ablation, where there were no changes of lung inflammation and only occasional patchy zones of interstitial edema and vascular congestion (arrow). (Hematoxylin and eosin; magnification ×100, inset ×400)
In the animals that underwent RFA, there were no significant changes in pulmonary histology in the majority of assessed animals, with occasional areas of mild patchy perivascular and airspace and patchy vascular congestion edema (Fig. 1B). No perivascular lymphocytic infiltrate or evidence of pulmonary inflammatory changes was seen in any animals.
Serum Levels of TNF-α and MIP-2
Serum TNF-α and MIP- 2 concentrations were measured by enzyme-linked immunosorbent assay in serum after cryosurgery and RFA (Figs. 2 and 3). MIP-2 is a major C-X-C chemokine in the rat (analogous to human interleukin-8) and functions to recruit polymorphonuclear leukocytes to sites of inflammation. It may be important in rats for directing the polymorphonuclear leukocyte alveolitis that is characteristic of acute lung injury. Although the levels of both cytokines were normal in untreated animals, the mean TNF-α concentration increased to 289 ± 56 pg/mL by 1 hour after cryosurgery and remained elevated for 6 hours. In contrast, only minimal elevation of TNF-α (18 ± 18 pg/mL, P < .01 vs. cryoablation at 1 hour) was detected in the serum of rats that underwent RFA ablation.
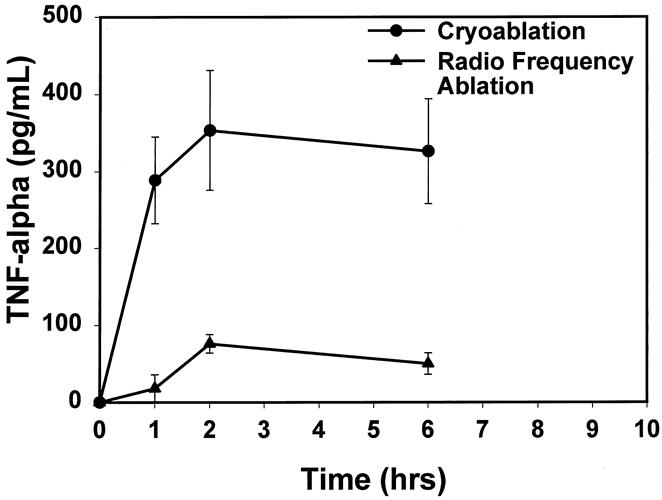
Figure 2. There was a significant increase in levels of tumor necrosis factor α starting 1 hour after cryoablation that remained elevated until 6 hours after the ablative procedure. No significant changes were noted in tumor necrosis factor α levels of animals undergoing radiofrequency ablation (P ≤ .01, cryoablation vs. radiofrequency ablation).
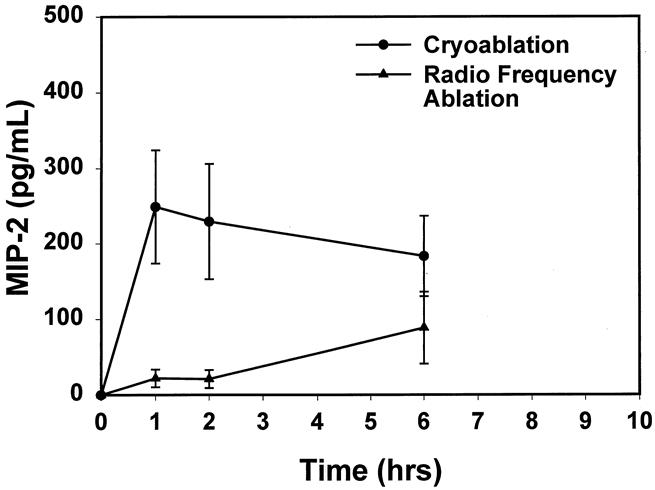
Figure 3. There was a prompt increase in the chemokine MIP-2 level after cryoablation, with no significant elevations found in animals undergoing radiofrequency ablation (P < .05, cryoablation vs. radiofrequency ablation, at 1 and 2 hours).
A similar pattern was observed for MIP-2. Mean MIP-2 levels increased to 249.0 ± 75 pg/mL by 1 hour after cryosurgery and remained elevated for 6 hours, whereas minimal elevation of MIP-2 (22 ± 12 pg mL, P < .05 vs. cryoablation at 1 hour) levels occurred with RFA treatment.
Rupture of Hepatocyte Plasma Membrane and Release of Cellular Contents
Electron microscopy of liver sections taken 15 minutes after cryoablation or RFA demonstrated markedly different effects on cellular architecture (Fig. 4). After cryoablation, the overall hepatic architecture at the ultrastructural level was maintained, with the normal arrangement of hepatocytes along the space of Disse. Cytoplasmic organelles were intact, with normal nuclei, endoplasmic reticulum, and mitochondria. Cryoablation, however, resulted in disruption of the hepatocyte plasma membrane, with large breaks such that hepatocyte organelles appeared to extend into the space of Disse with no clear zone of demarcation.
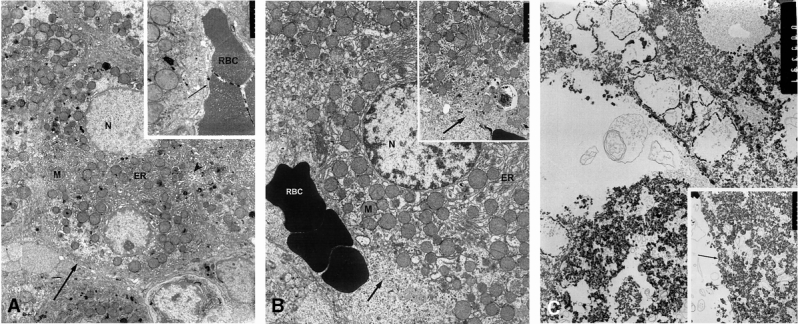
Figure 4. Electron micrographs from normal (A), cryoablated (B), and radiofrequency-ablated (C) liver tissue 15 minutes after ablative treatment. (A) The untreated liver contained hepatocytes with normal nuclei (N) and the usual complement of cytoplasmic organelles, with abundant endoplasmic reticulum (ER), mitochondria (M), an intact plasma membrane (arrows), and normal bile canaliculus (arrowhead) (RBC, erythroctyes in sinusoid). After cryoablation (B), the overall hepatic architecture at the ultrastructural level was maintained, with the normal arrangement of hepatocytes along the space of Disse (arrow). Cytoplasmic organelles were intact, with normal nuclei, endoplasmic reticulum, and mitochondria, and red blood cells (RBC) were seen in the region of the space of Disse. However, cryoablation resulted in extensive disruption of the hepatocyte plasma membrane, with large breaks such that hepatocyte organelles appeared to extend into the region of the space of Disse (arrow); however, there was not a well-defined hepatocyte plasma membrane at this location. In contrast, hepatocytes from radiofrequency-ablated liver tissue (C) had totally unrecognizable intracytoplasmic organelles, and the cytoplasm was filled with amorphous electron-dense material. The hepatocyte plasma membranes and space of Disse (arrow) appeared intact for the most part; there were very focal areas that had some amorphous material in the space of Disse, adjacent to either intact plasma membranes or very focal breaks in the plasma membrane. (Magnification: A, ×8,320, inset ×41,600; B, ×8,320, inset ×17,810; C, ×10,140, inset ×17,810)
In contrast, hepatocytes from RFA-treated liver tissue had totally unrecognizable intracytoplasmic organelles, and the cytoplasm was filled with amorphous electron-dense material. The hepatocyte plasma membranes and space of Disse appeared intact for the most part. There were focal areas in which this amorphous material was present in the space of Disse immediately adjacent to hepatocytes, but the plasma membrane was either intact or had very focal areas of possible disruption.
These findings suggested that in the case of cryoablation, plasma membrane disruption with otherwise intact cellular structures occurred. In contrast, RFA induced a coagulative-type denaturing of intracytoplasmic organelles and proteins with much less disruption of the cell surface, and possibly “resealing” of the hepatocyte plasma membrane.
NF-κB Activation in Liver and Lung
Because of the noted increases in TNF-α and MIP-2 in serum from animals treated with cryoablation but not RFA, we performed EMSAs to determine whether the increased cytokine levels correlated with NF-κB activation, because cytokine production is known to be closely regulated by the transcription factor NF-κB. Figure 5 shows EMSA gels from nuclear extracts of liver and lung samples taken at 1 hour after cryoablation and RFA. There was prominent activation of NF-κB at 1 hour in both liver and lung tissue from animals undergoing cryoablation, but not from RFA-treated animals. The specificity of detected binding on EMSA is shown by cold and nonspecific competition. The addition of 50 ng specific NF-κB oligonucleotide markedly diminished the NF-κB bands (cold competition = C), whereas the addition of 50 ng unlabeled oligonucleotide that did not contain the NF-κB motif (nonspecific competition = NS) did not affect NF-κB binding. These findings authenticate the identification of the EMSA bands identified as NF-κB.
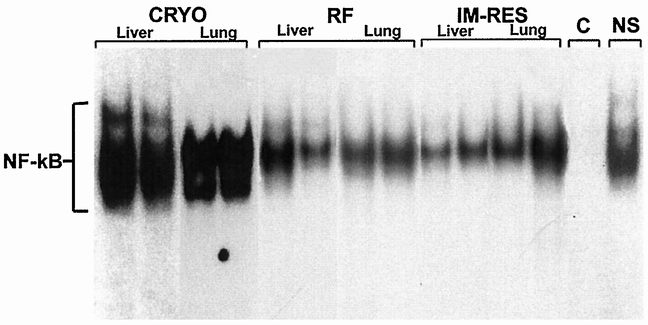
Figure 5. Composite electrophoretic mobility shift assay gel performed on tissue taken from the nonablated liver and lung from representative animals undergoing cryoablation (cryo) and radiofrequency ablation (RFA) killed 1 hour after treatment. Each lane represents a single animal experiment. These results demonstrate activated nuclear factor κB (NF-κB) in liver and lung tissue extracts from animals undergoing cryoablation but not from animals undergoing RFA. Also, immediate resection (IM-res) of the cryoablated liver before thawing prevented activation of NF-κB in liver and lung tissue. Addition of 50 ng unlabeled, specific NF-κB oligonucleotide markedly diminished all three bands (cold competition = C). The addition of 50 ng unlabeled oligonucleotide that did not contain the NF-κB motif (nonspecific competition = NS) did not affect NF-κB binding. These results confirm the authenticity of detected protein bands in NF-κB analysis.
Resection of Cryoablated Liver Before Thawing
In animals undergoing immediate resection of the cryoablated segment of liver, there were no areas of perivascular inflammatory infiltrates and there was only minimal evidence of perivascular edema; this was indistinguishable from animals undergoing RFA ablation (Fig. 6A). However, in animals in which a 15-minute complete thaw interval preceded resection of the ablated segment, a similar but less intense perivascular inflammatory infiltrate was present in the lung histology sections 1 and 2 hours after the initial cryoablation injury, in a pattern similar to animals undergoing cryoablation but without resection (Fig. 6B). In animals undergoing cryoablation followed by immediate resection, there was no activation of NF-κB in liver or lung tissue samples (Fig. 5).
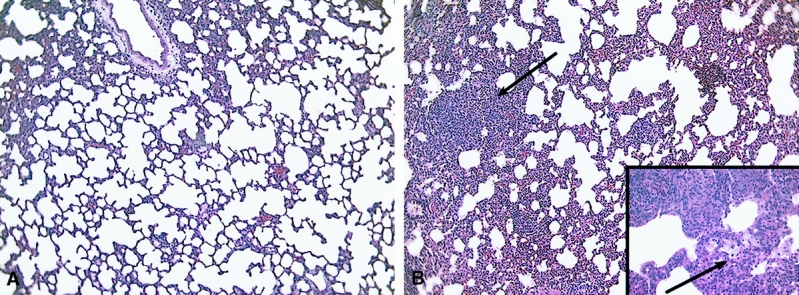
Figure 6. Photomicrographs of lung sections 1 hour after 35% hepatic cryoablation with immediate resection of the ablated segment (A) or after a 15-minute thawing interval before resection of the cryoablated segment (B). Immediate resection prevented the inflammatory infiltrate (A), whereas delayed resection (B) was associated with infiltration of lymphocytes, neutrophils, and macrophages (arrows) as in cryoablated but nonresected animals. (Magnification ×100, inset ×400)
DISCUSSION
Our results demonstrate that in a rodent model, 35% hepatic cryoablation results in acute lung inflammation that does not occur in association with radiofrequency-induced ablation of a similar volume of hepatic parenchyma. These changes are associated with NF-κB activation in the nonablated liver and lung and increased production of the NF-κB dependent cytokines TNF-α and MIP-2. Electron microscopy studies of the ablated liver tissue demonstrate a markedly different injury pattern at the ultrastructural level between the two ablation techniques. Cryoablation results in plasma membrane disruption and dispersion of intact cellular structures into the space of Disse that could reach the systemic circulation. In contrast, RFA appears to induce a coagulative destruction of intracytoplasmic organelles while maintaining the integrity of the cell surface. The cryoablation-induced lung inflammatory response appears to be associated with the thawing phase of cryoablation, because immediate resection of the ablated liver parenchyma before the thawing period prevented NF-κB activation and acute inflammatory changes in the lung.
Although hepatic resection remains the standard therapy for primary and metastatic tumors in the liver, there is increasing interest in nonresectional tumor ablative therapy. Hepatic tumors represent a major healthcare problem in the United States, with approximately 14,000 new cases of primary hepatic malignancies 19 and 45,000 new cases of metastatic colorectal cancer to the liver 20 each year. There is little doubt that surgical resection in carefully selected patients improves survival for those with primary liver cancer and hepatic colorectal metastases. Survival of patients with metastatic colorectal cancer confined to the liver who do not undergo treatment is a median of 3 to 24 months, with 5-year survival rates reported at 0% to 2%. 21 Alternative treatment with chemotherapy alone also provides poor results, with 10% to 30% response rates usually lasting less than 6 months, and 5-year overall survival rates of less than 5%. 1 However, complete tumor resection in patients with three or fewer metastatic foci results in a 30% to 35% 5-year survival rate, compared with less than 5% for patients not undergoing resection, 22 in nonrandomized studies. Hepatic tumor ablation with cryoablation and RFA has been applied to patients with unresectable tumors with results that appear to be similar to those of conventional surgical resection, 5,6,12 although most series still have relatively short follow-up and small numbers of patients, and there have been no controlled trials comparing these techniques.
At present, there are overlapping indications for cryoablation and RFA, and it remains unclear whether one of these techniques will emerge as superior. There are several differences in the technical aspects of these modalities that influence selection of the ablation method. Cryoablation has been available clinically for a longer time than RFA. This technique can generate large freeze zones (up to 10 cm in diameter with a single 10-mm probe), with a sharp ultrasonic demarcation between frozen and nonablated tissue. However, most cryoablation systems require placement of relatively large probes (3–10 mm), making laparoscopic use impractical and precluding percutaneous applications. Systemic side effects of cryoablation have been recognized and commonly include thrombocytopenia, myoglobinuria, and renal insufficiency. 10–12 Thrombocytopenia occurs in almost all patients undergoing hepatic cryoablation, but the incidence of ARDS increases when the volume of ablated liver approaches or exceeds 30% to 35%. ARDS is uncommon when the volume of ablated hepatic parenchyma is less than 30% to 35%. 10,13
In contrast to cryoablation, RFA involves the passage of an electrical current through a 15-gauge needle guide passed into the tumor, causing ionic charge shifts that generate heat. The heat is dispersed in a spherical shape around the needle tip and extended needle electrode array. Because the current can be delivered through a needle delivery system, this technique lends itself to laparoscopic use; it can also be used percutaneously under selected circumstances. Unlike cryoablation, tissue ablated with RFA becomes diffusely hyperechoic under ultrasound monitoring, but RFA does not generate a clearly demarcated ablation zone, so real-time monitoring is less precise than with cryoablation. Instead, thermocouples are placed at the ends of the extended electrode array to monitor surrounding temperatures. Thus, RFA is performed as a timed treatment, either at a specified target temperature 7,9 or a specified power setting. 8 Also unlike cryoablation, most radiofrequency systems at present can generate ablation zones of no more than 3 cm because of technology limitations. For this reason, tumors 3 cm or larger usually require multiple overlapping fields of ablation, which can make it difficult to ensure complete tumor ablation.
In addition, system availability and surgeon preference continue to play significant roles in technology selection. Many hepatobiliary centers now have both cryoablation and RFA available for use, depending on the clinical circumstances.
There is increasing evidence that the liver plays an important role in many systemic inflammatory disease states. SIRS can result from infectious or noninfectious causes and commonly includes the development of acute lung injury, defined clinically as ARDS. Cytokines, including TNF-α, interleukin-1, and other proinflammatory mediators, are known to be produced by Kupffer cells in the liver in response to a variety of stimuli. Previous studies have demonstrated that infusion of TNF-α produces changes in pulmonary endothelial cells and can produce changes similar to ARDS. 23,24 Ferrari-Baliviera et al 24 have demonstrated that infusion of TNF-α can produce interstitial and alveolar edema, hemorrhage, and leukocyte sequestration in the lung. We have shown similar histology findings that support a link between hepatic damage from cryoablation and acute pulmonary damage. Interestingly, a recent report by Seifert et al 25 confirmed the release of TNF-α and interleukin-6 after hepatic cryoablation in their rat model.
Although 35% hepatic cryoablation in our study was associated with NF-κB activation in the liver and lung and histologic evidence of neutrophilic lung injury, a similar ablative injury using RFA did not cause similar pulmonary inflammatory changes. These findings are consistent with clinical reports of RFA in which procedure-related complication rates have been low. In the largest report to date, 8 RFA was used in 123 patients, with no deaths and a complication rate of 2.4%. This is in striking contrast to the rate of cryoablation-associated complications in previous reports, which have ranged from 15% to 50%; common complications are pleural effusion, renal insufficiency, and coagulopathy. 10–12
Although the exact mechanism that results in NF-κB activation and associated cytokinemia and lung injury after cryoablation is unknown, we noted a significant difference in the ultrastructural changes associated with cryoablation versus RFA (Fig. 4). Cryoablation appears to cause large breaks in the plasma membrane, and intact intracytoplasmic organelles, including mitochondria, endoplasmic reticulum, and other structures, are dispersed into the space of Disse. RFA, in contrast, appeared to destroy intracellular structures completely (possibly with denaturing of cellular protein), left an intact cell membrane, and was not associated with NF-κB activation or upregulation of NF-κB dependent cytokines. This result suggests that passive release of an intracellular substance initiates the NF-κB activation cascade. In our study, this difference in ablative method led to marked parenchymal lung injury at 24 hours in rats undergoing hepatic cryoablation compared with minimal changes in animals undergoing RFA under similar treatment conditions (Fig. 7).
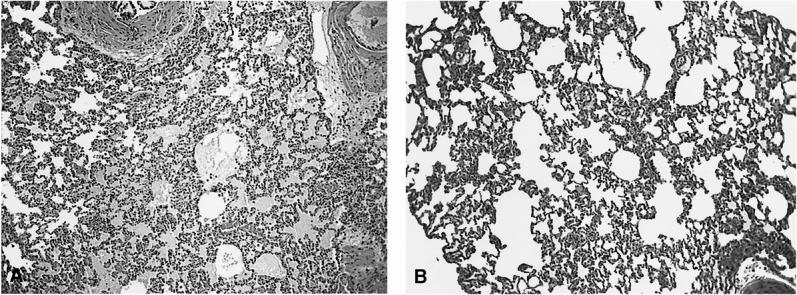
Figure 7. Lung sections taken at 24 hours from animals undergoing 35% cryoablation (A) demonstrated severe lung injury with parenchymal hemorrhage and alveolar and interstitial edema. In contrast, animals undergoing 35% radiofrequency ablation (B) had mild pulmonary edema with no evidence of acute lung injury. (Magnification ×100)
The findings of cryoablation-induced activation of NF-κB dependent cytokines suggests strategies for intervention to minimize the multisystem injury observed with this modality. We have performed preliminary experiments with TNF-α antibody blockade and other techniques that appear to ameliorate the deleterious effects of cryoablation treatment. 26 Our current research involves the use of genetically altered mice to identify specific pathways that result in SIRS. We believe that prevention of the systemic effects of cryoablation may be possible through NF-κB inhibition, specific cytokine blockade, and directed therapy against any identified mediators of injury.
Discussion
Dr. R. Scott Jones (Charlottesville, Virginia): My question is, first, because clinically there were patients that underwent cryo who also had some renal failure and problems with the kidney, have you yet or are you going to investigate the effects of hepatic cryo on renal function? That might be an opportunity, if you have not already done it, for some additional insights into the ill effects of this cryotherapy.
My second question, and probably the most important and bottom-line question: are you continuing to do cryotherapy on your service for these hepatic lesions, since there is a presumably equally effective and less dangerous technique available? Are you still doing cryotherapy? Do you continue to do cryotherapy, and what role will cryotherapy play in the future in the treatment of hepatic tumors?
Dr. J. Michael Henderson (Cleveland, Ohio): This is a very nice experimental study that pulls together some of our clinical biases and supports the use of RFA versus cryo, which as you showed has significant systemic effects compared with radiofrequency ablation.
You very nicely go through that whole sequence of bringing together the histologic differences between the two techniques through the systemic cytokine release to the mechanisms of those releases right at the beginning, and I compliment you for a first-class experimental design that takes those steps.
At the clinical level, the lung injury is one of the major issues, as you demonstrated with the X-rays of your patient being cryoablated. I would iterate again Dr. Jones’ question of the other systemic effects. The renal injury—I can’t believe you didn’t keep some of the kidneys to look at them as well. Do you have any data, either in this or any of the other studies you have done, that does look at the renal injury that may occur.
Equally, another clinically important injury is coagulopathy. Did you have the opportunity to measure fibrinogen or platelets in the model and document whether or not there were any benefits showing there is no coagulopathy with RFA?
Dr. Michael J. Edwards (Louisville, Kentucky): We, too, observed this phenomena that RFA patients were ready to go home earlier than the cryoablation patients. And one day at the VA Hospital, I turned to Neal Garrison and said, “This is what we are seeing.” And I said, “Do you have any idea why that might be true?”
So my question is why? Why do you think this is so? Neal’s answer was intriguing. He said, “Mike, you went to the same medical school as me. Don’t you know that when you want to denature protein, you heat it, and when you want to preserve it, you freeze it?” And he said, “I think it’s just that simple, that you are preserving the cytokines when you freeze them and you are denaturing them when you are heating them.” And I ask you, is that a valid hypothesis? Because if it is, it is intriguingly simple.
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): Since the response you observed resembles that to endotoxin, and since hepatic tissue contains a variable amount of endotoxin, are the changes that you have observed merely a response to endotoxin? In that same vein, have you been able to modify those changes by pretreatment with polymyxin or an antiendotoxin antibody?
Dr. George M. Watkins (Tampa, Florida): You know, the cryoablation people, patients, look like they are snakebit. Mr. President, you live in a den of vipers every day, and we mean that literally. These are rattlesnake bites—same sort of thing. Severe rattlesnake bite, you treat with steroids, all those symptoms tend to go away and the person gets well.
Now maybe people have done that, I don’t know. I think that may be in the literature; I’d like to ask about that. But more importantly, preoperative steroids have gotten a bad mark because they are such an invasive technique when we used to use them in the patients in ICU. These people are minimally intubated most of the time and have one single thing done. Has anyone, experimentally or clinically, used steroids, either preop or postop in patients or rats?
The second thing, the phenomena you see may be bloodborne or may be borne by the lymphatic system via the peritoneal circulation. Has anyone studied that by cross-circulation experiments or taking blood from one animal and putting it into another animal from the superhepatic system?
Dr. William C. Chapman (Closing Discussion): A number of questions have been raised regarding the role of cryotherapy versus RFA. The major question is whether or not the multisystem injury associated with cryoablation eliminates its use clinically. With the advent and introduction of RFA, which does appear clinically and, certainly, in our research models, to be a safer modality, will we effectively stop using cryoablation?
I don’t know the answer to this question. There are significant technical differences between cryoablation and RFA ablation that make these techniques not interchangeable at the present time. Cryoablation generates a freeze zone—a freeze-ball, if you will—that is very clear and easily seen ultrasonographically. This technique can allow the generation of freeze-balls of significant size, so that tumors of a large size can be cryoablated. On the other hand, radiofrequency ablation is limited now to only about a 3-cm ablation zone because of technology limitations. Additionally, the imaging that occurs, imaging changes in the heated tissue, is much more difficult to follow, so that most centers use a timed treatment after placing the RFA probe in the center of the tumor, so that the confidence level surrounding the ability to adequately treat areas with RFA is much less secure.
Several weeks ago, Dr. Anton Bilczek and his group from the John Wayne Cancer Institute presented their results using RFA and cryo for liver metastatic tumors greater than 3 cm in size, and reported a local recurrence rate of 41% in the patient group treated with RFA. So I think radiofrequency ablation from a technology standpoint at the present time is limited. Will that change in the future and will the technology allow treatment of larger areas of tumor? I think if that occurs, then RFA may be able to be substituted for many areas of cryoablation.
There were a number of questions regarding other sites of multisystem injury, and other effects that we may have seen in our animal model. In our work thus far, we have concentrated primarily on the effects on liver and lung, although we have begun to investigate studies using genetically altered mice. We have started studies with TNF-α and IL-1 knockouts to look at the effect of specific cytokines in this process. We have also done some preliminary studies in an HLL NF-κB luciferase promoter line of mice in which NF-κB acts as a surrogate marker that is detected with a luciferase enzyme. In these studies, we have seen significant evidence of activation in the liver, lung, spleen, and kidneys in this mouse model with cryoablation within 1 hour after the cryo procedure. So I think there is some evidence that similar effects to the lung injury are occurring at multiple systemic sites following cryoablation.
Dr. Henderson, you raised questions about the effects on coagulation cascade. We have looked at these effects with cryo and noted, in our model, similar effects on platelet count reduction, with thrombocytopenia and a marked decrease in fibrinogen levels that have not occurred with hepatic resection. We have not investigated the effect of those clotting parameters with RFA in this model.
Dr. Edwards raised a question about the potential mechanism of injury associated with cryoablation. The points you made regarding the possible release of intact protein from this cryoablated site to the lung and distant sites are intriguing. This is certainly a possible cause of this effect. Whereas it appears that all intracellular structures are denatured with RFA treatment, exactly which specific cellular components are responsible for this multisystem effect, I think, still remains in question.
Cytokines are generally felt not to be prestored in cells, so that cytokine increases in serum, particularly TNF and MIP-2 in our studies, are generally thought to require gene transcription and actual production of these cytokines. So I don’t think that it’s actual release of cytokines during this process, but exactly which component from these injured cells is responsible for the induction of this inflammatory response, I don’t think we know. But certainly this is something we are pursuing at the moment.
Dr. Pruitt raised questions regarding the possible role of endotoxin. We have not utilized endotoxin antibody in these particular studies. I would point out that the responses in terms of NF-κB activation in the liver and lung and increases in cytokines are very similar in this model to endotoxin models of systemic inflammation. So certainly the systemic inflammatory responses appear to be similar, but whether or not there is an actual endotoxin role, we can’t say. I would point out that certainly in most settings, the multisystem injury clinically associated with cryoablation is not thought to be due to sepsis. These patients do not have positive blood cultures or evidence of bacterially mediated injury.
Dr. Watkins raised questions about the possible role of steroids, either clinically or experimentally to lessen the effects of this systemic inflammatory response. It was interesting to me that several years ago when we were using cryoablation in a more aggressive way in our early experiences, one of our anesthesiologists did begin, on an anecdotal basis, to pretreat our patients with steroids by a bolus dose prior to treatment. There is certainly experimental evidence that this may have a beneficial effect. Steroids do inhibit activation of NF-κB, so this may provide a strategy to lessen the multisystem injury. We have not looked into this experimentally.
What impact has this work had on our clinical use of cryoablation? I would say at the outset that we use ablative techniques in a very limited way, in probably less than 10% of our patients. We have begun use of RFA. We continue to use cryoablation for the close or focally positive surgical margin after a major resection. This is one area where cryoablation is beneficial and RFA cannot be utilized for that purpose.
The ultimate use of one strategy (RFA vs. cryo) or the other is still a process that is evolving. We still use cryoablation in a limited way, although I think in the future radiofrequency ablation will play an increasingly important role.
Footnotes
Correspondence: William C. Chapman, MD, Dept. of Hepatobiliary Surgery and Liver Transplantation, 801 Oxford House, Nashville, TN 37232-4753.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Supported in part by the U.S. Department of Veterans Affairs through Merit Funding.
E-mail: will.chapman@surgery.mc.vanderbilt.edu
Accepted for publication December 1999.
References
- 1.Fong Y, Blumgart LH. Surgical options in the treatment of hepatic metastases from colorectal cancer. Curr Prob Surg 1995; 35:336–413. [DOI] [PubMed] [Google Scholar]
- 2.Shafir M, Shapiro R, Sung M, et al. Cryoablation of unresectable malignant liver tumors. Am J Surg 1996; 171:27–31. [DOI] [PubMed] [Google Scholar]
- 3.Seifert JK, Morris DL. Prognostic factors after cryotherapy for hepatic metastases from colorectal cancer. Ann Surg 1998; 228:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Majno P, Castaing D, Giovenardi R, Bismuth H. Treatment of irresectable liver tumors by percutaneous cryosurgery. Br J Surg 1998; 85:1493–1494. [DOI] [PubMed] [Google Scholar]
- 5.Zhou X, Tang Z. Cryotherapy for primary liver cancer. Semin Surg Oncol 1998; 14:171–174. [DOI] [PubMed] [Google Scholar]
- 6.Weaver ML, Ashton JG, Zemel R. Treatment of colorectal liver metastases by cryotherapy. Semin Surg Oncol 1998; 14:163–170. [DOI] [PubMed] [Google Scholar]
- 7.Siperstein AE, Rogers SJ, Hansen PD, Gitomirsky A. Laparoscopic thermal ablation of hepatic neuroendocrine tumor metastases. Surgery 1997; 122:1147–1155. [DOI] [PubMed] [Google Scholar]
- 8.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 1999; 230:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose DM, Allegra DP, Bostick PJ, et al. Radiofrequency ablation: a novel primary and adjunctive ablative technique for hepatic malignancies. Am Surg 1999; 65:1009–1014. [PubMed] [Google Scholar]
- 10.Sarantou T, Bilchik A, Ramming KP. Complications in hepatic cryosurgery. Semin Surg Oncol 1998; 14:156–162. [DOI] [PubMed] [Google Scholar]
- 11.Stewart GJ, Preketes A, Horton M, Ross WB, Morris DL. Hepatic cryotherapy: double-freeze cycles achieve greater hepatocellular injury in man. Cryobiology 1995; 32:215–219. [DOI] [PubMed] [Google Scholar]
- 12.Haddad FF, Chapman WC, Blair TK, Wright JK, Pinson CW. Clinical experience with cryosurgery for advanced hepatobiliary tumors. J Surg Res 1998; 75:103–108. [DOI] [PubMed] [Google Scholar]
- 13.Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg 1999; 23:109–114. [DOI] [PubMed] [Google Scholar]
- 14.Barnes PJ, Karin M. Mechanisms of disease: nuclear factor-(kappa)B—A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997; 336:1066–1071. [DOI] [PubMed] [Google Scholar]
- 15.Bohrer H, Qiu F, Zimmerman T, et al. Role of NF-κB in the mortality of sepsis. J Clin Invest 1997; 100:972–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackwell TS, Debelak JP, Venkatakrishnan A, et al. Acute lung injury after hepatic cryoablation: correlation with NF-κB activation and cytokine production. Surgery 1999; 126:518–526. [PubMed] [Google Scholar]
- 17.Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques 1994; 16:405. [PubMed] [Google Scholar]
- 18.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248–254. [DOI] [PubMed] [Google Scholar]
- 19.American Cancer Society. Cancer Facts & Figures. 1998.
- 20.Fong Y, Blumgart LH. Surgical options in the treatment of hepatic metastases from colorectal cancer. Curr Prob Surg 1995; 35:336–413. [DOI] [PubMed] [Google Scholar]
- 21.Stone MD, Cady B, Jenkins RL. Surgical therapy for recurrent liver metastases from colorectal cancer. Arch Surg 1990; 125:718–722. [DOI] [PubMed] [Google Scholar]
- 22.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery 1986; 100:278–284. [PubMed] [Google Scholar]
- 23.Goldblum SE, Henning B, Jay M, Yoneda K, McClain CJ. Tumor necrosis factor-α induced pulmonary vascular endothelial injury. Infect Immun 1989; 57:1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrari-Baliviera E, Mealy K, Smith RJ, Wilmore DW. Tumor necrosis factor induces adult respiratory distress syndrome in rats. Arch Surg 1989; 124:1400–1405. [DOI] [PubMed] [Google Scholar]
- 25.Seifert, JK, Finlay I, Armstrong N, Morris DL. Thrombocytopenia and cytokine release following hepatic cryosurgery [abstract]. Aust NZ J Surg 1998; 68:526. [Google Scholar]
- 26.Debelak JP, Blackwell TS, Benkatakrishnan A, et al. Attenuation of NF-κB dependent cytokine activation following hepatic cryoablation. Hepatology 1998; 28:254A. [Google Scholar]


