Abstract
Objective
This experiment evaluated enterally administered low molecular weight heparin (LMWH) combined with sodium N-[10-(2-hydroxybenzoyl)amino] decanoate (SNAD) for the treatment of induced venous thrombosis.
Summary Background Data
SNAD is a delivery agent that potentiates the gastrointestinal absorption of LMWH.
Methods
Forty female pigs were equally assigned to four groups: control (saline); enteral LMWH, 2,000 IU/kg; enteral SNAD, 50 mg/kg; and enteral LMWH, 2,000 IU/kg and SNAD, 50 mg/kg. Under fluoroscopic guidance, the infrarenal vena cava was occluded with a balloon catheter. Two milliliters of ethanol was injected into the distal vena cava. The inflated balloon catheter remained in situ for 5 days, at which time animals angiographically exhibiting thrombus were randomly assigned to the four groups. Study medications were dosed at 12-hour intervals by means of a gastrostomy tube placed previously. After 7 days of treatment, thrombus was extracted. A separate group of 10 animals was used to measure plasma antifactor Xa levels for 6 hours after enteral dosing of LMWH/SNAD.
Results
The amount of residual thrombus after treatment with enteral LMWH/SNAD was significantly decreased. Antifactor Xa levels were significantly elevated in the LMWH/SNAD group versus baseline.
Conclusion
The combination of enterally administered LMWH and SNAD given for 7 days appeared to decrease caval thrombosis in this model of deep vein thrombosis. Enteral LMWH/SNAD effected an increase in plasma levels of antifactor Xa.
The diagnosis of acute deep venous thrombosis (DVT) is suggested in approximately 250,000 people per year in the United States alone. 1 Patients may exhibit the classic signs and symptoms of calf pain and swelling and seek the advice of their physician. After a diagnostic duplex ultrasound, the patient is generally admitted to the hospital for intravenous anticoagulation with unfractionated heparin and subsequent initiation of oral warfarin. This traditional method of treating DVT has recently been challenged by a novel approach where patients are given twice-daily dosages of subcutaneous low molecular weight heparin (LMWH) and then discharged to complete a 5- to 7-day course at home with warfarin started concomitantly. Some centers have taken this approach one step further and initiated the entire treatment protocol on an outpatient basis. A home care nurse typically visits the patient and assists with the administration of the subcutaneous medication. A blood sample is drawn at appropriate intervals to measure the prothrombin time to modulate the administration of warfarin. 2–9
The advantages of this novel method of DVT treatment are its cost-effectiveness and the decreased hospital stay. However, the disadvantage of LMWH has been the subcutaneous route of administration. Administration of LMWH is costly, and many patients find it to be painful. The administration of LMWH as an oral agent by itself has been studied but has not been shown to be effective. The gastrointestinal (GI) absorption of LMWH by itself has not been reliable because of the relatively large size and the negative charge of this molecule. Emisphere Technologies (Tarrytown, NY) has developed a carrier compound that will allow the LMWH molecule to be administered orally. The carrier compound sodium N-[10-(2-hydroxybenzoyl)amino] decanoate (SNAD) is a novel delivery agent that facilitates the GI absorption of LMWH. When SNAD and LMWH are combined, they form a noncovalent interaction that allows the complex to pass through the GI mucosa. Once the LMWH/SNAD complex reaches the bloodstream, SNAD dissociates and thus the LMWH molecule is free to effect anticoagulation.
The primary hypothesis of this experiment was that the enteral administration of LMWH and its delivery agent SNAD would effectively treat vena caval thrombosis in the porcine model of DVT. Because LMWH partly effects anticoagulation by inhibiting factor Xa, the secondary hypothesis of this experiment was that the combination of enteral LMWH and SNAD would increase serum levels of antifactor Xa.
METHODS
Fifty female farm pigs (weight 25–30 kg) were used for this experiment. The basic surgical procedure was previously described by Hosaka et al 10 but has been extensively modified to provide a reliable animal model with consistently reproducible results. The surgical aspect of this experiment was divided into three parts:
1. Initiation of the caval thrombosis and placement of the gastrostomy tube;
2. Confirmation of caval thrombosis with fluoroscopy and removal of the balloon catheter; and
3. Harvesting the inferior vena cava and extraction of the residual thrombus.
On the first experimental day, the animals were given a preoperative antibiotic (flocillin 300,000 U given intramuscularly) and were sedated with acepromazine (0.4 mg/kg given intramuscularly). The animals were then brought to the operating room and placed under general endotracheal anesthesia with inhaled 1% isoflurane. The abdomen, left flank, and both groins were prepared in continuity with povidone–iodine solution. A transverse skin incision was made in the right groin overlying the femoral vessels. The femoral vessels were then identified, and the superficial femoral vein was isolated from its surrounding structures and controlled with 2–0 silk sutures. A transverse venotomy was made and a 4F introducer catheter (Cordis Corp., Miami, FL) was placed under direct vision into the femoral vein. A similar procedure was then performed on the left groin to isolate the left superficial femoral vein. A venotomy was then made on the left superficial femoral vein, and a 7.5F angioplasty balloon catheter was placed into the venous circulation. Using fluoroscopic guidance, a 3F cobra catheter (Cordis Corp.) was placed in the right femoral introducer sheath and into the inferior vena cava. The renal veins were selectively cannulated and fluoroscopically imaged with a 3- to 5-mL injection of contrast to delineate the extent of the infrarenal vena cava. At that time, the balloon catheter was advanced into the final position such that the tip of the catheter was at the level of the renal veins. The balloon catheter was then manually inflated to occlude the infrarenal vena cava entirely. This was confirmed by injecting contrast into the infrarenal vena cava. The cobra catheter was repositioned at a level just adjacent and inferior to the balloon catheter, where 2 mL absolute ethanol was injected during a 10-minute period. The cobra catheter was removed from the venous circulation. The introducer sheath and the balloon catheter were left in situ and affixed to the venous structures with 2–0 silk ties. The groin incisions were then closed in two layers.
At this point, the abdomen was opened to place the gastrostomy tube. An upper midline abdominal incision was made, the stomach was identified, and a purse-string suture was placed on the anterior surface of the stomach. Using electrocautery, a small gastrotomy was made and a 26F gastrostomy tube was placed in the lumen of the stomach. After securing the gastrostomy tube, a subcutaneous tunnel was created and the tube was directed onto the posterior left flank. The tube was then secured to the skin with a heavy silk suture.
The animals were then allowed to recover for 5 days before returning to the operating room for the second part of this experiment. Once again, the animals received antibiotics and were placed under general anesthesia as described above. After preparing the appropriate areas with povidone–iodine solution, the groin incisions were opened. The balloon catheter was deflated and removed. Through the existing right femoral introducer sheath, a cobra catheter was placed. A venogram was then performed to document the presence of intraluminal thrombus.
Animals that were noted to have thrombus in the inferior vena cava were then randomized to one of four experimental groups (n = 10 each). After randomization, each of the animals received the study medications at 12-hour intervals for 7 treatment days. All medications were administered through the gastrostomy tube. The group assignments were as follows:
• Group 1 (control) received an enteral bolus of saline (20 mL).
• Group 2 (enteral LMWH, 2,000 IU/kg) received LMWH (enoxaparin; Rhone-Poulenc Rorer Pharmaceuticals, Collegeville, PA).
• Group 3 (enteral SNAD, 50 mg/kg) was given a dose of the carrier molecule SNAD.
• Group 4 (enteral LMWH, 2,000 IU/kg and SNAD, 50 mg/kg) received an enteral bolus of LMWH combined with the carrier SNAD.
All animals that angiographically exhibited intraluminal thrombus were allowed to return to the recovery area. Animals that did not have evidence of intraluminal thrombus were killed because they were considered a failure of this animal model. In the recovery area, the animals received measured amounts of the study medications at 12-hour intervals throughout the 7-day study period. In addition, the animals were fasted approximately 4 hours before and after the administration of the enteral medication. The fasting schedule was initiated to maximize GI absorption of the study medications.
After the 7-day treatment period, the animals were returned to the operating room, where under general anesthesia the entire segment of the infrahepatic vena cava was harvested for extraction of the residual thrombus. Once harvested, the vena cava was opened longitudinally to remove the entire thrombus for measurement on a scientific scale. Veins that had no thrombus were counted as zero in the calculation of average thrombus weight.
A separate experiment was undertaken to measure plasma levels of antifactor Xa specifically. A group of 10 female farm pigs weighing 25 to 30 kg each were given general anesthesia and underwent placement of a right femoral venous introducer sheath as described above. The animals were given a single dose of enteral LMWH (2,000 IU/kg) and SNAD (50 mg/kg) by a gastrostomy tube at time 0. Blood samples were obtained from these animals at −30, 0, 15, and 30 minutes and 1, 2, 3, 4, and 6 hours after the administration of the test substance. The blood samples were collected in vials containing sodium citrate and placed in a centrifuge at 3,000 rpm for 10 minutes to separate the cellular components from the plasma. The separated plasma was then placed in a colorimetric quantitative assay for the presence of antifactor Xa (Chromogenix, MoIndal, Sweden).
Statistical analyses used were the chi-square and analysis of variance. The Institutional Animal Care and Use Committee of the Alton Ochsner Medical Foundation granted approval for this experiment.
RESULTS
At the initiation of this experiment, 40 female farm pigs were used to create an experimental thrombosis of the inferior vena cava. As a result of either balloon catheter dysfunction or animal death, 8/40 were removed from the experimental analysis. The final number of animals in each groups was as follows: group 1, n = 8; group 2, n = 8; group 3, n = 7; and group 4, n = 9. All of these animals had exhibited evidence of either partial or complete occlusion of the inferior vena cava on fluoroscopic examination before treatment.
Quantitative Assessment of Thrombus
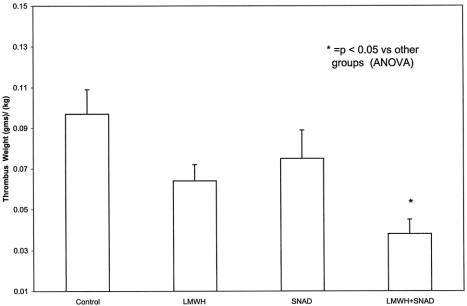
Because the animals were of varying size and weight, the absolute amount of thrombus extracted from the inferior vena cava was then divided by the weight of the animal on the day of randomization. The results of the thrombus weight standardized to the animal weight were as follows: group 1, 0.097 ± 0.012 g/kg; group 2, 0.064 ± 0.008 g/kg; group 3, 0.075 ± 0.014 g/kg; and group 4, 0.038 ± 0.007 g/kg. The amount of thrombus in the animals receiving enteral LMWH/SNAD was significantly less than the other three groups (P < .05). There was no significant difference in the amount of thrombus among the other three groups (Fig. 1).
Figure 1. Quantitative assessment of residual thrombus. Thrombus was extracted from the excised inferior vena cava. Animals that received low-molecular-weight heparin (LMWH) combined with sodium N-[10-(2-hydroxybenzoyl)amino] decanoate (SNAD) displayed significantly less thrombus than groups 1, 2, and 3. Error bars = SEM.
Quantitative Assessment of Serum Antifactor Xa
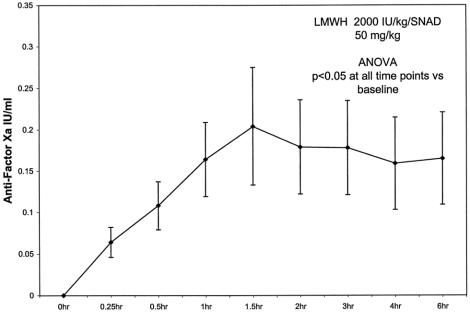
Antifactor Xa levels (in IU/mL) were as follows: baseline = 0.0, 15 minutes = 0.064 ± 0.018, 30 minutes = 0.108 ± 0.029, 1 hour = 0.164 ± 0.045, 1.5 hours = 0.204 ± 0.071, 2 hours = 0.179 ± 0.057, 3 hours = 0.178 ± 0.057, 4 hours = 0.159 ± 0.056, and 6 hours = 0.165 ± 0.056. The level of antifactor Xa rose above baseline, peaked at approximately 1.5 hours, and then remained above baseline for the rest of the 6-hour period. All values were significantly elevated versus baseline (Fig. 2).
Figure 2. Plasma antifactor Xa levels were measured in a separate group of ten animals. An enteral bolus of low-molecular-weight heparin (LMWH) combined with sodium N-[10-(2-hydroxybenzoyl)amino] decanoate (SNAD) was given to the animals at time 0 (*P < .05). Error bars = SEM.
DISCUSSION
In this study, the oral administration of LMWH and its carrier compound SNAD was applied to a porcine model of DVT to examine the effectiveness of this combination in the treatment of DVT. The animals given the enteral combination of LMWH and SNAD exhibited quantitatively less thrombus than the other study animals. In accordance with these findings, the plasma levels of antifactor Xa were significantly elevated from baseline in the animals that received LMWH/SNAD.
In contrast to unfractionated heparin, the smaller LMWH has little effect on inhibiting factor II but has a similar inhibitory effect on factor X. The ability of LMWH to inhibit factor X, as measured by the antifactor Xa levels, is the cornerstone of its anticoagulant properties. 11 By measuring the serum levels of antifactor Xa, we were able to test the anticoagulant properties of this enteral combination. Animals that received enteral LMWH/SNAD had significantly higher levels of antifactor Xa than controls. We have shown in a previous study that an enteral dose of LMWH combined with the carrier SNAD was associated with much higher levels of antifactor Xa than with LMWH alone. 12 This demonstrated that the addition of enteral SNAD significantly enhanced the GI absorption of LMWH.
There are several advantages to using LMWH for the treatment of DVT. In animal studies, LMWH has been shown to have less activity against platelets and to produce fewer bleeding side effects. It also has improved bioavailability. The biologic half-life of subcutaneously administered LMWH is 4 hours, considerably longer than that of unfractionated heparin. 11 This enhanced bioavailability obviates the need for repetitive and expensive laboratory monitoring and makes this a medication better suited to an outpatient treatment protocol. 9
The present disadvantage of LMWH is the subcutaneous route of administration. Not only is this costly, in that skilled nursing personnel must train the patient and must sometimes administer the drug, but it is also painful. The ability to introduce this medication orally would greatly enhance the utility of LMWH as a method of treating DVT.
Recently, several articles have been published that tout the efficacy of LMWH for both the prophylaxis and the initial treatment of DVT. 2–5 In patients diagnosed with DVT, a regimen of subcutaneous LMWH can be started, and then they can be sent home. In these studies, warfarin was administered after 24 hours of subcutaneous LMWH therapy. 2–5 LMWH was continued for approximately 5 days, or until the International Normalized Ratio (INR) reached 2 to 3. In one study, a subcutaneous dosage of LMWH was administered in the emergency room before discharge. 4
The prospect of home DVT treatment could be advanced with the use of an orally administered LMWH. Most of the studies documenting the efficacy and safety of home DVT treatment with parenteral LMWH state that several logistical details must be addressed before implementing this form of treatment; in essence, the details are related to patient education and cost. A study from the Mayo Clinic stated that a nurse educator was responsible for teaching the patients how to inject the medication appropriately. 13 In another study, a home care nurse routinely visited the patient and either directly injected the LMWH or supervised the patient in self-administration. 5 In either case, the authors recognized that there were logistical and cost issues that needed to be resolved. The cost of providing nursing care at home is approximately $200 per day. The cost of twice-daily subcutaneous administration of LMWH is approximately $40 per day. Therefore, at $240 per day, the cost of home treatment has not routinely been covered by most insurance carriers. 5 Most of these issues are related to the route of administration of LMWH and not the medication itself. It seems that if an oral preparation of LMWH were to be developed, the cost could be far less, and the various other logistical problems associated with the subcutaneous administration would be resolved.
In summary, LMWH given enterally in combination with the carrier SNAD resulted in a decrease in thrombus weight in a porcine model of DVT. Also, elevated levels of antifactor Xa were found in the animals receiving the LMWH/SNAD combination. DVT is currently treated using a variety of inpatient and outpatient modalities, and the application of an orally administered treatment regimen based on this novel carrier of LMWH could revolutionize the care of these patients. Our experience in the treatment of DVT in a large animal warrants further investigation of oral LMWH/SNAD for the treatment of DVT. Preclinical studies of the potential toxicities of the LMWH/SNAD combination are ongoing and should be completed before investigating the efficacy of this formulation on the clinical level.
Discussion
Dr. Thomas F. Dodson (Atlanta, Georgia): This work has the obvious potential to markedly change the method of delivery of low molecular weight heparin delivery for the home treatment of venous thrombosis. Given the historical nature of our society, let me just briefly digress and give you a few sentences on the discovery of heparin. As you may know, this was done by a physiologist at Hopkins named William Howell and a medical student at Hopkins named J. McLain. As the sole author of a landmark paper in 1916, the medical student, McLain, wrote on the thromboplastic action of cephalin. Howell, the physiologist, continued McLain’s work and, after analyzing the anticoagulant heparaphosphatid, named it heparin. A full account of this remarkable event was published in the Journal of Vascular Surgery in 1989.
Back to the matter at hand. The use of heparin laid the foundation for vascular and cardiovascular surgery. It provided an effective treatment for venous thrombosis, a discharge diagnosis of more than a quarter million patients from United States hospitals annually. In March 1996, an important paper and editorial about low molecular weight heparin appeared in the New England Journal of Medicine and documented the use of low molecular weight heparin as an outpatient modality. It stated that this could be used effectively and safely as an outpatient. In analyzing the author’s paper, I was able to find an earlier paper about the oral delivery of low molecular weight heparin to prevent jugular venous thrombosis in the rat. This appeared in the Journal of Vascular Surgery in September of this year. That paper also used the novel carrier SNAD that allowed gastrointestinal absorption of low molecular weight heparin.
I have two questions:
First, your method of inducing caval thrombosis by injecting absolute ethanol into the vena cava, similar to the technique in your first paper. This technique, however, seems a far cry from Virchow’s triad of stasis injury in hypercoagulability. Could you comment on this?
Secondly, in your early paper, you did not have any data on the toxicity of SNAD. I notice that you said in the conclusion of this most recent paper that you thought that the project was at a point where we could consider human trials. Do you have any information on the toxicity of this novel agent?
I enjoyed this paper very much and look forward to future contributions on this subject from the Ochsner Clinic.
Dr. Hugh H. Trout III (Bethesda, Maryland): As Dr. Bowen indicated, treatment of DVT by virtue of the availability of low molecular weight heparin is moving toward complete outpatient treatment. This treatment, however, is complicated by the current need to administer the low molecular weight heparin percutaneously. Treatment with an absorbable and effective oral low molecular weight heparin will greatly reduce the need for the percutaneous preparation.
I congratulate you on this fine presentation and paper, and I have two brief questions. One, much like Tom’s question: if there is a clinical experience, are there significant side effects of this approach? And finally, what is the FDA status of this approach? When might we be able to see or use this preparation in our patients?
Dr. Samuel R. Money (Closing Discussion): Dr. Dodson, I will address your questions first. You questioned our method of caval thrombosis. Basically, we have stasis by using the balloon catheter, and the absolute alcohol is injected very slowly over 10 minutes, which gives an injury to the venous lining. Therefore, we have two out of three of the triad.
The other thing that must be kept in mind is that pigs clot a lot more readily than human beings do. They are animals of the wild, and their PT runs about 7 to 9, and their PTT runs at about 15. So I think, by nature, they do run a little more coagulable than human beings.
The toxicity of SNAD was questioned by both you and Dr. Trout. Human studies with SNAD have not yet begun; however, SNAD has a sister molecule which is called SNAC, which has been developed and tested to be used with unfractionated heparin. There are some clinical trials going on with SNAC at this point. The toxicities of SNAC are that if one takes a much higher dosage, such as four or five times what we have given these animals, there is potential for some nausea and vomiting in about 5% to 10% of the patients. However, at lower doses it is significantly less.
Turning to Dr. Trout’s questions, the first question, do we have any clinical experience with SNAD: no, we do not. But we do have clinical experience with SNAC—not with SNAD, excuse me—and one study has been completed already on the prevention of DVT in an orthopedic total joint replacement population, comparing SNAC and unfractionated heparin with subcutaneous unfractionated heparin, and it has been shown equivalent. The goal to get through FDA testing is to have two clinical studies separated; therefore, a second clinical study is underway. If all goes well, we should see unfractionated heparin and SNAC in combination probably within a year to 2 years. I believe, as most other people who are in the field believe, that SNAC is not the home run we are all looking for, but low molecular weight heparin and SNAD is, because low molecular weight heparin’s half-life is significantly longer than unfractionated heparin.
If you could dose somebody with oral low molecular weight heparin in a carrier agent, not have to check their blood and have them on a BID or acute 12-hour dosing schedule, it would be a lot more usable clinically.
So I think that, in answer to Dr. Trout’s question, unfractionated heparin in a carrier agent will reach the market, but a year or so after that, low molecular weight heparin in its carrier agent SNAD will hit the market.
Footnotes
Correspondence: Samuel R. Money, MD, Division of Vascular Surgery, Alton Ochsner Medical Foundation, Ochsner Clinic, Dept. of Surgery, 1514 Jefferson Highway, 8N, New Orleans, LA 70121.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Supported by Emisphere Technologies.
E-mail: SMONEY@Ochsner.org
Accepted for publication December 1999.
References
- 1.Meissner MH, Strandness DE. Pathophysiology and natural history of acute deep venous thrombosis. In: Rutherford RB, ed. Vascular Surgery. Philadelphia: WB Saunders; 1999: 1920–1937.
- 2.Crowther M, Hirsh J. Low-molecular-weight heparin for the out-of-hospital treatment of venous thrombosis: rationale and clinical results. Semin Thromb Hemost 1997; 23:77–81. [DOI] [PubMed] [Google Scholar]
- 3.Harrison L, Mc Ginnis J, Crowther M, Ginsberg J, Hirsh J. Assessment of outpatient treatment of deep-venous thrombosis with low molecular weight heparin. Arch Intern Med 1998; 158:2001–2003. [DOI] [PubMed] [Google Scholar]
- 4.Innes GD, Dillon EC, Holmes A. Low-molecular-weight heparin in the emergency department treatment of venous thromboembolism. J Emerg Med 1997; 15:563–566. [DOI] [PubMed] [Google Scholar]
- 5.Wells PS, Kovacs MJ, Bormanis J, et al. Expanding eligibility for outpatient treatment of deep venous thrombosis and pulmonary embolism with low-molecular-weight heparin: a comparison of patient self-injection with home care injection. Arch Intern Med 1998; 158:1809–1812. [DOI] [PubMed] [Google Scholar]
- 6.Grau E, Real E, Pastor E, Viciano V, Aguilo J. Home treatment of deep vein thrombosis: a two-year experience at a single institution. Haematologica 1998; 83:438–441. [PubMed] [Google Scholar]
- 7.Grosset AB, Spiro TE, Beynon J, Rodgers GM. Enoxaparin, a low-molecular-weight heparin, suppresses prothrombin activation more effectively than unfractionated heparin in patients treated for venous thromboembolism. Thromb Res 1997; 86:349–354. [DOI] [PubMed] [Google Scholar]
- 8.Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681. [DOI] [PubMed] [Google Scholar]
- 9.Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998; 3:41–46. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka J, Roy S, Kvernebo K, Enge I, Laerum F. Induced thrombosis in the pig inferior vena cava: a model of deep venous thrombosis. J Vasc Interv Radiol 1996; 7:395–400. [DOI] [PubMed] [Google Scholar]
- 11.Drug and Therapeutics Bulletin 1998; 36:25–29. [DOI] [PubMed]
- 12.Salartash K, Gonze MD, Leone-Bay A, et al. Oral low-molecular-weight heparin and delivery agent prevents jugular venous thrombosis in the rat. J Vasc Surg 1999; 30:526–532. [DOI] [PubMed] [Google Scholar]
- 13.Litin SC, Heit JA, Mees KA. Use of low-molecular-weight heparin in the treatment of venous thromboembolic disease: answers to frequently asked questions. Mayo Clin Proc 1998; 73:545–550. [DOI] [PubMed] [Google Scholar]