Abstract
Objective
To determine the suitability of a single-layer continuous technique for intestinal anastomosis in a surgical training program.
Summary Background Data
Several recent reports have advocated the use of a continuous single-layer technique for intestinal anastomosis. Purported advantages include shorter time for construction, lower cost, and perhaps a lower rate of anastomotic leakage. The authors hypothesized that the single-layer continuous anastomosis could be safely introduced into a surgical training program and that it could be performed in less time and at a lower cost than the two-layer interrupted anastomosis.
Methods
The study was conducted during a 3-year period ending September 1999. All adult patients requiring intestinal anastomosis were considered eligible. Patients who required anastomosis to the stomach, duodenum, and rectum were excluded. Patients were also excluded if the surgeon did not believe either technique could be used. Patients were randomly assigned to one- or two-layer techniques. Single-layer anastomoses were performed with a continuous 3–0 polypropylene suture. Two-layer anastomoses were constructed using interrupted 3–0 silk Lembert sutures for the outer layer and a continuous 3–0 polyglycolic acid suture for the inner layer. The time for anastomosis began with the placement of the first stitch and ended when the last stitch was cut. Anastomotic leak was defined as radiographic demonstration of a fistula or nonabsorbable material draining from a wound after oral administration, or visible disruption of the suture line during reexploration.
Results
Sixty-five single-layer and 67 two-layer anastomoses were performed. The groups were evenly matched according to age, sex, diagnosis, and location of the anastomosis. Two leaks (3.1%) occurred in the single-layer group and one (1.5%) in the two-layer group. Two abscesses (3.0%) occurred in each group. A mean of 20.8 minutes was required to construct a single-layer anastomosis versus 30.7 minutes for the two-layer technique. Mean length of stay was 7.9 days for single-layer patients and 9.9 days for two-layer patients; this difference did not quite reach statistical significance. Cost of materials was $4.61 for the single-layer technique and $35.38 for the two-layer method.
Conclusions
A single-layer continuous anastomosis can be constructed in significantly less time and with a similar rate of complications compared with the two-layer technique. It also costs less than any other method and can be incorporated into a surgical training program without a significant increase in complications.
Intestinal anastomosis has been successfully performed for more than 150 years using a variety of techniques, materials, and devices. Of these, the method that has proven successful in most situations and in the hands of most surgeons has been the two-layer anastomosis using interrupted silk sutures for an outer inverted seromuscular layer and a running absorbable suture for a transmural inner layer. The only appreciable shortcoming of the two-layer technique is that it is somewhat tedious and time-consuming to perform. Recently, several reports have appeared advocating a single-layer continuous anastomosis using monofilament plastic suture. 1–11 This anastomosis requires less time to fashion, costs less than any other method, and may have a lower risk of leakage. Because of these potential advantages, we hypothesized that the single-layer technique could be introduced into our surgical teaching program with no increase in anastomotic failure and that it could be performed in less time than the two-layer technique.
METHODS
All adult patients requiring intestinal anastomosis at Denver Health Medical Center from September 1996 to September 1999 were considered eligible. Patients requiring anastomosis to the rectum or third or fourth portion of the duodenum were excluded because the single-layer continuous method is awkward when both ends of the bowel cannot be fully mobilized. Patients requiring gastrointestinal anastomosis were excluded because of the presumably increased risk of hemorrhage from the stomach. Patients were also excluded if the surgeon did not believe that either technique could be used because of technical concerns such as edema or scarring.
Patients were assigned to either the one- or two-layer technique in a prospective, randomized fashion. Randomization was performed using random permuted blocks of size 10. Opaque, sealed envelopes indicating the technique to be used were placed in the operating room and were drawn sequentially when the need for intestinal anastomosis became apparent. The study was approved by the Colorado Multiple Institutional Review Board, which also granted a waiver of consent because both methods were in common use in our institution and had similar results.
All two-layer anastomoses were constructed using interrupted 3–0 silk Lembert sutures for the outer layer and a running 3–0 polyglycolic acid suture for the transmural inner layer. All single-layer anastomoses were constructed using a continuous 3–0 polypropylene double-needle suture that began at the mesenteric border (Fig. 1). All layers of the bowel wall except the mucosa were incorporated. Each bite included 4 to 6 mm of the seromuscular wall; the larger bites were used at the mesenteric border to ensure an adequate seal. Each stitch was advanced approximately 5 mm. To avoid ischemia of the anastomosis, the surgeon had to ensure that only enough pressure was applied to the suture while following to approximate the ends of the bowel and render the anastomosis watertight. The time recorded for construction of the anastomosis began with the placement of the first stitch and ended with cutting the excess material from the last stitch.
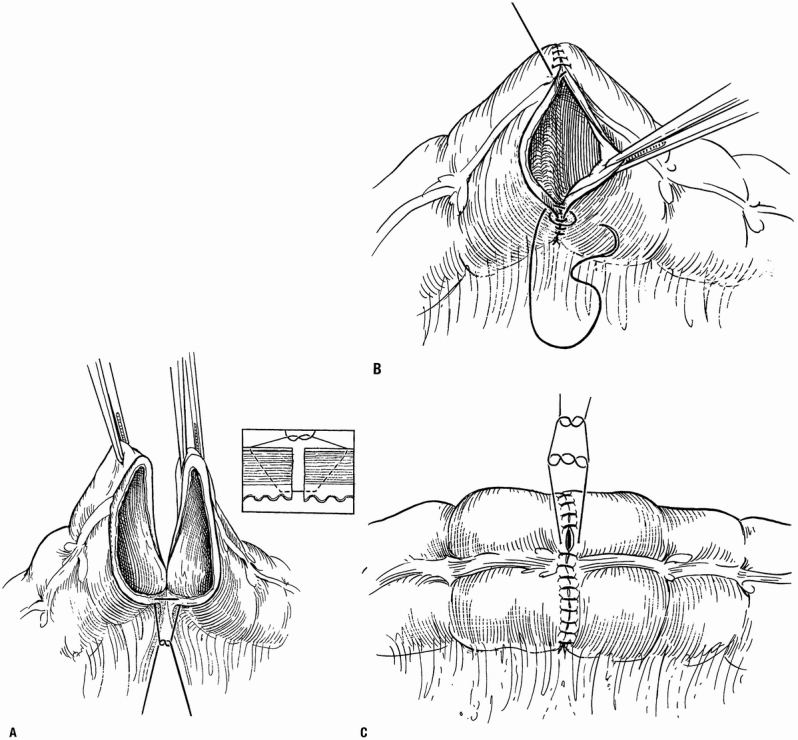
Figure 1. (A) The one-layer continuous anastomosis is begun at the mesenteric border using a 3–0 double-needle suture. The stitches include all layers except the mucosa. (From Burch JM. Injury to the colon and rectum. In: Mattox KL, Feliciano DV, Moore EE, eds. Trauma. New York: McGraw-Hill; 2000, reproduced with permission.) (B) Stitches are placed 4 to 6 mm from the edge of the bowel and are advanced about 5 mm with each stitch. Both limbs of the suture are carried away from the mesenteric border; this ensures that suture placement at the mesenteric border is clearly visualized and precise. (C) The suture is tied at the antimesenteric border. Care must be taken to avoid a purse-string effect.
Anastomotic failure was defined as a fistula documented radiographically or by the finding of a nonabsorbable material (charcoal) draining from the wound after oral administration, or a visible disruption of the suture line during reexploration. The complication of abscess without fistula was also included in the analysis because it is potentially related to the anastomosis.
Calculations for the outcome variables of leak, abscess, and time for construction of the anastomosis used the number of anastomoses in each group for the denominator. Calculations of the length of stay used the number of patients for the denominator. Calculations of the cost of materials were based on the actual hospital costs for the suture material and the standardized utilization of suture material by the surgeons. For two-layer anastomoses, three packets of 3–0 silk sutures, each containing five needled sutures, and two packets of 3–0 polyglycolic acid sutures were used. Each packet of silk sutures cost $9.41, and each packet of polyglycolic acid suture cost $1.88. For single-layer anastomoses, one package of double-needled 3–0 polypropylene suture was used. Each packet of polypropylene suture cost $4.61. All procedures were performed by postgraduate year 3 to 5 residents from the University of Colorado Health Sciences Center assisted by an attending surgeon, or by the attending surgeons themselves.
Data were analyzed based on the intention-to-treat principle. Continuous data were analyzed using the Student t test. The Fisher exact test and the Pearson chi-square test were used to analyze categorical data. P < .05 was considered to indicate statistical significance. All data analysis was performed on an IBM-compatible PC using SPSS 10.0 for Windows (SPSS Inc., Chicago).
RESULTS
One hundred twenty-five patients were enrolled in the study. Fifty-nine patients were randomized to single-layer anastomoses and 66 to two-layer anastomoses. For six patients who required more than one anastomosis, the same method was used for each anastomosis. This resulted in 65 single-layer continuous and 67 two-layer interrupted anastomoses. The groups were evenly matched by age, sex, diagnosis, and location of the anastomosis (Table 1). Anastomotic leaks, intraabdominal abscesses, length of time required for anastomosis, length of stay, and material costs are shown for both groups in Table 2. Four patients in the study died, but no deaths were related to the anastomosis. Of the three patients in whom fistulas developed, one patient had abdominal carcinomatosis; one had diffuse peritonitis from an iatrogenic injury of the cecum that occurred during a radical resection of a gynecologic malignancy; and the third patient had been treated with an abbreviated laparotomy for trauma and was undergoing a planned reoperation.
Table 1. COMPARISON OF STUDY GROUPS
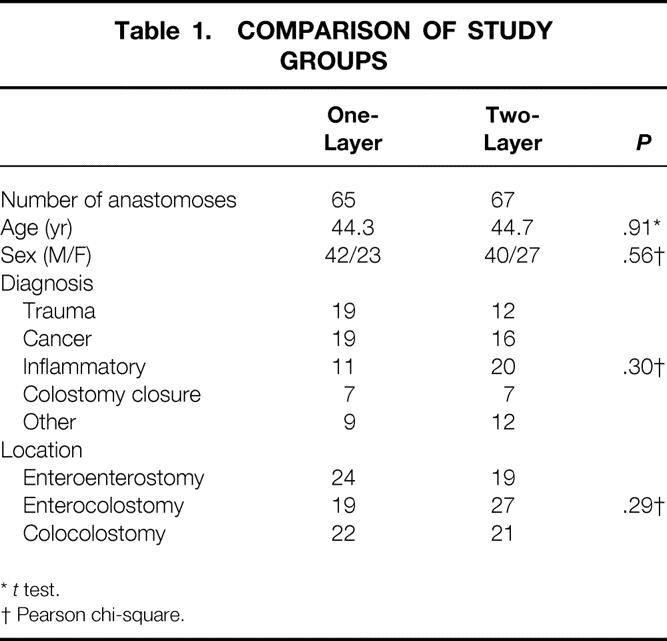
*t test.
† Pearson chi-square.
Table 2. OUTCOME VARIABLES FOR THE STUDY GROUPS
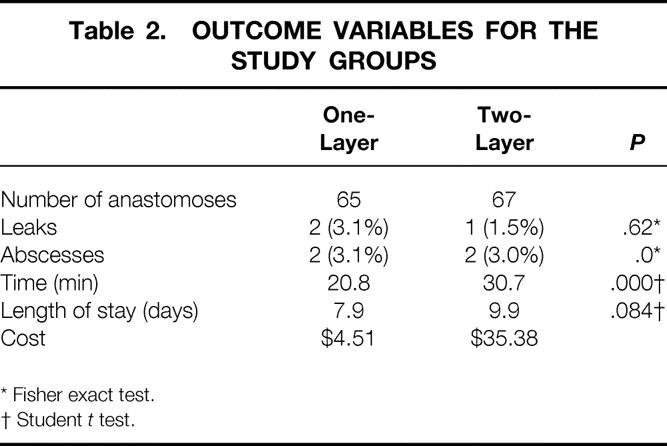
* Fisher exact test.
† Student t test.
DISCUSSION
The two-layer interrupted anastomosis has its origins in the early 19th century through the experimental work of Travers 12 and of Lembert, 13 who advocated careful approx- imation of the serosal surfaces of the bowel and devised a method of suturing to accomplish this. In 1836, Dieffenbach performed the fist successful anastomosis of the small intestine using Lembert’s method. 14 In 1880, Czerny 15 advocated the addition of an inner layer to reduce the risk of leakage and to achieve a precise mucosal approximation. Since then, the technique has remained essentially unchanged except for the evolution of suture material for the inner layer.
The single-layer interrupted anastomosis was never entirely abandoned and has periodically attracted renewed interest. 16,17 The single-layer continuous anastomosis is a contemporary innovation first described by Hautefeuille in 1976. 1 In the United States, the first mention of this technique was by Allen et al, 18 who presented their results with its use before the Texas Surgical Society in 1979. It was then popularized by a colon and rectal surgical group based in Houston, Texas. 5,11,19
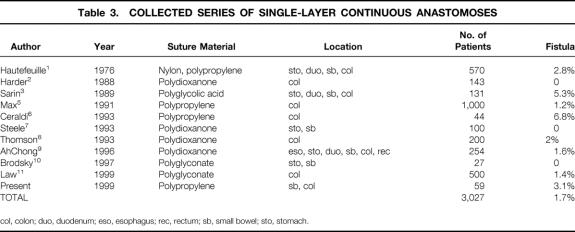
The present study demonstrates that a single-layer continuous anastomosis is similar in terms of safety to the two-layer technique, but that it can be constructed in a significantly shorter time and at a lower cost. These results also imply that the technique can be safely introduced into a surgical training program without a painful learning curve. The ultimate test of the suitability of a technique for intestinal anastomosis is its ability to heal without leakage. This complication has catastrophic consequences for the patient’s health as well as the cost of care. Ischemia, tension on the anastomosis, and poor technique are clearly responsible for anastomotic failure and are all under the direct control of the surgeon. It is not surprising, therefore, that both Feilding et al 20 and Tuson and Everett 21 found that leakage rates varied significantly between surgeons and tended to be lower with more experienced surgeons. Other traditional risk factors such as diabetes, steroids, anastomotic method, blood loss, and nutritional factors have not clearly been predictive of anastomotic failure. 22 Numerous studies in the literature comparing techniques (e.g., one-layer vs. two-layer, hand-sewn vs. stapled, and end-to-end vs. end-to-side) have failed to demonstrate a clear superiority of one over another. 22 In fact, the only technique that has been unequivocally demonstrated to be unacceptable is the everted anastomosis. 23 Given that, for a new technique to be acceptable, it only needs to be demonstrated safe by a number of different surgeons in a large number of patients. Table 3 shows a collected series of single-layer continuous anastomoses, and a leakage rate of 1.7% in 3,027 patients fulfills this criterion.
Table 3. COLLECTED SERIES OF SINGLE-LAYER CONTINUOUS ANASTOMOSES
col, colon; duo, duodenum; eso, esophagus; rec, rectum; sb, small bowel; sto, stomach.
The mean time saved by creating the single-layer anastomosis, 10 minutes, may seem relatively insignificant. However, our study design did not include the time required to prepare the bowel for anastomosis, which is considerably less for the one-layer technique. To accomplish a two-layer anastomosis, at least 1 cm of the serosal surface must be circumferentially cleared of mesentery, appendices epiploica, and omentum before beginning the anastomosis. With the single-layer method, less circumferential clearing is required, and in many instances no clearance is necessary. Further, most of the anastomoses in this study were performed by residents, who were often unfamiliar with the technique or were performing their first intestinal anastomosis. The senior authors of this paper (J.M.B., E.E.M.) have used the single-layer technique exclusively for many years and can routinely perform an anastomosis in 8 to 10 minutes. This time has been documented by other surgeons using the single-layer continuous method. 5,11 In contrast, it is difficult for an experienced surgeon to create a two-layer anastomosis in less than 20 to 25 minutes. Therefore, the overall time savings may be closer to 20 to 25 minutes, including bowel preparation, in experienced hands.
It was surprising to see the considerable difference in cost of materials for the anastomoses. Most of the difference is accounted for by the cost of the silk suture packets, at $9.41 each. Because at least three packets are required (15 sutures), the cost for the outer layer alone is $28.33 compared with $4.51 for one packet of polypropylene suture. Even more dramatic is the difference in cost when compared with a stapled anastomosis: the disposable staple gun costs $115.26 and two refills cost an additional $123.54, for a total of $238.80! In today’s cost-conscious environment, the use of staples for anastomoses seems irresponsible if a hand-sewn anastomosis can also be safely used in a similar time interval.
Another surprising finding was the 2-day difference in the mean length of stay. Although it did not quite reach statistical significance, it may be related to an intrinsic difference between the two methods: the single-layer anastomosis always has a larger lumen. It is possible that gastrointestinal function may return to normal in a shorter time with the single-layer method, although further studies would be required to confirm this speculation.
The apparent success of the single-layer continuous anastomosis may be attributed to several factors. Because less mesentery is cleared for the single-layer anastomosis, the cut edge of the bowel is more likely to have an adequate blood supply. Another factor is related to the properties of a continuous monofilament plastic suture line. Although it is certainly possible to create an ischemic continuous anastomosis by applying too much tension while following the suture, Hautefeuille 1 has argued that this is easier to avoid with a continuous suture because there is no point in the anastomosis where the bowel is completely devoid of its blood supply. In contrast, this can easily occur to the tissue enclosed by an interrupted suture. Further, the surface of monofilament plastic suture is slick and may permit areas of relative excess extension to equilibrate with surrounding areas of less tension by minute movements of tissue with respect to the suture material. Bailey et al 24 have speculated that the continuous single-layer suture, which resembles a circular coiled spring, may be able to expand and contract depending on intraluminal forces. This, they argue, may also explain the rarity of stenoses of the suture line.
The major weakness of the study is its relatively small sample size. It is therefore difficult to make definitive statements regarding the relative risk of uncommon outcomes, such as anastomotic leakage. To detect a significant difference in leakage rates based on our data, a power analysis indicated that 1,500 patients would be required. We did not believe we could realistically enroll this many patients; instead, a multiinstitutional trial would be necessary. However, our results and those in the literature are consistent and reassuring.
We conclude that the single-layer continuous anastomosis requires less time to construct and has a similar risk of leakage compared with the two-layer technique. It also costs less than any other method and can be safely introduced into a surgical training program with no apparent increase in complications. For these reasons, we believe the single-layer continuous anastomosis is superior to the two-layer interrupted technique.
Discussion
Dr. Martin Dalton (Macon, Georgia): I commend the authors of this excellent paper for their temerity in taking on the resolution of this rather controversial problem in general surgery.
In the time of William Halsted, this was indeed a contentious portion of surgery—difficult to teach, difficult to expand, and he had the foresight to take this to the laboratory and try to resolve this problem. Because not only was it contentious, the results were terrible. If I could see that first slide. [Slide] This is what Halsted came up with and published in 1887. He apparently was the first to realize the importance of the submucosa. Because of the problem with infection, he avoided going into the lumen of the bowel and used one layer of “plain quilt” stitches, which, as best I can tell from reading this article, was simply a horizontal Lembert-type suture.
My personal interest in this stems from a paper presented at this meeting in 1966 and published in the Annals in 1967, wherein we found not only equal but superior results to interrupted proline sutures for both GI and colo-colonic anastomoses.
I would like to ask Dr. Burch how he coerces his residents into doing handsewn anastomosis. My residents are so enamored of stapling that it is difficult to get them to do any handsewn anastomosis, single or multi.
Dr. William W. Turner (Jackson, Mississippi): Did the authors note any difference in anastomotic complications such as ileus, edema, or stricture formation, that might implicate a disadvantage of the two-layer anastomosis involving the inversion of more intestinal mass than with a single-layer anastomosis?
I noticed that the anastomoses were performed with permanent suture. Was that a personal choice, and do the authors have an objection to performing these anastomoses with absorbable suture such as PDS?
In follow-up to Dr. Dalton’s comments, it is interesting that my residents seem to want the opportunity to sew the GI tract these days, since they get to sew it so infrequently.
Dr. Hiram C. Polk, Jr. (Louisville, Kentucky): I think that this is another example of Jon and Gene Moore assuming the responsibility for doing first-class prospective clinical trials in a county hospital–trauma setting. That’s something I think Dr. Stone had done for years, and I think that mantle has now passed to Denver. This is one of a continuing example of papers in which they have been able to show that they can study real-world problems like this and produce some interesting results. I guess I believe the outcome, a poor man’s meta-analysis of this. If you read what has been published now, it has to convince you that a single layer of nonabsorbable suture is safe. It has the same leak rate, and you saw some of the advantages.
I have some simple questions for Jon. Number one, how long does it take? I understand how long the learning curve is for a resident. I wonder how long it takes to teach a psychologically senior staff person who has been raised on a two-layer anastomosis to make the change. Secondly, what do you do about purse-string little tricks, and especially not picking up the suture with something that might fracture the braid in it?
The projection that it would take 1500 patients to prove this is a good one, and we are going to have to live with this result the way it is, because I doubt if anybody will want to do more or tackle this.
The issue of time use is really important. The last two papers on the program this afternoon deal with hospital cost issues, and I think they are where we live right now. If you can save 10 or 15 minutes per anastomosis with this, with that amounting to a couple hundred dollars of OR cost and even more of OR charges, you are looking at something we are just about going to have to implement whether we like it or not.
Dr. Jon M. Burch (Closing Discussion): Dr. Dalton, we too think that if you leave the residents alone to perform their choice of anastomosis, they probably would go with the stapled anastomosis—at least I think that was probably true before we instituted this trial. As the trial went on, the residents actually became enamored with performing the single-layer anastomosis, and invariably, when we would pull the other card, there would be a groan, “Oh, no.”
We did not find a difference, Dr. Turner, with regards to the frequency of ileus or stricture. There is no question that the single-layer anastomosis results in a larger lumen. We did find a curious difference in the length of stay, which was 2 days. Although it didn’t reach statistical significance as we defined it—the P value was .08—I’m not sure that it is necessarily related. We are going to look at that in more detail.
The literature contains about eight papers now which have demonstrated that the PDS or Maxon-style sutures, that is, the absorbable monofilament plastic sutures, can be used successfully in these anastomoses. We selected polypropylene, the nonabsorbable version, simply because we wanted to standardize the technique, and this was the method that I had originally learned from Dr. Max in Houston.
Dr. Polk, in regards to teaching senior members of our group to perform this procedure, I personally instructed every one of them, and they got one chance to see how it was done. After that, they were on their own, and we did not have any early leaks. So there does not appear to be a learning curve.
The avoidance of the purse-string effect is an important issue. Certainly, these are very slick sutures. What I like to do is pull up slightly on the sutures as the second knot goes down and pull them up and apart. That tends to lock the suture without actually pulling any additional suture out of the tissue. However, I think it is more important to avoid pulling hard on the suture while following.
Footnotes
Correspondence: Jon M. Burch, MD, Dept. of Surgery, Denver Health Medical Center, Box 0206, 777 Bannock St., Denver, CO 80204-4507.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
E-mail: jburch@dhha.org
Accepted for publication December 1999.
References
- 1.Hautefeuille P. Reflexions sur les sutures digetives: a propos de 570 sutures accomplies depuis 5 ans au surjet monoplan de monobrin. Chirurgie 1976; 102:153–165. [PubMed] [Google Scholar]
- 2.Harder F, Vogelbach P. Single-layer end-on continuous suture of colonic anastomoses. Am J Surg 1988; 155:611–614. [DOI] [PubMed] [Google Scholar]
- 3.Sarin S, Lightwood RG. Continuous single-layer gastrointestinal anastomosis: a prospective audit. Br J Surg 1989; 76:493–495. [DOI] [PubMed] [Google Scholar]
- 4.Irwin ST, Krukowski ZH, Matheson NA. Single-layer anastomosis in the upper gastrointestinal tract. Br J Surg 1990; 77:643–644. [DOI] [PubMed] [Google Scholar]
- 5.Max E, Sweeney B, Bailey HR, et al. Results of 1,000 single-layer continuous polypropylene intestinal anastomoses. Am J Surg 1991; 162:461–467. [DOI] [PubMed] [Google Scholar]
- 6.Ceraldi CM, Rypins EB, Monahan M, et al. Comparison of continuous single-layer polypropylene anastomosis with double-layer and stapled anastomoses in elective colon resections. Am Surg 1993; 59:168–171. [PubMed] [Google Scholar]
- 7.Steele RJC. Continuous single-layer serosubmucosal anastomosis in the upper gastrointestinal tract. Br J Surg 1993; 80:1416–1417. [DOI] [PubMed] [Google Scholar]
- 8.Thomson WHF, Robinson MHE. One-layer continuously sutured colonic anastomosis. Br J Surg 1993; 80:1450–1451. [DOI] [PubMed] [Google Scholar]
- 9.AhChong AK, Chiu KM, Law IC, et al. Single-layer continuous anastomosis in gastrointestinal surgery: a prospective audit. Aust NZ J Surg 1996; 66:34–36. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky JT, Dadian N. Single-layer continuous suture for gastrojejunostomy. Am Surg 1997; 63:395–398. [PubMed] [Google Scholar]
- 11.Law WL, Bailey HR, Max E, et al. Single-layer continuous colon and rectal anastomosis using monofilament absorbable suture (Maxon): study of 500 cases. Dis Colon Rectum 1999; 42:736–740. [DOI] [PubMed] [Google Scholar]
- 12.Travers B. Enquiry into the Process of Nature in Repairing Injuries of the Intestine. London: Longman, Rees, Orme, Brown, and Green; 1812.
- 13.Lembert A. Memoire sur l’enteroraphie avec la description d’un procede nouveau pour pratiquer cette operation chirurgicale. Rep Gen Anat Physiol Path 1826; 2:100. [Google Scholar]
- 14.Leonardo RA. History of Surgery. New York: Froden; 1943.
- 15.Czerny V. Zur Darmresektion. Berl Klin Wschr 1880, 17:637. [Google Scholar]
- 16.Gambee LP, Garnjobst W, Hardwick CE. Ten years’ experience with a single-layer anastomosis in colon surgery. Am J Surg 1956; 222–227. [DOI] [PubMed]
- 17.Bronwell AW, Rutledge R, Dalton ML. Single-layer open gastrointestinal anastomosis. Ann Surg 1967; 165:925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen TW, Salem RJ, Stirman JA. Continuous suture for single-layer enteroanastomosis. Read before the Texas Surgical Society, Austin, TX, Oct. 1, 1979.
- 19.Bailey HR, LaVoo JW, Max E, et al. Single-layer continuous colorectal anastomosis. Aust NZ J Surg 1981; 51:473–476. [DOI] [PubMed] [Google Scholar]
- 20.Feilding LP, Stewart-Brown S, Blesovsky L, et al. Anastomotic integrity after operations for large-bowel cancer: a multicentre study. Br Med J 1980; 9:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuson JRD, Everett WG. A retrospective study of colostomies, leaks and strictures after colorectal anastomosis. Int J Colorectal Dis 1990; 5:44–48. [DOI] [PubMed] [Google Scholar]
- 22.Pickleman J, Watson W, Cunningham J, et al. The failed gastrointestinal anastomosis: an inevitable catastrophe? J Am Coll Surg 1999; 188:473–482. [DOI] [PubMed] [Google Scholar]
- 23.Goligher JC, Morris C, McAdam WAF, et al. A controlled trial of inverting versus everting intestinal suture in clinical large-bowel surgery. Br J Surg 1970; 57:817. [DOI] [PubMed] [Google Scholar]
- 24.Bailey HR, LaVoo JW, Max E, et al. Single-layer polypropylene colorectal anastomosis: experience with 100 cases. Dis Colon Rectum 1984; 27:19–23. [DOI] [PubMed] [Google Scholar]