Abstract
Objective
To examine how the choice of surgical technique influenced perioperative outcomes in liver transplantation.
Summary Background Data
The standard technique of orthotopic liver transplantation with venovenous bypass (VVB) is commonly used to facilitate hemodynamic stability. However, this traditional procedure is associated with unique complications that can be avoided by using the technique of liver resection without caval excision (the piggyback technique).
Methods
A prospective comparison of the two procedures was conducted in 90 patients (34 piggyback and 56 with VVB) during a 2.5-year period. Although both groups had similar donor and recipient demographic characteristics, posttransplant outcomes were significantly better for the patients undergoing the piggyback technique. The effect of surgical technique was examined using a stepwise approach that considered its impact on two levels of perioperative and postoperative events.
Results
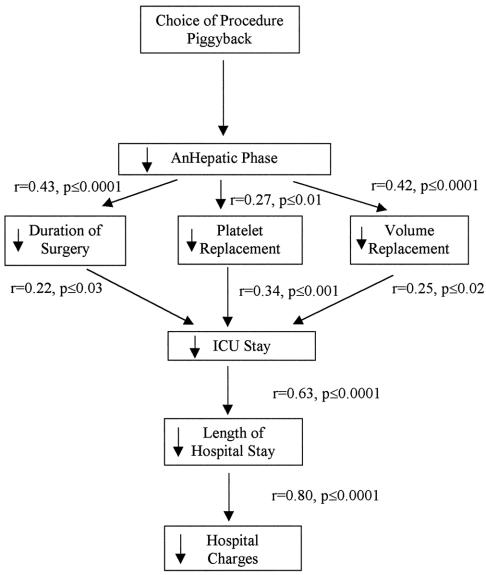
The analysis of the first level of perioperative events found that the piggyback procedure resulted in a 50% decrease in the duration of the anhepatic phase. The analysis of the second level of perioperative events found a significant relation between the anhepatic phase and the duration of surgery and between the anhepatic phase and the need for blood replacement. The analysis of the first level of postoperative events found that the intensive care unit stay was significantly related to both the duration of surgery and the need for blood replacement. The intensive care unit stay was in turn related to the second level of postoperative events, namely the length of hospital stay. Finally, total charges were directly related to length of hospital stay. The overall 1-year actuarial patient and graft survival rates were 94% in the piggyback and 96% in the VVB groups, respectively.
Conclusions
These data demonstrate that surgical choices in complex procedures such as orthotopic liver transplantation trigger a chain of events that can significantly affect resource utilization. In the current healthcare climate, examination of the sequence of events that follow a specific treatment may provide a more complete framework for choosing between treatment alternatives.
The results of orthotopic liver transplantation (OLT) in patients with end-stage liver disease continue to improve 1 because of refinements in surgical technique, advances in perioperative monitoring and management, and more effective immunosuppressive protocols. OLT is unique in that the recipient undergoes a substantial hemodynamic challenge during surgery, which is reflected in the complications and postoperative course. Venovenous bypass (VVB) is used in most cases of OLT to facilitate hemodynamic stability. 2,3 Despite its usefulness, VVB is associated with unique complications such as thromboembolic complications, air embolus, wound seromas, and infections, and the added costs of the machine, catheters, and extra personnel. 4–6 In addition, caval excision and mechanical pumping has been associated with global capillary injury, leading to third-spacing of fluid after surgery. 4–6
However, VVB may not be necessary in all patients undergoing OLT. Instead, hemodynamic stability could be maintained through the use of alternate approaches such as the caval preservation (piggyback) technique. 7,8 Calne in 1968 9 was the first to describe the piggyback technique with preservation of the recipient’s vena cava and anastomosis of the donor’s vena cava with the recipient’s hepatic veins. The advantages of the piggyback technique include maintenance of caval flow during explantation, leading to reduced fluid resuscitation requirements, better maintenance of core body temperature, and improved cardiac hemodynamic stability. The disadvantages of caval preservation may include the need to dissect the cava completely from the liver, with prolongation of the hepatic excision phase. Another potential disadvantage is the need for clamping of the portal circulation during the caval anastomosis. OLT with the piggyback technique is used routinely in reduced-size liver transplantation and also in pediatric living-related liver transplantation, because in both these procedures the recipient cava must be preserved.
With the increasing use of this technique by centers in the United States and Europe in adult OLT, 10–15 we attempted to study whether the technique had a significant impact on resource utilization in liver transplantation. Liver transplantation continues to be one of the most expensive treatment options in medicine, thus mandating a thorough examination of the cost effectiveness of various approaches to the procedure. The timeliness of the topic has been stressed in a recent review of 711 liver transplants that concluded that examination of resource utilization in liver transplantation may result in significant programmatic and societal savings. 16 Our data demonstrated that the choice of surgical technique can have significant implications on resource utilization without significantly affecting the outcomes of liver transplantation.
METHODS
Between January 1997 and July 1999, 90 consecutive patients with end-stage liver disease prospectively underwent OLT either by the conventional technique with VVB (n = 56) or by the piggyback technique without inferior vena cava (IVC) clamping, VVB, and portacaval decompression (n = 34). Patient assignment to the two study groups was determined by the operating team, usually before the start of the procedure. Sixteen patients who underwent OLT during the same time period were excluded from the study. These patients were individuals receiving a partial liver (in situ splitting, n = 4, 2 adults and 2 pediatric), a living-related adult-to-adult liver transplant (n = 7), a pediatric cadaveric transplant (n = 3), a combined liver and kidney transplant (n = 1), and a retransplant. Three perioperative deaths (two in the conventional group and one in the piggyback group) were also excluded from the data collection but included in the outcome analysis.
Data Collection
The following donor data were collected: age, sex, cause of death, intensive care unit (ICU) stay, need for pressor drugs before organ donation, and presence of any vascular anomalies in the liver allograft. Recipient pretransplant data included Childs-Pugh score and UNOS status at the time of transplant, cause of liver disease, creatinine and creatinine clearance levels, and presence of prior upper abdominal surgery. Intraoperative data included operating time, anhepatic time, cold ischemia time, mean blood pressure during anhepatic phase, mean blood pressure after reperfusion, lowest core body temperature, number of transfusions with red blood cells (banked blood plus cell saver), fresh-frozen plasma, platelet, cryoprecipitate, total fluid volume replacement during surgery, and urine output. Postoperative data included ICU length of stay as well as total length of hospital stay and hospital charges.
Surgical Technique
In the conventional group, bypass catheters were placed by a percutaneous puncture in the femoral vein and by cutdown in the axillary vein. A portal vein catheter was placed directly in the portal vein after it was divided for explanting the native liver. Vascular clamps were placed on the infra- and suprahepatic vena cava and VVB was started as the recipient liver, together with the retrohepatic cava, 17 was removed. A pump (Bio Medicus Model 520D, Bio Console Model 540, Minneapolis, MN) was used to pump portal and caval blood into the axillary vein through GOTT (Medtronics, Minneapolis, MN) tubing.
In the piggyback technique, the liver was mobilized and the bile duct and arterial branches to the liver were divided. The liver was then dissected free from the IVC and all of the small hepatic branches to the IVC were ligated. The entrance of the right hepatic vein to the IVC was encircled and divided, and the stump was repaired with 4–0 prolene (Fig. 1), leaving the liver attached to the body only through the portal, middle, and left hepatic veins. After the liver allograft was prepared at the back table, vascular clamps were applied to the recipient’s portal vein and the confluence of the middle and left hepatic veins, and the recipient’s liver was removed. The cuff of the upper cava of the allograft was anastomosed to the confluence of the middle and left hepatic veins with running 4–0 prolene (Fig. 2), followed by the portal vein anastomosis. No lower caval anastomosis was necessary because the lower cava of the allograft was repaired at the back table around a 16F rubber catheter. Once the portal vein anastomosis was completed, portal flow was established, and the initial 100 to 200 mL of the blood perfused to the liver was allowed to bleed through the inferior cava of the allograft through the red rubber catheter. The catheter was then removed and the suture line tightened. Once all bleeding was secured, the hepatic arterial and bile duct anastomosis was constructed in the usual fashion.
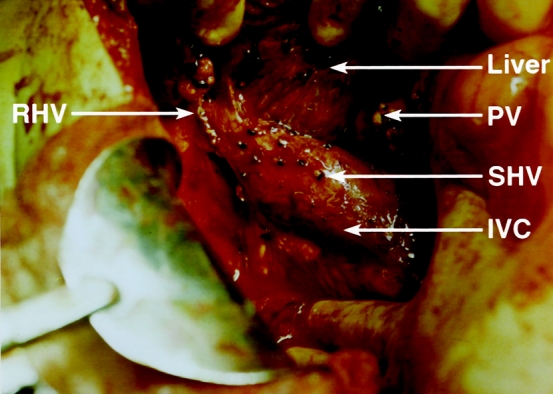
Figure 1. Liver has been dissected completely from the inferior vena cava (IVC) and is attached to the body only through the left and middle hepatic veins and the portal vein (PV) in preparation for piggyback orthotopic transplantation. Note the repair site of the right hepatic vein (RHV) and the ligature on all small hepatic veins (SHV).
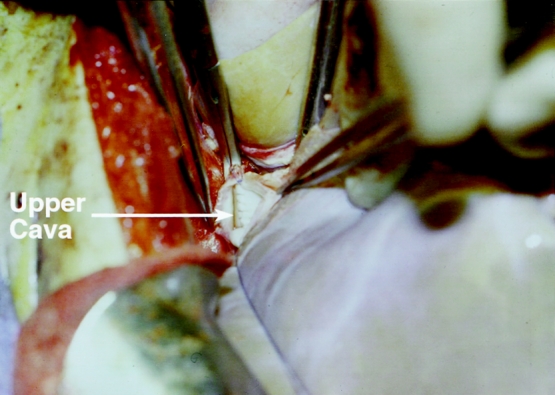
Figure 2. Upper cava anastomosis to the confluence of the middle and left hepatic veins in piggyback orthotopic transplantation. Note the equal size of the upper cava of the graft and the confluence of the middle and left hepatic veins.
Statistical Analysis
Unpaired t tests and chi-square analysis were used to determine differences in study outcomes between groups. Data from this univariate analysis were then used to identify variables for incorporation in a subsequent multivariate analysis to review the study outcomes for the potential influence of key intervening variables. All data are presented as means ± standard errors, and an a priori level of significance was set at 0.05.
RESULTS
The mean age for donors in the study population was 31.3 ± 2 years. Most donors were male (53%), the average preprocurement ICU stay was 3.1 days, and most (90%) had one or more pressor drugs used for periods of 12 to 48 hours. The cause of donor death included head injuries sustained in motor vehicle accidents (n = 30), intracerebral hemorrhage (n = 29), gunshot wound to the head (n = 25), and intracranial tumors (n = 6). Vascular abnormalities were reported in seven patients. There were no significant differences in donor parameters between the piggyback and the VVB group (Table 1).
Table 1. DONOR CHARACTERISTICS
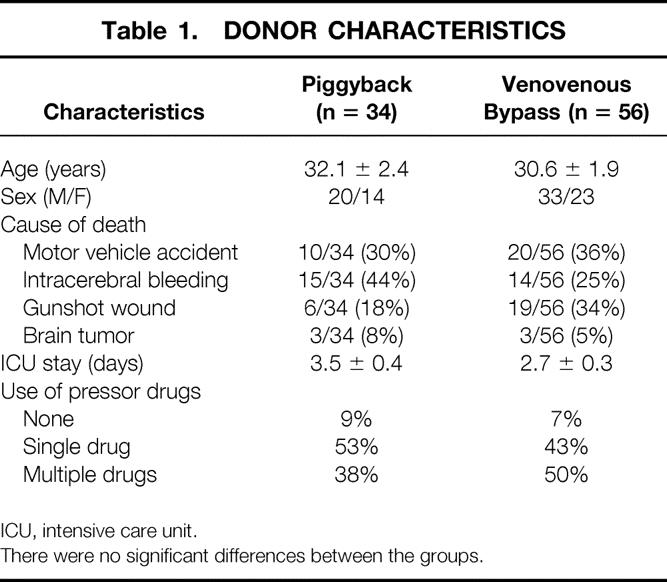
ICU, intensive care unit.
There were no significant differences between the groups.
Recipient characteristics reflected the general demographics of patients with chronic liver failure requiring transplantation. Mean age was 49.5 years, with slightly more men (56/90) and with most (36/90) having liver failure secondary to chronic hepatitis C. Patients had a mean Childs-Pugh score of 10 at the time of transplantation, with the majority having UNOS status IIb and III. Again, recipient risk factors and demographics did not differ between the two groups of study patients (Table 2).
Table 2. RECIPIENT CHARACTERISTICS
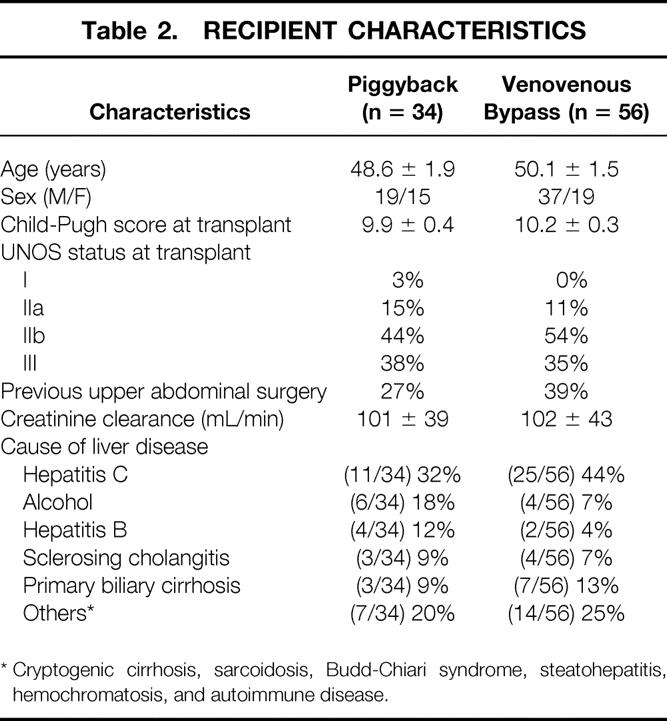
* Cryptogenic cirrhosis, sarcoidosis, Budd-Chiari syndrome, steatohepatitis, hemochromatosis, and autoimmune disease.
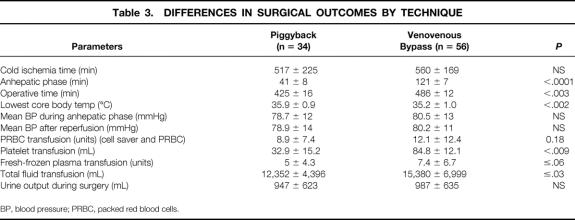
There was no difference in the cold ischemia time between the piggyback recipients (517 minutes) and the VVB group (560 minutes). Hemodynamic stability during surgery was equally maintained in both groups, as was urinary output (Table 3). The most significant difference between the groups occurred, as expected, in the duration of the anhepatic phase, which was reduced by approximately 60% in the piggyback recipients. A statistically significant difference in the total duration of surgery of approximately 1 hour in favor of the piggyback group was also seen. Physiologically important differences occurred between the two groups. These included a higher core body temperature in the piggyback group, associated with a significant decrease in the requirements for fluid, plasma, platelets, and red blood cell transfusion (see Table 3). After surgery, patients treated by the piggyback technique had a 30% shorter ICU stay and a similar reduction in their overall hospital stay. As a result, a significant reduction in hospital charges was seen in the piggyback group (Table 4).
Table 3. DIFFERENCES IN SURGICAL OUTCOMES BY TECHNIQUE
BP, blood pressure; PRBC, packed red blood cells.
Table 4. POSTOPERATIVE OUTCOMES BY SURGICAL TECHNIQUE
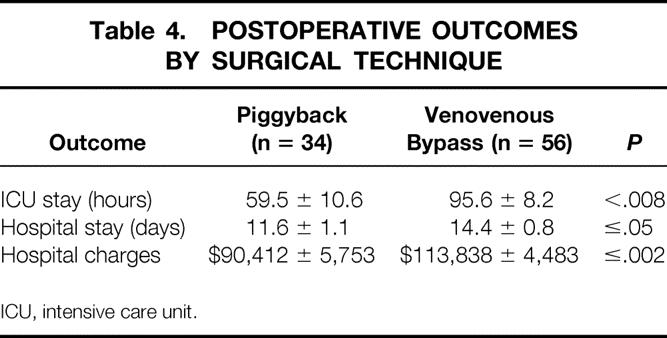
ICU, intensive care unit.
Because Childs-Pugh score and donor age are key factors that are known to influence surgical and postsurgical events, we also conducted a multivariate analysis adjusting for the effects of these variables. These data are summarized in Table 5 and indicate that all study outcomes remained significantly (P ≤ .05) influenced by choice of surgical procedure, even after adjusting for the effects of donor age and Childs-Pugh score. These effects remained intact even in the presence of a significant effect of donor age on volume replacement (P ≤ .02), length of ICU stay (P ≤ .02), length of hospital stay (P ≤ .03), and hospital charges (P ≤ .03); and Childs-Pugh score on length of ICU stay (P ≤ .02), length of hospital stay (P ≤ .008), and hospital charges (P ≤ .001).
Table 5. INFLUENCE (P VALUES) OF DONOR AGE, CHILD-PUGH SCORE, AND SURGICAL PROCEDURE ON STUDY OUTCOMES

ICU, intensive care unit.
Overall, the actual 1-year patient and graft survival rates for the total sample, the conventional group, and the piggyback group were 94%, 96%, and 94%, respectively (not significant). There was no primary allograft nonfunction in any of the groups during the time period of the study. In 13% of cases where the piggyback technique was attempted, the procedure was changed to conventional VVB because of concerns regarding the diameter of the middle and left hepatic veins. Liver congestion or caval outflow compromise were not encountered.
To determine the impact of the intraoperative choice of technique on outcomes and resource utilization, a stepwise approach that considered the impact of the type of procedure on various levels of perioperative and postoperative events was applied. This in turn was extended to determine the ultimate influence of the choice of procedure on the total charges of the transplant events. We first examined whether differences in various outcomes were operator-dependent, because the number of piggyback and conventional procedures was not equally divided among the surgeons. An analysis for surgeon effect indicated that outcome variation was independent of the surgical team or the individual lead surgeon. Using the results of the univariate analysis (see Tables 1 through 4), a model was constructed that postulated the differences in the outcomes that represented a chain of events set into motion by the choice of the piggyback procedure based on the relation of logical cause and effect (Fig. 3). The procedure choice resulted in a significant reduction in the length of the anhepatic phase and was in turn significantly correlated with intraoperative events (duration of surgery, blood and fluid replacement). The intraoperative events were significantly correlated with postoperative outcomes, which in turn correlated strongly and significantly with the total charges of the procedure.
Figure 3. Impact of surgical choice on outcome of orthotopic liver transplantation.
DISCUSSION
In this study, more than 85% of patients were able to undergo OLT using the caval-preservation technique. Not only did this avoid use of the VVB, but we were also able to maintain stable hemodynamic conditions, preserve core body temperature, and decrease the requirement for fluids, blood, and blood product transfusions. Although the two patient groups were similar and received organs from donors with similar characteristics, patients who underwent OLT with the piggyback technique spent a significantly shorter time in the ICU and were discharged more rapidly from the hospital. This decreased ICU and hospital stay enabled us to demonstrate significant cost savings when VVB was not used (a mean of $23,500 per patient). Our model indicated that these outcomes were associated in stepwise fashion to the choice of surgical procedure. We were able to reduce the cost of an otherwise expensive procedure while maintaining comparable graft and patient survival rates.
The most immediate impact of the piggyback technique is on surgical events, particularly the duration of the anhepatic phase. Because of the significant hemodynamic alterations occurring during clamping of the cava, performance of hepatic excision without caval clamping and with caval preservation resulted in better maintenance of core body temperature. By maintaining a significantly higher core body temperature and avoiding the external shunting of blood through the bypass catheter and pump, intraoperative coagulopathy and third-spacing of fluid were significantly reduced. The reduced blood and fluid loss decreased the need for transfusion, which in turn further maintained core temperature. Another powerful impact of reducing of the anhepatic phase was related to its effect on decreasing the total length of surgery. This occurred despite the increased time required to dissect the liver from the IVC. We believe that the extra dissection time required in the piggyback procedure is usually offset by the extra time required in the conventional procedure for placement and removal of the bypass catheters and achievement of retroperitoneal hemostasis when the cava is dissected from the adrenal and retroperitoneal tissue. The piggyback procedure is further shortened by the need for one less anastomosis, because the infrahepatic closure is usually performed on the bench. Additional time savings may be possible because the upper caval anastomosis is easier in the piggyback technique because the anastomosis is performed in a slightly more anterior plane compared with the conventional caval anastomosis (see Fig. 2). Bleeding from the posterior suture line is also handled more efficiently because it is easier to lift the liver and check posterior bleeding because the liver is attached to the vena cava by only one anastomosis. As a result of all of these technical factors, a significant improvement in surgical outcome was demonstrated in the piggyback group of patients.
Of course, technical pitfalls can complicate the outcome of the piggyback procedure and reduce its impact on first- and second-level surgical events. For example, several different methods of caval anastomosis have been reported in the literature. Belghiti et al 10 suggested side-to-side caval–caval anastomosis for piggyback transplantation in conjunction with repairing the upper and lower caval orifices of the allograft on the back table. We have not used this technique because we believe it would involve partial clamping of the recipient IVC, which might eliminate the advantages we achieved in our patients by maintaining unobstructed caval flow. The technique described by Belghiti was introduced in an attempt to eliminate the risk of upper caval outflow compromise and liver congestion (Budd-Chiari syndrome) that was seen with some of the early attempts at caval preservation. In fact, others 18 have suggested performing an end-to-side anastomosis between the lower cava of the allograft to the patient’s IVC if this complication arises (rescue caval–caval anastomosis). We were able to obviate these potential problems and had no instances of hepatic congestion, outflow obstruction, or hemorrhage complication, as reported by others, 19,20 by carefully assessing the adequacy of outflow from the middle and left hepatic veins (see Fig. 2). Doing this, we had to abandon 13% of the attempted piggyback procedures. In these instances, we elected to proceed with conventional hepatic resection and VVB.
Other technical pitfalls that could affect outcome can be easily avoided by the experienced surgeon. For example, as in major hepatic resections, all small hepatic veins draining into the inferior cava should be meticulously ligated. Larger hepatic veins should be repaired by running prolene stitches without narrowing of the cava. The stump of the middle and left hepatic veins should not be left too long in an attempt to make the anastomosis easier, because a long stump would lead to an increased likelihood of torsion/kink and outflow obstruction.
Another concern regarding the piggyback technique centers around the management of the portal vein during the procedure. The need to delay the transection of the portal vein until after completion of the hepatic–caval disconnection is thought to increase the technical difficulty of the caval dissection. In fact, some have advocated the use of a temporary portacaval anastomosis to facilitate the dissection of the liver from the IVC and to keep portal pressure and splenic circulation intact during the implantation. 11,13 We did not encounter any difficulty in dissecting the liver from the IVC, although we routinely performed this dissection without dividing the portal vein (see Fig. 1). Further, by maintaining portal flow to the liver up to the moment of hepatic excision, we ensured the hemodynamic stability of the patient and minimized portal vein occlusion. In fact, we estimate that based on the time needed to perform the upper caval anastomosis, 10 to 15 minutes of portal vein clamping was all the extra time needed to complete the piggyback technique. Because most OLT patients have established portal collaterals through natural routes, this short extra period of portal occlusion does not appear to have caused any detrimental effects.
Because our analysis clearly documents a positive impact of the procedure on perioperative and postoperative events and on the total cost of the transplant procedure, further understanding of the technical aspects of the piggyback procedure is important to help realize the full potential of this technique.
Our analysis indicated that perioperative events, particularly the total duration of surgery and amount of blood replacement, significantly correlated with ICU stay, which in turn had a strong correlation to total hospital stay. The relation between the amount of blood transfusion and the overall survival after liver transplantation has been reported previously. 21 These relations appear interactive for the most part, but they have not before been correlated to the duration of anhepatic phase and to caval-preservation techniques. The power of this observation is expressed in the impact of these factors on total charges for the transplant procedure: patients undergoing the piggyback procedure had a significant reduction in hospital charges (mean $23,500). Our study found that these significant savings could be achieved in more than 85% of all patients undergoing liver transplantation. If the piggyback procedure were to produce similar savings in other centers, it could yield an annual savings of more than $80 million nationwide.
One should approach these extrapolations with extreme care, however, despite the relatively large numbers of patients, because our data were retrospective and represented an early experience with the piggyback technique. As with all nonrandomized studies, there is a potential for selection bias, particularly in assigning patients to the two treatments. In addition, establishing cause and effect through retrospective studies is particularly difficult, and this should be considered when examining the study results. Further study of this procedure, preferably in a multicenter prospective fashion, is needed to verify these data.
In conclusion, we demonstrated that a liver transplantation technique that has been in use since 1968 but has been less popular than the conventional VVB can be successfully incorporated into a contemporary liver transplant program. The piggyback technique without VVB or IVC clamping creates a hemodynamically stable transplant environment, shortens the ICU and hospital stay, and ultimately leads to a substantial cost savings.
Acknowledgments
The authors thank Mrs. Ginger Hoskins and Ms. Jo Lariviere for expert secretarial assistance and Mid-South Transplant Foundation for providing transplant donor data.
Discussion
Dr. John C. McDonald (Shreveport, Louisiana): This nice contribution by the Memphis group is an effort to compare the classical technique of venovenous bypass and resection of the inferior vena cava of the recipient to the more recent so-called “piggyback” procedure, in which the inferior vena cava of the host is preserved.
The data presented are clear. Without the necessity of venovenous bypass, the anhepatic time is shortened, fewer blood products are necessary, and recovery time is shorter. Although the results are clear, they do not answer the question—which perhaps was not the question—asked. But they do not answer the question of whether IVC preservation technique can replace the traditional approach. These patients were not randomized, and that, I think, is a serious flaw in the study.
Thus, I ask: you told us something about conversions, but you did not tell us what factors influenced you to choose which technique you used. We have found that the piggyback technique is more difficult when the liver is large; however, in live donor transplants, there is no other option. This mandate may lead to more skill in inferior vena caval preservation.
Finally, one of the original descriptions of the piggyback technique recommended a temporary portacaval shunt to prevent intestinal congestion. We have not done that, but I am not sure that we shouldn’t. What is your opinion on the temporary portacaval shunt?
Dr. Timothy Lane Pruett (Charlottesville, Virginia): As all of you are aware, liver transplantation has been a procedure that has gone through a lot of evolution in its clinical practice.
The venovenous bypass was developed initially to help hemodynamic stability, but things have changed. Not everybody does cutdowns to access the veins anymore. We have routinely moved to all percutaneous techniques, and a lot of the comorbid attention which was associated with venovenous bypass has decreased. We have also used, even in the standard techniques, a caval-sparing procedure in which the liver is removed completely off, leaving the retrohepatic cava intact without interruption of flow. So it’s changed a lot without having to excise because you stay out of the adrenal.
With that as a caveat, we also agree that you should try at all costs to stay out of the retroperitoneum, and we agree with your overall technique. But my question, again, would parallel pretty much what Dr. McDonald has said. Out of your 90 patients, only 34 of those had the piggyback technique—meaning, of course, that 56 were left to the standard technique. I think the question is basically going to be, why so few?
We have found that the stiff livers, big livers, are tough. In people with TIPS, it has been very difficult to get the right hepatic vein cleaned. So the question is going to be not so much, should you do all one or the other, but when do you bring the bypass machine into the room? When do you plan on clamping the cava, causing local venous occlusion and return? And when would you plan on doing a programmatic use of the venovenous bypass? Is it with your old patients?
When you looked at the people you didn’t do, you clearly said they were pretty much the same. But I would suspect that there would be—as it was nonrandomized and the surgeons made the decisions—probably a bit of a predisposition to use patients who were maybe a little bit older, putting them on venovenous bypass, or who were maybe a little more hemodynamically at risk before going into the operation. So if you could elucidate that, I’d appreciate it.
Dr. J. Michael Henderson (Cleveland, Ohio): I will freely admit that when I started doing liver transplants, I routinely bypassed everyone, then I stopped bypassing everyone, and now I’m back to bypassing everyone. We go through phases of change, and I think the change, as Dr. Pruett just alluded to, really relates a lot to the ease of now doing bypass again. When it comes to teaching—and certainly, as I take fellows and residents through a liver transplant—it makes me a lot more comfortable and them a lot more comfortable to do them bypass.
The specific questions related to this presentation come back to how were the patients selected. One gets the feeling that the more difficult patients are the patients who got bypassed?
Let me ask about the anhepatic phase. How was this really timed? It appeared when you did the piggyback you were leaving the portal vein intact for the duration of dissection, leading to a short anhepatic phase? Whereas, in the venovenous group, you were anhepatic from the moment you went on bypass?
Another comment and question relates to the overall gaining of experience of the team as you moved into the piggyback arena. Was this really done later in the experience? And was it really just the experience of the team? I think factors other than venovenous bypass led to the improvement in that group.
Dr. Gazi B. Zibari (Shreveport, Louisiana): The authors have presented their results of orthotopic liver transplantation in 34 patients using the piggyback technique without venovenous bypass, compared to 56 patients who underwent orthotopic liver transplantation with the conventional technique using venovenous bypass. They have shown that the patients in the piggyback group had a significantly shorter anhepatic phase, shorter ICU and hospital stays, and incurred significantly lower hospital charges. These are important advantages in the current environment of competitive contracts and cost containment. Additionally, they have shown over 90% 1-year patient and graft survival in both groups. I have several questions for the authors.
In 13% of the cases where the piggyback technique was attempted, then changed to the conventional venovenous bypass technique because of concerns pertaining to the inadequate diameter of the middle and left hepatic veins, did you consider incorporating the right hepatic vein to increase the diameter of the hepatic vein orifice and/or perform a side-to-side cavocaval anastomosis with partial caval occlusion?
Have you performed a cost and outcomes analysis of this 13% group who were converted to conventional venovenous bypass?
Do you have any information about donor/recipient weight ratio in your study? Our past experience prompted this question. Three years ago, one of our patients underwent orthotopic liver transplantation using this piggyback technique. However, the donor liver was much larger than the recipient liver. This patient developed a thrombus in the retrohepatic cava. Since then, reports of the same complication have surfaced from other centers. Could you comment on this problem?
Finally, are there any relative or absolute contraindications to this procedure, such as primary liver tumors and extremely large livers?
Dr. Amadeo Marcos (Richmond, Virginia): I also have questions related to the anhepatic phase. It is not clear to me how much is warm ischemic time during the anhepatic phase. Do you spend a significant portion of the 120 minutes, the mean time you showed today, obtaining hemostasis before you proceed to remove the liver? If that is the case, then maybe carrying the dissection farther before going on bypass would decrease your anhepatic phase, which was the medical reason behind the success of the piggyback technique.
There is no question of the benefit and cost effectiveness of the technique, so it should be encouraged.
Again, the study is not randomized. If it would have been randomized, maybe you could have sorted out the indications for preserving the cava and for proceeding without venovenous bypass. Do you feel there are still patients that need to be put on bypass?
Dr. William C. Chapman (Nashville, Tennessee): I noted in the manuscript that there was a 44% increased incidence of prior upper abdominal surgery in patients who had venovenous bypass as compared to the group that had the piggyback technique. While this did not demonstrate a statistical difference, I wonder if that difference may be an important factor in some of the findings you have shown. Patients with prior upper abdominal surgery represent the very group where there is a longer dissection time interval and an increased incidence of bleeding that may have influenced the results from your study.
My second question relates to other factors that may have been in play during the time interval of this study. So I wondered, were there clinical pathways that were being implemented, either by your group or your nursing group within the hospital, which may also have resulted in decreased ICU stays, hospital length of stays and decreased costs?
Dr. A. Osama Gaber (Closing Discussion): I would like to thank all the discussants for their excellent points, and I will try to answer them grouped together.
Clearly, this is not a randomized study, and I think that the observation had to be made first that the choice of the technique influences the outcomes. I clearly believe that this is an issue that begs for randomization, and I think we will proceed to doing that.
We have a team of five surgeons who perform the procedure. Two of us were very enthusiastic about doing this new procedure in preparation for our living donor program; three of us were reluctant. Because two of us together always do the operations, the team sort of bargained ahead of time and decided whether or not we were going to do piggyback. But these were truly concurrent, so that the last patient in the piggyback and the last patient in the venovenous bypass occurred within, I think, a couple of weeks of each other. So it wasn’t where you staggered one technique then the other one. It’s just that gradually more and more people started doing the piggyback technique.
The definition of the anhepatic phase is also important. We counted anhepatic phase from the time the cava was clamped and the liver was removed to the time that liver was perfused, and I think that the difference may be related to the fact that when we used the venovenous bypass, we did the arterial anastomosis on the bypass. When we did the piggyback, we, of course, perfused after we had the portal vein and the cava attached and then did the arterial anastomosis. Despite these differences, however, there was a significant difference in all the physiological other factors—the body core temperature and so forth. I think these are clearly related to using the extracorporeal circulation. We cannot ignore that fact.
The other questions relate to whether there are some patients who should or shouldn’t have this procedure. Shifts in paradigm, basically, is what we have been talking about in surgical therapies, and I think the whole theme today is that you can’t totally say one procedure or one approach is bad and the other one is good. There are patients who are suited for one or for the other, and you have to be able to choose the procedure based on your findings.
Based on our current thinking, the size of the vein is important. We tried to avoid making an extra slit in the hepatic veins and avoided partial clamping of the cava, because we think that physiologically it would be easier, if we are going to do some of these reconstructions and some of the shunts that Dr. McDonald talked about, it would be better then to just go on bypass, take your time and do the standard operation. Clearly, there are patients that are going to have this happen, like Dr. Pruett said. The patient with the two or three tips on top of each other, is better off being on bypass, having the clamps really high up on the cava, fishing these things out. We have not done a piggyback operation on patients with Budd-Chiari syndrome, and clearly, it would be not very logical to think that when you already have clotting of those veins that you are going to use them for reanastomosis. What we are saying is in the majority of the patients, it is possible to do this procedure, and we think that it affects the outcome.
The incidence of previous upper abdominal surgery was 39% in the venovenous bypass group, 27% in the other group, and that was not different statistically. There was a tendency for it to be more in the venovenous bypass group. Since we have completed this study, we are almost now routinely using the piggyback technique for all of our patients, regardless of whether or not they have had previous upper abdominal surgery. I think that the results continue to be the same. The main thing—and what I told Dr. Shokouh-Amiri when he first started doing this—is that nobody should be on a mission to prove that one thing is better. The main thing is to choose the best procedure for that patient. That can only be done by sound clinical judgment in the operating room. You don’t want to prove that one procedure has an advantage over the other and harm the patients in the process of trying to prove that.
Footnotes
Correspondence: M. Hosein Shokouh-Amiri, MD, Dept. of Surgery, University of Tennessee–Memphis, 956 Court Ave., Suite C208, Memphis, TN 38125.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Reprints will not be available from the authors.
E-mail: HAMIRI@UTMEM.EDU
Accepted for publication December 1999.
References
- 1.Seabert EC, Belle SH, Beringer KC, Schivins JL, Detre KM. Liver transplantation in the United States from 1987–1998: updated results from the Pitt-UNOS Liver Transplant Registry. In: Cecka JM, Terasaki PI, eds. Clinical Transplants 1998. Los Angeles: UCLA Tissue Typing Laboratory; 1998. [PubMed]
- 2.Shaw BW, Martin DJ, Marquez JW, et al. Venous bypass in clinical liver transplantation. Ann Surg 1984; 200:524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulsen N, Valek TR, Blessing W, et al. Hemodynamics during liver transplantation with veno-venous bypass. Transplant Proc 1987; 21 (1):2417. [PubMed] [Google Scholar]
- 4.Khoury G, Martin MD, Mann M, et al. Air embolism associated with veno-venous bypass during orthotopic liver transplantation. Anesthesiology 1987; 67:848. [DOI] [PubMed] [Google Scholar]
- 5.Navalgund A, Kang Y, Sarner J, Jahr J, Gieraerts R. Massive pulmonary thromboembolism during liver transplantation. Anesth Analg 1988; 67:400. [PubMed] [Google Scholar]
- 6.Ellis J, Lichtor J, Feinstren S, et al. Right heart dysfunction, pulmonary embolism and paradoxical embolization during liver transplantation. Anesth Analg 1989; 68:777. [PubMed] [Google Scholar]
- 7.Cherqui D, Lauzet J-Y, Rotman N, et al. Orthotopic liver transplantation with preservation of the caval and portal flows: technique and results in 62 cases. Transplantation 1994; 58:793. [PubMed] [Google Scholar]
- 8.Jovine E, Mazziotti A, Grazi GL, et al. Piggyback versus conventional technique in liver transplantation: report of a randomized trial. Transplant Intl 1997; 10:109. [DOI] [PubMed] [Google Scholar]
- 9.Calne RY, Williams R. Liver transplantation in man: observations on technique and organization in 5 cases. Br Med J 1968; 4:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belghiti J, Panis Y, Sauvanet A, Gayet B, Fekete F. A new technique of side-to-side caval anastomosis during orthotopic hepatic transplantation without inferior vena caval clamping. Surg Gynecol Obstet 1992; 175:270. [PubMed] [Google Scholar]
- 11.Belghiti J, Noun R, Sauvanet A. Temporary portacaval anastomosis with preservation of caval flow during orthotopic liver transplantation. Am J Surg 1995; 169:277. [DOI] [PubMed] [Google Scholar]
- 12.Lerut JP, Molle G, Donataccio M, et al. Cavocaval liver transplantation without veno-venous bypass and without temporary portacaval shunting: the ideal technique for adult liver grafting? Transplant Intl 1997; 10:171. [DOI] [PubMed] [Google Scholar]
- 13.Tzakis AG, Reyes J, Nour B, et al. Temporary end-to-side portacaval shunt in orthotopic hepatic transplantation in humans. Surg Gynecol Obstet 1993; 176–180. [PMC free article] [PubMed]
- 14.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg 1989; 210:649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busque S, Esquivel CO, Concepcion W, So SKS. Experience with the piggyback technique without caval occlusion in adult orthotopic liver transplantation. Transplantation 1998; 65 (1):77–82. [DOI] [PubMed] [Google Scholar]
- 16.Showstack J, Katz PP, Lake JR, et al. Resource utilization in liver transplantation: effects of patient characteristics and clinical practice. JAMA 1999; 281:1381–1386. [DOI] [PubMed] [Google Scholar]
- 17.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Am Surg 1968; 168:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stieber AC, Gordon RD, Bassi N. A simple solution to a technical complication in “piggyback” liver transplantation. Transplantation 1997; 64:654–655. [DOI] [PubMed] [Google Scholar]
- 19.Navarro F, Le Moine M-C, Fabre J-M, et al. Specific vascular complications in orthotopic liver transplantation with preservation of the retrohepatic vena cava: review of 1361 cases. Transplantation 1999; 68:646. [DOI] [PubMed] [Google Scholar]
- 20.Parrilla P, Sanchez-Bueno F, Figueras J, et al. Analysis of the complications of the piggyback technique in 1,112 liver transplants. Transplantation 1999; 67 (9):1214–1217. [DOI] [PubMed] [Google Scholar]
- 21.Palomo Sanchez JC, Jimenez C, Moreno Gonzalez E, et al. Effects of intraoperative blood transfusion on postoperative complications and survival after orthotopic liver transplantation. Hepatogastroenterology 1998; 45 (22):1026—1033. [PubMed] [Google Scholar]