Abstract
Objective
To review the anatomical variations of the right lobe encountered in 40 living liver donors, describe the surgical management of these variations, and summarize the results of these procedures.
Summary Background Data
Anatomical variability is the rule rather than the exception in liver and biliary surgery. To make effective use of liver segments from living donors for transplantation, surgical techniques must be adapted to the anomalies.
Methods
Donor evaluation included celiac and mesenteric angiography with portal phase, magnetic resonance angiography, and intraoperative ultrasonography and cholangiography. Arterial anastomoses were generally between the donor right hepatic artery and the recipient main hepatic artery. Jump-grafts were constructed for recipients with hepatic artery thrombosis, and double donor arteries were joined to the bifurcation of the recipient hepatic artery. The branches of a trifurcated donor portal vein were isolated during the parenchymal transection, joined in a common cuff, and anastomosed to the recipient main portal vein. Significant accessory hepatic veins were preserved, brought together in a common cuff if multiple, and anastomosed to the recipient cava. The bile ducts were individually drained through a Roux-en-Y limb, and stents were placed in most patients.
Results
Forty right lobe liver transplants were performed between adults. No donor was excluded because of prohibitive anatomy. Seven recipients had a prior transplant and five had a transjugular intrahepatic portosystemic shunt (TIPS). Arterial anomalies were noted in six donors and portal anomalies in four. Arterial jump-grafts were required in three. Sixteen had at least one significant accessory hepatic vein, and one had a double right hepatic vein. There were no vascular complications. Multiple bile ducts were found in 27 donors. Biliary complications occurred in 33% of patients without stents and 4% with stents.
Conclusions
Anatomical variations of the right lobe can be accommodated without donor complications or complex reconstruction. Previous transplantation and TIPS do not significantly complicate right lobe transplantation. Microvascular arterial anastomosis is not necessary, and vascular complications should be infrequent. Biliary complications can be minimized with stenting.
The first living donor liver transplant using the right hepatic lobe was reported in 1994. 1 The decision to proceed with right lobectomy was forced by the intraoperative discovery of prohibitive left lobe vascular anatomy. This single report demonstrated both the feasibility of donor right lobectomy and some of the advantages of right lobe grafts. This technique was approached cautiously, however, and it has been applied more liberally only in the past 2 years. The primary motivation for the further development of this technique, and the main advantage of right lobe transplantation, is the larger size of the graft. There are more subtle features that make the use of right lobes attractive, however.
Anatomical variations of the liver vasculature and bile ducts are common, 2,3 and their recognition and management are critical in living donor liver transplantation. Replaced hepatic arteries are desirable anomalies and are easily managed. The anatomy of segment IV is particularly complex and variable, however, and it frequently complicates left lobe and lateral segment transplantation. 4,5 The right lobe can be resected without disturbing segment IV and without consideration of most of the variations. Although anomalies of the right lobe are also commonly encountered, a relatively limited number significantly complicate resection and transplantation.
We recently performed our fortieth living donor liver transplant using the right hepatic lobe. The general surgical technique for donor hepatectomy and the overall results of the first 25 procedures have been reported elsewhere. 6 The donor anatomical variations we have encountered and their surgical management are described in detail in this article. Some of the technical modifications dictated by recipient anatomy are also included.
METHODS
Assessment of Donor Anatomy
Right lobe mass was estimated before surgery with magnetic resonance imaging (MRI) and was corrected for the degree of steatosis as determined by percutaneous liver biopsy. Each percentage point of fat was assumed to decrease the functional right lobe mass by 1%. A corrected graft-to-recipient body weight ratio (GRBW) of 0.8% was the lowest accepted limit for right lobe transplantation. Right lobes were weighed after resection for calculation of the actual GRBW.
Celiac and mesenteric angiography with portal phase defined the vascular anatomy of both the graft and the remnant left lobe. Particular attention was paid to arterial and portal blood supply to segment IV arising from the right.
Magnetic resonance angiography and intraoperative ultrasonography were used to image the hepatic veins. Magnetic resonance angiography was primarily used to demonstrate the extrahepatic portions. Intraoperative ultrasonography mapped both the intrahepatic course of the midhepatic vein and the relation of the midhepatic vein to the right hepatic vein, defining an appropriate plane of transection. Accessory hepatic veins with significant flow were also identified with ultrasonography.
Preoperative magnetic resonance cholangiography identified major biliary anomalies and excluded extrahepatic biliary pathology. Intraoperative cholangiography performed though the cystic duct demonstrated biliary anatomy in detail.
Donor Hepatectomy
Many of the details of the surgical technique have been previously reported. 6 Intraoperative cholangiography and ultrasonography preceded dissection of the vascular and biliary structures. The right hepatic artery was first identified in its usual course behind the common bile duct. It was approached only from the right to minimize the chance of devascularization of the duct. When two main arteries to the right lobe were present, one could usually be identified in front and the other behind the common bile duct. No attempt was made to trace these vessels to their origin, and division was to the right of the common bile duct. If inflow to segment IV arose predominantly from the right, dissection of the right hepatic artery was carried only as far as the branch to segment IV, and the right hepatic artery was divided distal to it. If arterial inflow to segment IV arose either from the left hepatic artery or from both the left and right hepatic artery, dissection was carried to the bifurcation of the main hepatic artery. Likewise, when the primary source of portal flow to segment IV originated on the right, dissection was carried only to this branch. If it arose from the left, dissection was carried to the bifurcation and was extended to expose a portion of the left portal vein. Access to the left portal vein and the bifurcation facilitated clamping and assessment of the inflow to the remnant left lobe. 6 In the case of trifurcation of the portal vein (Fig. 1), isolation of the branches was not possible until the portal vein was exposed by transection of the parenchyma and the hilar plate (unroofing of the portal vein). No attempt to obtain a common trunk was made by dividing too near the bifurcation. Inflow to the remnant left lobe was verified with a Doppler probe before division of the portal branch. Sufficient length was left so the orifice could be closed without encroaching on the main or left portal branches. Heparin (40 U/kg) was administered to donors before clamping and division of the vasculature.

Figure 1. A portal phase angiographic image from a donor with trifurcation of the portal vein. The two branches to the right lobe can be seen.
The technique for isolating the right hepatic vein has been described elsewhere. 6 When two right hepatic veins were present, thorough intraoperative ultrasonography was necessary to identify the plane between the most medial right hepatic vein and the midhepatic vein. Isolation of the individual branches was simplified by exposure of the inferior vena cava (IVC) after the parenchymal transection. Accessory veins were preserved if their diameter was greater than 5 mm or if they appeared significant by ultrasonography.
The bile ducts draining the right lobe were identified by cholangiography. The ducts were divided after transection of the anterior two thirds of the parenchyma. The course of the transection of the last one third of the parenchyma was adjusted to protect the ducts in their intraparenchymal course and to leave sufficient tissue around them. If more than one duct was identified, the plane of transection was determined by the most superior duct. 6 Division was far enough from the common duct to prevent narrowing with closure of the orifice. In all cases, only sharp dissection was used near the ducts, and activity near the common bile duct was avoided.
Recipient Surgery
The recipient surgery consisted of a total hepatectomy with preservation of the IVC. Total venovenous bypass was used in all cases.
If multiple accessory hepatic veins were preserved, a common cuff was constructed from the individual veins at the back table when technically possible. Exclusion of the retrohepatic vena cava with vascular clamps facilitated venous anastomosis. The donor right hepatic vein was anastomosed to the orifice of the recipient right hepatic vein. Double right hepatic veins were joined in a common trunk and anastomosed to the orifice of the recipient right hepatic vein as well. If the diameter of the orifice of the recipient right hepatic vein was inadequate, it was enlarged by creating a slit. Accessory veins were anastomosed directly to the IVC in an end-to-side fashion while the cava was still clamped (Fig. 2).

Figure 2. The inferior vena cava and right lobe are depicted. The inferior vena cava is clamped above and below the orifice of the right hepatic vein and the sites of anastomosis of the accessory hepatic veins. A bloodless field is created without interfering with construction of the anastomosis.
The donor right portal branch was generally anastomosed to the recipient main portal branch unless, because of significant size mismatch, the right portal branch was more appropriate. Double donor portal venous branches were joined to form a common trunk and anastomosed to the recipient main portal vein. Sufficient length of the main portal vein was salvaged from patients with transjugular intrahepatic portosystemic shunts (TIPS).
Arterial anastomosis was performed under 2.5× loupe magnification using interrupted 7–0 prolene suture. Microvascular anastomosis was not performed in any recipient. Anastomoses were constructed between the donor right hepatic artery and the recipient main hepatic artery unless inflow from the recipient vessel was compromised. The bifurcation of the main hepatic artery served as the site of anastomosis when there were double donor arteries. Jump-grafts between the donor hepatic artery and the recipient aorta were constructed from recipient saphenous vein in patients undergoing retransplantation for hepatic artery thrombosis.
The bile ducts were individually drained through a retrocolic Roux-en-Y limb. In most patients, the main duct was stented and externally drained with a Turcotte catheter, and minor ducts were internally stented with modified Turcotte catheters 6 (Fig. 3). Turcotte catheters were generally left in place for 4 months after transplantation.

Figure 3. The placement of biliary stents is depicted. A Turcotte catheter is placed in the main bile duct and externally drained. The distal end of the Turcotte catheter is used to stent the minor bile ducts internally.
Follow-Up
Recipients underwent elective ultrasonography on postoperative days 1, 2, 3, and 7. Donors and recipients underwent elective MRI on postoperative days 7, 14, 30, 60, and 180 to assess liver regeneration.
RESULTS
Between March 1998 and September 1999, 135 potential donors were evaluated. Of these, 40 underwent right lobectomy. Only 42% of the potential donors were still candidates after preliminary noninvasive testing; most were excluded based on blood type or serology. One fourth of the potential donors who were not excluded based on noninvasive testing were excluded because of steatosis on liver biopsy that resulted in a corrected GRBW of less than 0.8%. Only one potential donor was excluded based on angiographic findings (incidental celiac trunk stenosis). No donor was turned down because of cholangiographic or intraoperative findings. The mean follow-up of the series was 281 ± 134 days (range 10–535).
Donors
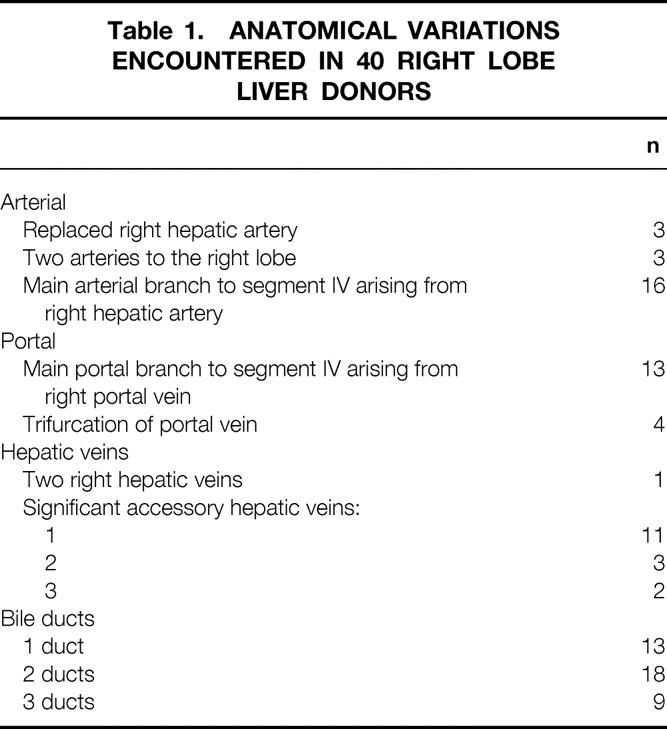
Mean age was 37 ± 10.9 years (range 21–55) and body weight was 78 ± 14.8 kg (range 55–124). Mean intraoperative blood loss was 652 ± 525 mL (range 221–1,900), with no banked blood products given to any of the 40 donors during or after surgery. Operative time averaged 7.1 ± 1.2 hours (range 5.9–12.1), and the average right lobe mass obtained was 865 ± 159 g (range 610–1250). Only seven donors required an overnight stay in the intensive care unit. Complications in five patients included pressure sores in three, atelectasis in two, phlebitis in one, and prolonged ileus in one. Mean hospital stay was 5.1 ± 1.5 days (range 3–10), with no hospital readmissions. The viability and regeneration of segment IV were verified by MRI in all donors. There have been no late complications to date. Table 1 summarizes the donor anatomical variations encountered. Management was previously described.
Table 1. ANATOMICAL VARIATIONS ENCOUNTERED IN 40 RIGHT LOBE LIVER DONORS
Recipients
The cause of liver disease was hepatitis C in 22, alcohol in 11, hepatitis B in 2, and hepatocellular carcinoma in 2, among others. Three of the seven recipients who had a prior cadaveric liver transplant required retransplantation because of late hepatic artery thrombosis, two of the seven for chronic rejection, one for long-term recurrent hepatitis C, and one for recurrent sclerosing cholangitis. Five recipients had a TIPS. UNOS status at transplant was III for 6 recipients, IIB for 26, IIA for 6, and I for 2. Mean age at transplant was 49 ± 14 years (range 19–67), and body weight was 84 ± 18.7 kg (range 51–140). Cold ischemic time was 1 hour 2 minutes ± 22 minutes (range 36 minutes to 1 hour 34 minutes), and warm ischemic time was 35 ± 21 minutes (range 21–48). The average corrected GRBW ratio was 1.1 ± 0.21% (range 0.62–1.7%). The average initial hospital stay was 11.2 ± 8.2 days (range 6–56), the readmission rate was 0.9 ± 0.9% (range 0–5%), and the total hospital stay was 22 ± 22 days (range 6–84). There were no instances of primary nonfunction or delayed graft function.
Biliary complications occurred in six recipients, sepsis in seven, upper gastrointestinal bleeding in three, and seizures in two. Biliary stents were placed in all but the first 12 recipients at the time of transplantation. Biliary complications occurred only in the first group of 15 recipients. There were no primary vascular complications in any of the 40 recipients. Thrombosis of the hepatic vein secondary to compression from an iatrogenic subcapsular hematoma occurred in one patient; this graft was ultimately lost, and this case has been described elsewhere. 7 Graft survival was 85%, and patient survival was 87%.
Four of the five recipients of grafts from living donors who died were status IIA at transplant. The immediate cause of death was overwhelming sepsis in all status IIA recipients who died. Vancomycin-resistant Enterococcus was a causative organism in all four, and Aspergillus was isolated in three of the four as well. One recipient died of complications associated with multiple myeloma. All grafts were functional at the time of recipient death at 33, 65, 73, 162, and 181 days after transplant.
DISCUSSION
Anastomosis of the right hepatic and accessory hepatic veins was facilitated by excluding a segment of the IVC with vascular clamps. Expansion of the orifice of the right hepatic vein (slitting of the cava) was often necessary and would have been difficult with a clamp in place. Side-biting or standard vascular clamps placed close to the site of anastomosis also tend to distort the orifice and alter the perceived length of the vessel. Many grafts had more than one accessory hepatic vein. Individual anastomosis of each was avoided by first bringing those in close proximity together in a common cuff at the back table. Warm ischemic time was not significantly prolonged, and significant flow through these vessels was observed both during surgery and on follow-up ultrasonography. The accessory veins recover some of the outflow sacrificed by leaving the midhepatic vein with the donor.
In contrast to the experience with left lateral and left lobe grafts, 8 interposition grafts and branch patches were not necessary for portal reconstruction. In every case, the diameter of either the recipient main portal trunk or right branch was well matched to the donor. The placement of the right lobe in its natural anatomical position also brings the vasculature of the graft in close proximity to that of the recipient, and even relatively short vessels are adequate. In all recipients with a TIPS in place (Fig. 4), it was possible to salvage at least a short segment of the portal vein, and jump-grafts were not necessary.
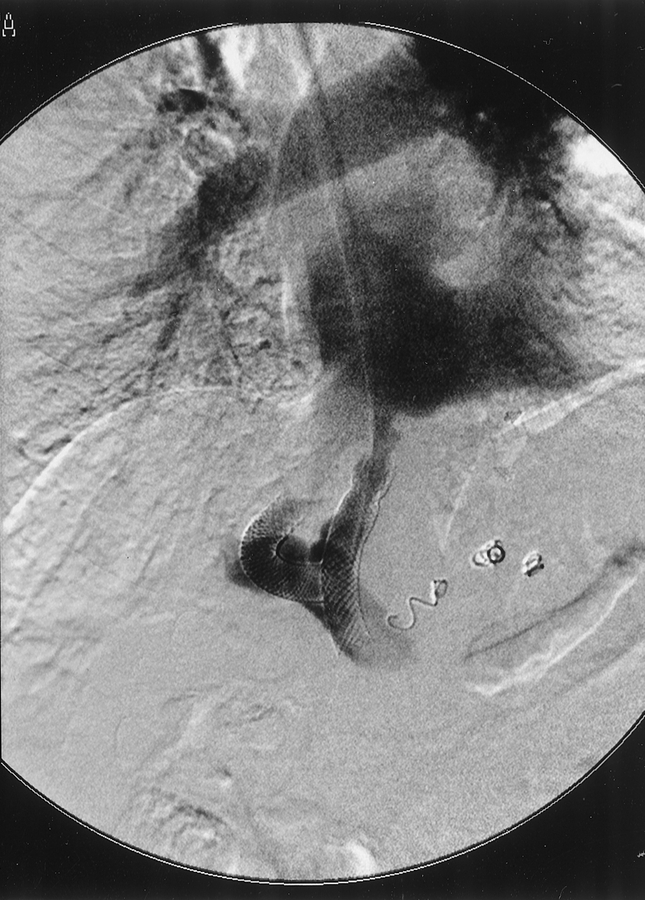
Figure 4. A portal phase angiographic image from a patient with a transjugular intrahepatic portosystemic shunt (TIPS) in place. The distal end of the TIPS can be seen near the superior mesenteric vein. The portal vein was salvaged from this patient.
In our experience, anastomosis of the hepatic artery was relatively straightforward, and there were no complications. Acute arterial thrombosis was a relatively frequent complication of left lobe and left lateral segment living donor transplantation until microvascular anastomosis was introduced. 9,10 Arterial anastomosis was aided only by loupe magnification in all cases in this series. The contrasting complication rate can be attributed only to the larger diameter of the vasculature supplying the right lobe. The diameter of the artery was greater than 3 mm in every patient, the size at which microvascular anastomosis has been recommended. 10 The technical difficulty associated with microscopic reconstruction can be avoided with right lobe transplantation without an increased risk of vascular complications.
Biliary reconstruction has proven to be the most challenging part of the recipient surgery. Early in this series, biliary complications occurred at a rate similar to that observed with left lateral segment and left lobe grafts. 11,12 Biloma secondary to leakage from the cut edge was most commonly observed, but stricture and anastomotic leak also occurred. Bile leaks from the cut edge were probably attributable to back-pressure caused by transient edema at the anastomosis. Turcotte catheters positioned across the anastomoses dramatically decreased the incidence of this type of complication, and no problems were associated with their removal. Multiple ducts were quite common, and even when a single duct was present, its division at a safe distance from the donor common bile duct often resulted in the creation of two ducts. Although the diameter of these ducts was often narrow, modified Turcotte catheters were placed without significant difficulty.
In summary, our experience with this technique demonstrates the advantages of the use of right lobe over left lobe grafts. More patients can benefit from right lobe transplantation because of the larger size of the graft. The surgical management of both donor and recipient anatomical variations is considerably simpler than with left-sided grafts, and it appears that few if any anomalies will be technically prohibitive.
Discussion
Dr. Richard J. Howard (Gainesville, Florida): This elegant paper is well worthy of note. There are currently over 14,500 patients on the waiting list for a liver transplant. Dr. Marcos showed 12,500. That was at the end of 1998; now there are 14,500. Last year, there were about 4,500 liver transplants done. So there is a tremendous disparity between need and supply. And in 1998, over 1400 patients died on the waiting list, waiting for a transplant.
So as with kidney transplantation, living donors are the most viable way of increasing the donor supply for the foreseeable future. The waiting list is increasing at 15% a year. The number of cadaveric donors is increasing 3% to 5% a year, so every year we are getting further and further behind. But there can’t be a learning curve for living donor liver transplantation, because the consequences of an ill-trained or untrained surgeon are so great. It’s estimated that for living kidney transplantation, the mortality rate is about three out of 10,000—probably acceptable. But of the estimated 1,000 living liver donor transplants that have been done, there are already at least two reported deaths, and we don’t know how many nonreported deaths. But the mortality rate has to be higher, and it has to be more of the procedures.
Many programs will be driven to—and I use that in the best of terms—to doing living donor transplantation in this country. We anticipate doing that in the coming year ourselves, just because of the growing waiting list and the increasing number of deaths in transplantation.
The American Society of Transplant Surgeons has recently circulated for comment a position paper dealing with living donor liver transplantation, in which they advise that each program have an oversight committee to review the results of living donor donation on an ongoing basis. I’d like to ask the authors whether they have such a committee. The proposal by the ASTS also suggests having a living donor program approved by the institutional review board.
Another question for the authors is, has it been difficult to get insurance companies to pay for living donor procedures? What have they done to try to get reluctant insurance companies to pay for that?
They exclude donors with too much steatosis, but what do they mean by too much? What percentage of steatosis would exclude a donor?
Finally, do they ever have difficulty with a sufficient length of portal vein in recipients who either have a clotted portal vein or who have tips down into the portal vein where extraction occasionally means having to resect part of the portal vein? Is that ever an issue?
Dr. Timothy Lane Pruett (Charlottesville, Virginia): This is a remarkable approach on how to deal with a significant problem. The Japanese have been doing it for a while. It started off with kids, but now it is clear that the population in greatest need of organs that don’t become available are adults, and it’s going to become an increasing part on all of our hands to deal with this.
When we started doing it, we started noting—and I’m just going to ask Amadeo for a few clarifications—that there were lots of accessory veins that come through. Professor Tanaka talks about an accessory hepatic vein coming into the cava of greater than a centimeter, which are the ones you should preserve. I would ask, first of all, what is the size that you use for reimplantation? Is it of equal importance that there are a host of veins that come out of segment 5 and 8 and head over into the middle hepatic vein, some of which are of substantial size? Do you ever reimplant any of the crossing veins that go out of the right lobe and into the middle hepatic vein? Do you use conduits with saphenous vein and the like?
Is there ever a time when you would use the left lobe for an adult? Basically, this is one of the issues that we are going to grapple with as we have larger and larger potential donors and smaller and smaller recipients, usually a son to a mother. Is there a time when you envision that the left lobe would be the safer of the lobes and give you the adequate amount of volume? Clearly, you mentioned for the technical factors that the right lobe is actually easier to use, but there may become a time when the left is the preferential donor source.
I would like to reiterate Dr. Howard’s question about the steatosis. We have been plagued with that as well, as to how much fat is enough. Do you routinely biopsy all of your patients, particularly folks who come from families with viral hepatitis, even with negative serologies? Our exclusion rate is actually a bit higher than yours when one excludes the ABO incompatibility. It has been surprising how many people who are potential donors will be excluded for a variety of reasons, most of which have to do with hepatitis.
In any case, I think your work is a tremendous contribution to the advancement for the care of a very significant and growing population of people in this country, and I look forward to continuing to hear from you.
Dr. A. Osama Gaber (Memphis, Tennessee): I’d like to congratulate Dr. Marcos for an excellent series. Living donor liver transplantation using the right lobe has been popularized in this country, actually, by his group, and they do set the standard that we will all have to follow in terms of results and outcomes.
I want to say that the importance of presenting the work and the solution that they have found for difficult problems cannot be understated.
Let me start first by a caution. I’m just going to echo what has been said before, that the magnitude of this operation for the donor cannot be justified by the recipient’s need. An environment like that described by Dr. Marcos, where the technical ability of the surgical team and the care and the thought put into this process results in consistent success with the donor surgery, is the only environment in which this operation can be done.
So it is very important to recognize that we are trying to help the recipients, but we cannot have other than perfection for the donor. I very much appreciate what he has said about every decision, in everything that has to be cut, you bias it to the recipient’s disadvantage. That is very, very important, because you are very tempted during the surgery not to do that. Actually, we have two surgeons working together; one of them represents the recipient, and the other represents the donor. And I tell you that the buck stops with the donor surgeon, because he is the one who says, no, we are not going to go there, and that’s what needs to happen.
Second, I want to ask Dr. Marcos a question. Clearly he is setting the standard, and what he presents, other people are going to try to do. If I look at his picture and I look at his description, all of those tiny little veins behind the cava, which he tries so much to save and anastomose, in our experience has not been necessary. I think that there is an accessory right hepatic vein that one would save, but all of these small veins that he shows, we call them the Virginia veins in our transplant program. We don’t attempt to save them at all. Our experience with the piggyback technique has shown that they are not important to save. I think this is important—it’s going to make it easier on a whole lot of other people to do this procedure if he can either produce some data to us that shows that it is important to save them, or he stops saving them, because it’s going to make the operation a lot easier.
Thirdly, I just want to show him one thing that we have done recently that he may find very interesting. [Slide] We have done all of our procedures without using venovenous bypass, and in the last two cases, we actually anastomosed the common bile duct primarily. We found that if you dissect the hepatic artery in the recipient exactly like you were to do in the donor, leaving all the connections between the common bile duct and the hepatic artery intact, then you can do a primary duct-to-duct anastomosis, and this is an ERCP of one of our recipients 2 weeks later. You can see the primary duct-to-duct anastomosis working very, very well.
He has stressed the correct point, that you have to leave enough viable liver tissue around the bile duct to be able to have the bile duct survive, and we think that if you deal with the recipient operation in the same way that you would deal with the donor operation, by making sure that the vascularization of the bile duct is intact, we may actually avoid some of the Roux-en-Ys, particularly in the patients who have a single bile duct.
Dr. J. Michael Henderson (Cleveland, Ohio): The MCV group needs enormous credit for getting adult living donors up and running in this country. What you have heard today is a very important contribution.
The emphasis today has clearly been on some of the anatomical variations. As you start in this business, you learn that this is important: a clear definition of the anatomy, of understanding just where the vessels are before you operate of what you are going to need to save, where you’re going to divide the vessels.
Questions I would focus on relate to:
You describe three ways of looking at the arterial venous anatomy: angiography, MRI, and intraoperative ultrasound. As you have gained the experience, do you think you will continue to need to use all three, or will there be a way of simplifying this?
The second question relates to biliary imaging. Intraoperative cholangiography has become your standard. You are not using ERCP. Are you seeing any situations in which you do want to have preoperative knowledge? For example, if your donor has had a lap-chole, are you still doing intraoperative cholangiography?
In your donors, you have been doing follow-up MRIs. Are you doing MR cholangiography in your donors? I think it’s important for information to be published as to what the donor data are showing. I understand you have follow-up to 6 months in that group of patients. I’d be interested to know what that shows.
Dr. Amadeo Marcos (Closing Discussion): Thank you for all those comments. I want to address them one by one. First, Dr. Howard’s experience and insight into living donors are very well taken and appropriate.
We submitted the protocol for follow-up of donors and recipients through an IRB and got approval. We have a transplant committee that oversees the evolution of these patients. Whenever there is a question about the donor work-up, we bring it to our committee, which has representatives outside the transplant division. It is very important what the ASTS is suggesting, and I have had the good fortune to be part of that committee.
There is no institution in America with the muscle to control which centers should do living donors. The individual centers and chairs are critically important in the decision to embark on this procedure. A committee within each center should be assigned to consider all the issues and come to a decision.
Insurance was a tough battle. Besides its medical contribution, MCV fought, one by one, those insurance companies that were still saying that this was an experimental procedure and shouldn’t be covered. At the end, even Medicare covered living donors and accepted it. As for those companies that refused, we encouraged the patients to go and sue them. They did, and we provided some of our medical information. At the end, we do not have an insurance company that does not cover a living donor in Virginia, and we are actually working with the VA to have them cover living donors through the federal government.
How much steatosis? We don’t have a predetermined amount of fat. We do the liver biopsy on all donors, and then subtract the amount of fat from the calculated mass. If the corrected mass gives a GRBW less than 0.8, then we exclude the donor. So if the donor is smaller than the recipient, as little as 5% of fat can exclude them. If the donor is bigger than the recipient, 30% fat may be acceptable—those grafts actually worked pretty well. So fat does not mean what it usually means for cadaveric organs and we do not, therefore, have a predetermined amount of fat. We subtract it from the calculated mass.
About the portal vein size and length, although we had a lot of TIPS in our series, we have not needed any jump grafts. That is one of the main advantages of this operation, and actually, the recipient operation is very enjoyable because all the vessels are sitting right there in front. So we have not used jump grafts.
On Dr. Pruett’s comments, we consider anything above 5 mm to be significant. Right lobes have outflow limitation, and that’s the main principle behind extended right lobectomy that comes from Hong Kong. I think it is a very dangerous operation to do in donors. The price to pay, I think, is to keep these vessels open. Sewing them to the cava does not add a lot to warm ischemia. We follow those veins on ultrasounds and they work. I think it is very important in the first 2 weeks, while the livers are regenerating, to keep a good outflow.
Right or left, with an American, is usually not a problem because most of our recipients need a right lobe for adequate mass, though there will be some recipients who can benefit from a left lobe. I think right lobectomy is easier and safer to do on a donor than a left lobectomy. The disadvantage of a right lobectomy for the donor will be that you are leaving behind less mass, about 40% of liver mass, as compared to when you do a left lobectomy, when you leave behind 60% of mass. But if you preserve segment 4 as I showed today, then you will be all right with the right lobe. The right lobe is easier to resect and I think it has less anatomical variance, especially the right hepatic vein. The right hepatic vein is usually single whereas there are always two veins with the left lobe.
Dr. Gaber, I strongly agree with you about exactly what donor safety is, and one of the main goals of this operation is that the donor comes first. You should go to surgery thinking that you might find something that you don’t like, and you might have to stop the operation rather than go ahead with the hepatectomy.
Again, my comments on the access remains the same. I do not favor duct-to-duct anastomosis of the bile ducts, and I believe that the Roux-en-Y provides extraarterial inflow to the duct. The inflow may be compromised when you do a right lobectomy, even when you take all precautions. So I’d rather have my duct to a Roux-en-Y. We also find a lot of multiple ducts. Of course, you lose the advantage of access through ERCP, but if you use of Turcotte catheters, you can still do cholangiograms. Also, we leave the Roux-en-Y with a loop of bowel close to the fascia, so that you can explore and instrument it in the OR if needed.
Dr. Henderson’s comments: we have done angio, MRI, and ultrasound as part of the work-up and we are still doing the same tests. I think these tests give complementary rather than repetitive information. The details of segment 4 cannot be seen by MRA, so angio will have to stay.
I haven’t considered substituting ERCP for cholangiography because of the risk involved and the low rate of exclusion for biliary anomalies.
We do MRIs, MRAs, and MRCs on donors and recipients with the intention of diagnosing any problem with the bile ducts before it becomes clinically significant. We have not seen any problems, but we do follow them with MR.
Footnotes
Correspondence: Amadeo Marcos, MD, Living Donor Liver Transplant Program, Division of Transplantation, Medical College of Virginia, P.O. Box 980057, Richmond, VA 23298-0057.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
E-mail: amarcos@hsc.vcu.edu
Accepted for publication December 1999.
References
- 1.Yamaoka Y, Washida M, Honda K, et al. Liver transplantation using a right lobe graft from a living related donor. Transplantation 1994; 57:1127–1141. [PubMed] [Google Scholar]
- 2.Huang TL, Cheng YF, Chen CL, Chen TY, Lee TY. Variants of the bile ducts: clinical application in the potential donor of living-related hepatic transplantation. Transplant Proc 1996; 28:1669. [PubMed] [Google Scholar]
- 3.Couinaud C. The Surgical Anatomy of the Liver Revisited. Paris: Maugein & Cie; 1989:84–89, 96–101, 108–117.
- 4.Couinaud C. A “scandal”: segment IV and liver transplantation. J Chir 1993; 130 (11):443–446. [PubMed] [Google Scholar]
- 5.Krupski G, Rogiers X, Nicolas V, et al. The significance of the arterial vascular supply of segment IV in living liver donation. Rofo Fortschr 1997; 167 (1):32–36. [DOI] [PubMed] [Google Scholar]
- 6.Marcos A, Fisher R, Ham J, et al. Right lobe living donor liver transplantation. Transplantation 1999; 68:798–803. [DOI] [PubMed] [Google Scholar]
- 7.Marcos A, Fisher R, Ham J, et al. Emergency portacaval shunt for control of hemorrhage from a parenchymal fracture following adult-to-adult living donor liver transplantation. Transplantation 2000 (in press). [DOI] [PubMed]
- 8.Saad S, Tanaka K, Inomata Y, et al. Portal vein reconstruction in pediatric liver transplantation from living donors. Ann Surg 1998; 227:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatano E, Terajima H, Yabe S, et al. Hepatic artery thrombosis in living related liver transplantation. Transplantation 1997; 64:1443–1446. [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Nagata I, Yamagata S, et al. The introduction of microvascular surgery to hepatic artery reconstruction in living donor liver transplantation: its surgical advantages compared with conventional procedures. Transplantation 1992; 54:263–268. [DOI] [PubMed] [Google Scholar]
- 11.Egawa H, Uemoto S, Inomata Y, et al. Biliary complications in pediatric living related liver transplantation. Surgery 1998; 124:901–910. [PubMed] [Google Scholar]
- 12.Cronin D, Alonso E, Piper J, et al. Biliary complications in living donor liver transplantation. Transplant Proc 1997; 29:419–420. [DOI] [PubMed] [Google Scholar]


