Abstract
Objective
To assess the efficacy of cultured epidermal autografts (CEA) for closure of burn wounds in pediatric burn patients with full-thickness burns of more than 90% total body surface area.
Summary Background Data
Paucity of donor sites in massive burns makes the use of expanded skin of paramount importance. CEA techniques have been used in burned patients with differing and controversial results. The true impact and the efficacy of such techniques in massive burns remain uncertain.
Methods
Patients with full-thickness burns of more than 90% body surface area treated between May 1988 and May 1998 were studied. Patients grafted with CEA were compared with patients grafted with conventional meshed autografts. Rates of death and complications, length of hospital stay (LOS), hospital cost, acute readmissions for reconstruction, and quality of scars were studied as outcome measures.
Results
Patients treated with CEA had a better quality of burn scars but incurred a longer LOS and higher hospital costs. Both groups had comparable readmissions for open wounds, but patients treated with CEA required more reconstructive procedures during the first 2 years after the injury. The incidence of sepsis and pneumonia in both groups was comparable.
Conclusions
Conventional meshed autografts are superior to CEA for containing hospital cost, diminishing LOS, and decreasing the number of readmissions for reconstruction of contractures. However, the use of CEA provides better scar quality such that perhaps future research should focus on bioengineered dermal templates to promote take and diminish long-term fragility.
Severe full-thickness burns covering more than 90% of the total body surface area (TBSA) continue to pose an immense challenge to even the most experienced burn teams. In the past few decades, the burn-related death rate has declined dramatically, and this can be attributed in part to early excision and closure of the burn wound. Currently, even children sustaining full-thickness burns of more than 90% TBSA have a better than 50% rate of survival. 1
The approach to skin coverage in the massively burned patient depends on the type and extent of injury. Burns of less than 30% TBSA can be covered with autograft skin at one operation. In full-thickness burns of more than 30% TBSA, however, the autograft donor site is quickly exhausted, so that alternative skin coverage is necessary. This is particularly true in patients with massive burns, in whom a paucity of donor sites makes skin substitutes and the use of expanded skin of paramount importance. For years, these patients have been treated with traditional methods of widely expanded meshed autografts with an overlay of cadaveric allograft. 2,3 More recently, the use of cultured epidermal autografts (CEA) has been advocated for wound closure in massive burn injury. 4 Nevertheless, cost, long-term fragility, and the lack of an optimal dermal equivalent for CEA have restricted its routine use. 5,6 Recurrent open wounds, increased rates of burn scar contractures, and troublesome rehabilitation are also arguments precluding CEA use. The use and the efficacy of CEA in full-thickness burns of more than 90% TBSA, therefore, are still uncertain. We studied our patient population with full-thickness burns of more than 90% TBSA to determine factors affecting survival and the outcomes in patients treated with CEA.
METHODS
Study Population
All pediatric patients with full-thickness burns more than 90% TBSA treated at the Shriners Burns Hospital in Galveston, Texas, between January 1988 and January 1998 were examined. Patients who were treated with CEA were identified and compared with a control cohort of patients not treated with CEA. Only patients surviving more than 3 weeks (time for CEA grafting) were analyzed for measures comparing CEA versus no CEA.
Surgical Technique
All patients were resuscitated according to the Galveston formula (5,000 mL Ringer’s lactate/m2 body surface area burned + 2,000 mL Ringer’s lactate/m2 body surface area) given in continuous increments during the first 24 hours. Within 24 hours of admission, patients underwent wound excision of all full-thickness burns and were covered with autografts from available donor sites and cadaver allografts. Based on the attending surgeon’s choice, during the first operation a full-thickness skin biopsy was harvested for CEA culture (Genzyme, Cambridge, MA). Patients returned at weekly intervals to the operating room for further autografting and replacement of allografts. After approximately 3 weeks, CEA grafts were ready for transplantation. Take of the CEA was assessed 1 week after application by the attending surgeon. Patients who received CEA were returned to the operating room only for further autografting to areas of graft loss or epidermolysis. Autografts were initially meshed 4:1 and applied to large surfaces. As the patient’s recovery progressed and the area open diminished, 2:1 meshing patterns and sheet grafts were used.
During the entire hospital stay, all patients received nutritional supplementation through a nasoduodenal feeding tube with Vivonex TEN (Sandoz Nutrition, Minneapolis, MN), an elemental formula containing 82.3% carbohydrate, 3% fat (linoleic acid), and 14.7% protein. Caloric intake was given at a rate to deliver 1,500 kcal/m2 body surface burned + 1,500 kcal/m2 body surface area. Rehabilitation was started on admission in a passive and active program.
Study Design
Patients treated with CEA were compared with patients treated with conventional autografting techniques to assess the efficacy of CEA. Data collected included the total number of operations, area grafted with CEA, CEA take, CEA grafts lost and subsequently autografted, complications, length of hospital stay (LOS), overall hospital cost, reconstructive operations during the first 2 years, and quality of scars.
Surface areas were calculated by plotting the height and the weight of the patient in a standard nomogram. The areas grafted with CEA, areas lost, and areas autografted were calculated by measuring the total amount of CEA (cm2) and autograft size applied at surgery. Donor site healing was assessed by the number of days required for removal of a standard donor-site dressing. 7,8 Hospital costs were estimated for individual patients by multiplying hospital days by $2,000 7 and adding the costs of CEA. Sepsis was defined as pathologic bacteremia on blood culture, and pneumonia was defined as lung infiltrate on chest x-ray combined with fever and a pathologic organism isolated on a class III sputum.
Burn scars were assessed 2 years after the injury by three separate investigators. Each observer analyzed all patients, and all three sets of observations were compared with the rest of the observers’ rating scores. Time from injury to reconstruction and number of reconstructions performed for burn scar contractures and cosmesis in the first 2 years after injury were quantified.
Scars were assessed for surface, border height, thickness, and color difference. The burn scar rating scale introduced by Yeong et al 9 was used to determine the degree and quality of scars. These scars were assigned a score in each of the former categories. Scores could range from −1 to 4. A score of 0 in any of the categories was considered normal, or a quality matching normal skin. A score of −1 meant atrophy, depression of borders, or hypopigmentation. Scores from 1 to 4 signified increasing degrees of severity of hypertrophy, sharp borders, and hyperpigmentation. To compare the different scar rating results in the two groups over time, rank values were assigned to all observations in columns from smallest to largest. Equal values were tied in rank, and an averaged rank was assigned to all tied values. This rank was the average of the ranks that would have been assigned to all the tied values if they were not tied. The rank was transformed to integer values for data analysis.
Data are shown as mean ± standard error for parametric data and median and range for nonparametric data. Statistical analysis was performed with an unpaired t test and the Mann-Whitney rank sum test, the Fisher exact test, and the kappa test for interrater agreement. Significance was accepted at P < .05.
RESULTS
Patient Population
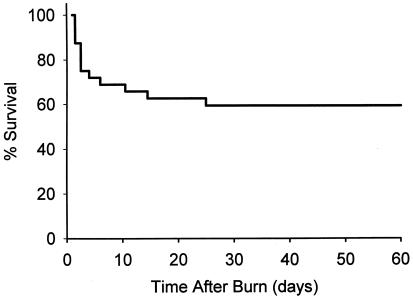
During the 10 years from May 1988 to May 1998, 32 pediatric patients with full-thickness burns of more than 90% TBSA were treated at the Shriners Burn Hospital in Galveston, Texas. Twelve patients died before day 21 (estimated day of CEA grafting), and only one patient died after day 21. The raw death rate of this severely burned cohort of patients was 40%; conversely, the survival rate was approximately 60% (Fig. 1). The mean age of all patients admitted during the study period (survivors and nonsurvivors) was 8.2 ± 1.9 years, with the percentage TBSA burned of 94.1% ± 2% and a percentage of full-thickness burns of 92.5% ± 1.5%. All patients had inhalation injury, which was diagnosed by bronchoscopic examination on admission.
Figure 1. Kaplan-Meier survival graph for children with more than 90% total body surface area burns.
Patient demographics for the CEA and non-CEA (traditional grafting techniques) groups are shown in Table 1. Patient groups were comparable. When the initial injury characteristics were compared with those of patients who died before day 21, there were no differences in terms of delay in resuscitation or burn shock. Patients who died, however, had a 70% incidence of anoxic brain injury and a 20% incidence of brain death. These brain injuries were significantly higher in nonsurvivors compared with patients who survived (P < .001).
Table 1. PATIENT DEMOGRAPHICS
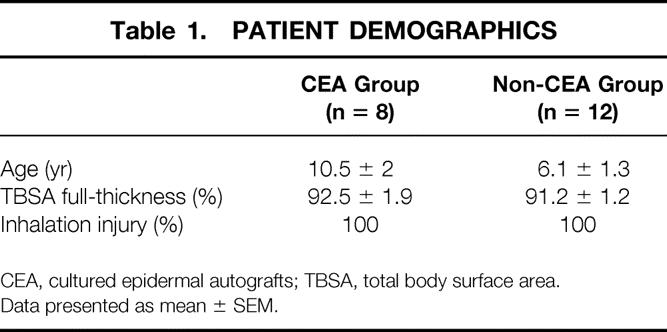
CEA, cultured epidermal autografts; TBSA, total body surface area.
Data presented as mean ± SEM.
Comparison of CEA and Non-CEA Groups
Twenty patients survived to day 21. Eight patients were treated with CEA and 12 with traditional (non-CEA) techniques. All patients in the CEA group survived, whereas one patient in the non-CEA group died on day 35 of pulmonary thromboembolism (100% survival vs. 91%, P = 0.796). Before day 30, patients in the CEA and non-CEA groups underwent a similar number of operations (5.2 ± 0.7 vs. 4.1 ± 0.5), but the number of operations after day 30 in the acute hospital stay was significantly higher in the CEA group (7.1 ± 1.1 vs. 3.6 ± 0.6;P = 0.03, Table 2). Donor-site healing times for all operations in the acute hospital stay were similar in both groups (7 ± 0.1 days in CEA vs. 6.6 ± 0.2 days in non-CEA), and the incidence of sepsis and pneumonia in both groups was comparable (three cases of pneumonia and two of sepsis in the CEA group vs. two cases of pneumonia and two of sepsis in the non-CEA group;P > 0.05). Patients treated with CEA had a significantly longer hospital LOS (P = 0.03), and the addition of the increased LOS and the cost of CEA produced a significantly greater hospital cost for the children treated with CEA (see Table 2).
Table 2. COMPARISON OF GROUPS
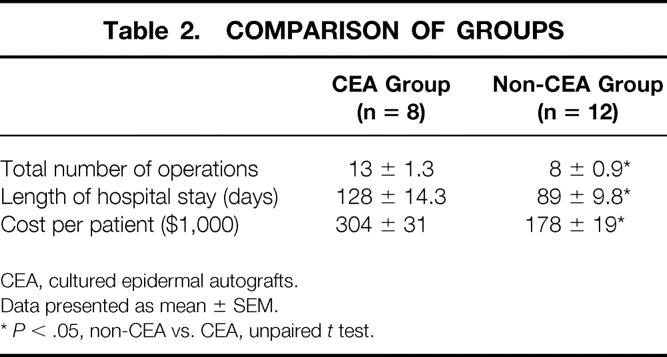
CEA, cultured epidermal autografts.
Data presented as mean ± SEM.
*P < .05, non-CEA vs. CEA, unpaired t test.
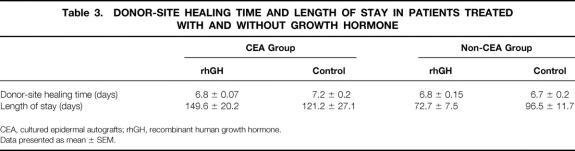
Three patients (37.5%) in the CEA group and four patients (33.3%) in the non-CEA group received recombinant human growth hormone. The dosage was 0.2 mg/kg/day in both groups. Subgroup analysis among patients treated with or without the hormone showed no significant differences. Donor-site healing times and LOS are shown in Table 3.
Table 3. DONOR-SITE HEALING TIME AND LENGTH OF STAY IN PATIENTS TREATED WITH AND WITHOUT GROWTH HORMONE
CEA, cultured epidermal autografts; rhGH, recombinant human growth hormone.
Data presented as mean ± SEM.
CEA Group Results
Patients were grafted with CEA on postburn day 30 ± 2.7. Two patients had en bloc fascial excision, two patients had tangential excision, and three patients had a combination of both types of excision performed in the first surgical procedure. A mean of 44% ± 7% TBSA was covered with CEA, with an initial CEA take of 60% ± 8%. Fifty percent ± 8% of all areas covered with CEA needed to be autografted later in the acute hospital stay to achieve definitive coverage. We noticed no differences in take or regrafting for anatomical distribution or age. When CEA take and regrafting rates were compared in different burn-wound beds, CEA take was significantly better in fascial than tangentially excised wounds or engrafted dermis from allograft (78% vs. 45% take rate, P < .001). Fascial excisions covered with CEA grafts, however, were more fragile and had a higher rate of blistering and regrafting requirement (66% of regrafting in fascial excision vs. 34% in tangential excision and allografts;P < .001). Thus, dermal wounds, either autologous or homologous dermis, had the best final take of CEA.
Quality of Scars and Reconstructive Procedures
Patients in the CEA group had a clinically more troublesome rehabilitation, although acute readmissions for open wounds and rehabilitation problems were similar in both groups. Nevertheless, patients treated with CEA needed more reconstructive procedures in the first 2 years for functional problems (2.3 ± 1.4 vs. 0.9 ± 1.8;P = .04). When the quality of scars was compared between groups, however, patients treated with CEA had scars with a significantly smoother surface and less pigmentation than traditional meshed autografts, thus supporting the evidence that CEA grafts produce better cosmesis than widely expanded autografts. Border height and thickness were no different between groups (Table 4).
Table 4. COMPARISON OF BURN SCARS 2 YEARS AFTER INJURY
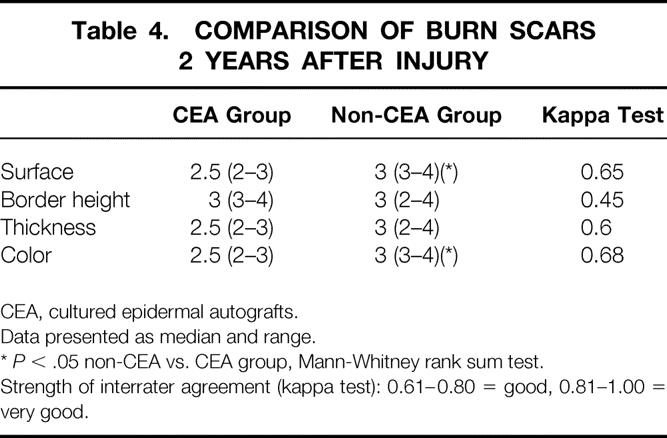
CEA, cultured epidermal autografts.
Data presented as median and range.
*P < .05 non-CEA vs. CEA group, Mann-Whitney rank sum test.
Strength of interrater agreement (kappa test): 0.61–0.80 = good, 0.81–1.00 = very good.
DISCUSSION
Cultured epidermal autografting continues to be a controversial and expensive technique for the closure of burn wounds. 4 The availability of cultured skin and clinical applicability were explored in the early 1980s. 10 Initial reports were followed by larger series of patients treated with CEA, although short-term results have been inconsistent. 11 Unsolved problems include patient selection, 4,11,12 graft take, 5,13 dermal replacement, 5,14,15 and long-term durability. 6,16
Large cutaneous burns create acute physiologic derangements and extensive cutaneous losses that, even in experienced hands, require repeated operations for débridement and grafting, creating a prolonged process for permanent coverage. The extent of the burn limits donor sites and increases the likelihood of burn wound sepsis, especially with delayed wound closure, which extends the period of metabolic stress and makes temporary burn wound coverage necessary. 17 Under these circumstances, the advent and availability of cultured epithelial cells and the successful clinical application of such techniques in burned patients 10,18–20 produced an initial enthusiasm for CEA among practitioners and gave rise to multiple clinical applications with short series and noncomparable data. 16 Mean burn size in these patients averaged 60% TBSA, 4,11,12,17,19 with different graft take and long-term results. Few clinical trials, however, comparing traditional techniques with CEA engrafting were carried out. Munster et al 12 found in their initial experience with CEA reduced rates of death and complications compared with an historical group. No significant variables in addition to CEA grafting were found that would explain this finding. Munster, in a prospective controlled trial, 4 corroborated these findings with a significant reduction in the death rate in the CEA group. Drawbacks of these studies were the special patient selection and a mean burn size of 69% TBSA. The cost of care for CEA patients was significantly higher than for the control group, which brought into question the use of CEA in patients who could have had their wounds closed with an otherwise less expensive technique. This was also our experience. Patients treated with CEA incurred higher hospital costs without significant decreases in the death or complication rate in this small series.
The use of total burn wound excision in the first 24 hours after admission and temporary wound closure with homografts has proved effective in reducing complications in severely burned victims. 3,21 We believe that the use of this surgical approach in our patient population is primarily responsible for the low death rate in the patients who survived more than 30 days after the burn. Survival in the overall population with burns of more than 90% TBSA was also improved by this technique. Moreover, Wolf et al 1 determined that in massive pediatric burns, the major determinants of death are age, burn size, delayed resuscitation, and burn-associated sepsis or multiorgan failure; the type of surgical wound closure had no effect whatsoever. Patients with limited donor sites were also most apt to die in this study, but the effect of such a determinant in the current cohort of patients afflicted with burns of more than 90% TBSA must be very low. Although the ideal setting for CEA application may be for patients with near-total loss of the skin (>90% TBSA), our experience and that of others emphasize current problems with CEA that must be solved for further application of this otherwise promising technique of tissue engineering.
Sheridan and Tompkins 16 used CEA to treat five pediatric burn patients who had burns of more than 90% TBSA, with a successful initial engrafted rate (51%) and delayed loss rate (60%) similar to ours. They concluded that although CEA techniques played an important role in the management of massive burns, CEA should be limited to defined areas while the rest of the wound continues to be covered with traditional techniques. Rue et al 22 also failed to identify any positive impact on wound closure in extensively burned patients, with a low long-term durability of such grafts. Initial take of CEA in our series was similar to others in the literature, 5,16 and so were the areas blistered and regrafted with traditional autografts. The best take was on fascially excised wounds, but these areas were also those with the worst long-term durability. Our experience was similar to that of McAree et al 17 : in their study, the best CEA initial take was on fascia (72%), but deep dermal wounds had the best average final take (95%). The fragility of CEA during the initial postoperative period and a few months after discharge on fascially excised wounds makes these areas more prone to blistering and contractures. 4,6,16,22 It is also possible that some dermal wounds, although initially covered with CEA, may eventually heal by means of autogenous keratinocytes left in the dermal appendages, which would have greater inherent stability.
Patients treated with CEA in our cohort had similar rates of open wounds and readmissions, but the incidence of contractures and reconstructive procedures was significantly higher. Some of this increase in surgical procedures could be accounted for by additional coverage procedures for large areas initially closed with CEA grafts that were lost because of fragility, whereas the wounds in the group covered with conventional grafts responded to conservative wound management. In addition, the implementation of an active and aggressive rehabilitation program is subjectively troublesome and delayed because of the nature of the CEA graft and the tendency to blister under minimal mechanical trauma. 17 Although as early as 6 days after surgery the epithelial graft has differentiated into a fully stratified epithelium with all four layers present in normal proportion, a basal lamina is not continuous until 4 weeks after grafting, and it is not until 5 months after grafting that the maturation is complete, with a functional dermal–epidermal junction. 23 Any minimal trauma, therefore, during this long maturation will provoke blisters and open wound formation, delaying the start and progression of the rehabilitation program. Therefore, we propose that these patients are inherently more prone to the development of contractures and thus require more surgical intervention. The development and application of hydrophobic pressure garments have proved beneficial in reducing surface maceration and shearing injuries, 24 but the exact effect of such therapy in severely injured patients remains uncertain.
Other approaches that have been explored to improve the take, shorten the long-term fragility, and reduce the tendency to hypertrophy and contracture of CEA is the use of dermal equivalents. In fact, in our cohort of patients, deep dermal wounds and allodermis had a significantly better final take (although fascial wounds had the best initial take) than other wounds, which supports the notion that composite bioengineered skin would provide the best long-lasting wound closure.
Dermal replacement with engrafted allodermis has been used effectively for dermal replacement. 5,25 Initial take consistently exceeded 80%, producing a supple skin that was durable and resistant to trauma and infection. The problems and difficulties of initial engrafting and the removal of the alloepidermis have made researchers search for new dermal equivalents that would provide long-lasting skin with an easier and quicker application. Types I and IV collagen, fibronectin, gelatin, and laminin do not have a significant effect on growth and colony-forming efficiency when included in extracellular matrices. 26 However, when different substrates are combined to form an extracellular matrix, direct regeneration of normal skin morphology can be achieved. Collagen and chondroitin-6-sulfate, 27 fibroblasts attached to collagen-glycosaminoglycan substrates, 28 and cultured autologous keratinocytes with Integra (Johnson and Johnson, Arlington, TX) 29 provide a dermal–epidermal junction and a quality of skin similar to that produced with traditional allodermis engrafting techniques or with an acellular human dermis as dermal matrix (Alloderm, Life Cell, Newark, NJ). 30 The optimal dermal substitute, however, is still to be defined.
Despite the problems with long-term durability of the cultured skin and the tendency for contractures, the cosmetic quality of scars in the CEA group appeared to be better than the control group. Given that the control group comprised patients with burn wounds closed with conventional autografts, the assumption that the quality of scars in the conventional group would be better is defensible because of the presence of some dermis in the autograft. However, hypertrophy, thickness, and raised surface were more obvious in the conventional group. An explanation lies in the long-term analysis of the wound covered with CEA, which shows eventual formation of a true dermis, unlike the interstices of meshed grafts, where this phenomenon seldom occurs. Five years after grafting, the underlying connective tissue has remodeled in a distinctly bilayered structure with collagen and elastic fibers and a vascular architecture resembling dermis. 23,31 The fact that widely expanded meshed grafts were used in the control group would explain the observation that patients grafted with CEA had smoother, thinner, and less pigmented scars.
In conclusion, in a prospective cohort of severely burned pediatric patients (>90% TBSA full-thickness burns), patients treated with CEA had a significantly longer LOS and higher hospital costs than patients treated with traditional widely meshed autografts. No differences in the death or complications rate were found between groups. Patients in both groups had similar rates of acute readmissions for open wounds, but patients in the CEA group had more contractures and reconstructive procedures. Patients treated with traditional techniques had more hypertrophic and hyperpigmented scars, whereas the CEA group had scars with better cosmesis.
Future research directions include determination of the optimal dermal replacement for cultured keratinocytes and shortening of the culture time. Tissue engineering techniques and cell transfection with cDNA coding for growth factors are also promising grounds of research. These developments would produce faster recovery with a shorter LOS and better long-lasting quality of bioengineered skin to permit the start of rehabilitation programs sooner and to decrease reconstructive needs.
Discussion
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): Dr. Herndon and his colleagues have confirmed the limitations of cultured epidermal autografts as used in the treatment of 20 pediatric-age patients with burns involving more than 90% of the body surface.
The real triumph recorded in this report is the fact that the 20 study patients represent a 60% survivorship of 32 patients with such extensive burns treated at Dr. Herndon’s burn center over the past 10 years. Contrast that with the situation 40 years ago when the LA 50 for pediatric-age burn patients was less than 50% of the body surface. That quantum improvement in survival means that there will be more patients with so few donor sites that timely closure of the wound becomes a problem. Since all skin substitutes provide only temporary coverage, attention has focused on culture-derived tissue in recent years. The results reported this morning represent an improvement over those in previous studies conducted by Dr. Rue and Dr. Cioffi, both of whom are in the audience, at the U.S. Army Institute of Surgical Research, in which engraftment, most discouragingly, was inversely proportional to the extent of the burn.
Consequently, my first question is, have there been technological or surgical technique developments that improve the take of CEA?
Your findings seem to be paradoxical in at least two respects, and we need additional information to resolve those apparent discrepancies.
You note that patients treated with CEA had a better quality of scars, but also required more reconstructive procedures during the first 2 years after injury. If the scars are better, why was more reconstruction needed?
You also note that CEA take was better than 78% on fascial excisional wounds, as compared to 45% when it was applied to either auto or allodermis. Again, those wounds with the better take required more regrafting, that is, 66% of the wounds at the fascial level versus only 34% of the wounds at the dermal level. Does that simply reflect survival of skin appendages in the autodermis and “normal” healing of a partial thickness injury?
You have reported the beneficial effects of human growth hormone in wound healing in burn patients, and we need to know whether any of the patients in this study received growth hormone and whether such treatment was equally represented in the two study groups.
Lastly, several techniques to enhance the use of CEA have been proposed, i.e., chimeric CEA and the in vitro seeding of a dermal analogue with CEA before application. We would be interested in your experience with those techniques, and your speculations about how to improve both the usefulness and effectiveness of CEA.
Dr. William G. Cioffi, Jr. (Providence, Rhode Island): Reinwald and Greene’s 1975 report detailing the methodology allowing for serial culture of human keratinocytes unleashed a flurry of interest of the use of culture-derived products for achieving rapid wound closure in massively burned individuals. A 1984 New England Journal of Medicine article by Gallico and O’Connor (1984;311:448–451), in which they reported permanent coverage of burn wounds in two children with greater than 90% total body surface area burns, highlighted the potential feasibility of this approach. Unfortunately, like many new methods, early unbridled enthusiasm is often replaced by cautiously guarded optimism, and such is the case surrounding the utility of CEA.
In 1993, my codiscussants, Dr. Rue and Dr. Pruitt, and I reported on a series of patients in whom we applied CEA. In summary, our results were dismal. One, it was almost $15,000 for every 1% of the body surface area covered. Two, there was an inverse correlation, as Dr. Pruitt pointed out, between body surface area burn and take of the cells. And, finally, our best results occurred in small burns in whom we applied CEA on dermal excisions.
Thus, Dr. Herndon and colleagues’ assessment of CEA in massively burned children is of particular clinical relevance. They have analyzed the most important outcomes to include take, length of stay, scar quality, and requirement for further operation. Although this is a nonrandomized trial, the results are hard to argue with, and it would appear that CEA is of least benefit in those who need it most. I have four questions for Dr. Herndon.
One, what was your indication for CEA use, in the sense that you had many children with greater than 90% burns in whom you chose not to use it?
Two, you have reported extensively on the use of growth hormone in massively burned kids with excellent outcome. How many kids in each group received growth hormone, and do you think it affected the results in any way?
Third, increased early costs may be offset if late function is improved. What data do you have concerning late function in terms of rehabilitation of these children?
And, finally, as Dr. Pruitt alluded, before discarding this technology, do you have any suggestions on how we might improve its utility either by altering its culture technique or the use of dermal analogues?
In sum, Dr. Herndon and colleagues are to be congratulated for their excellent results in a series of massively burned children. They have also pointed out, unfortunately, the lack of utility of CEA in its current form.
Dr. Loring Rue (Birmingham, Alabama): The Galveston group have presented their experience over the past 11 years in the care of extensively burned children. Dr. Herndon and others have made remarkable achievements in improving upon the survival of these patients, in that a 50% mortality is now realized with burn sizes of 90% of the body surface area or greater. As was presented in the manuscript, a major factor contributing to death in these patients is pulmonary-related. It is quite appropriate that attention is given to improving upon the quality of life of these surviving patients, specifically as it pertains to optimization of wound closure and functional outcome.
Unlike prior reports of the CEA technology, this paper focused on the long-term results of using cultured cells with respect to need for readmission for reconstructive needs and the quality of scar. Only institutions like Dr. Herndon’s, which provide longitudinal care of these challenging patients, can provide such a report.
I have two basic questions for the authors. One of the principal factors contributing to length of stay for extensively burned patients who undergo conventional autografting, often with wide expansion ratios such as 9:1, is the time it takes for interstitial closure. Further, as the authors have described, the quality of scar in these wounds is not optimal due to the lack of a dermis in these interstitial areas. Could Dr. Herndon speculate whether there could be a role for cultured epithelial autografts as an overlay of widely meshed conventional skin grafts to hasten interstitial closure and optimize the cosmetic and functional result? With the growth factors present in the cells, would this not be a better solution than the use of cadaver overlay and certainly provide more durable wound closure? Might this be a more cost-effective approach, as opposed to combining living dermal analog products with CEAs?
My second question is, can you provide a cost breakdown associated with cultured cells in that patient group? In a previous report from the U.S. Army Institute of Surgical Research, cultured cells were associated on average with a cost of $13,000 per 1% of the body surface area covered. If my calculations are correct from the manuscript, it appears as though on average, 12% of the body surface are was definitely closed with cultured autografts per patient. At the ISR rate, this would equate to over $150,000 of average cost or half the total cost per CEA patient as reported in the manuscript. Further, is there any cost efficacy to be realized by developing institutional “cottage industry” cell culturing labs over purchase from the commercial enterprises providing these cells?
Dr. David N. Herndon (Closing Discussion): Dr. Pruitt asked why more reconstruction was required with CEA if the scars were better. My reply is that the quality of the scars where the CEA actually took was better, but the areas where the CEA was lost to shear required more skin grafting and more reconstruction. In addition, when we say quality of the scar, we mean primarily its appearance, which in general was smoother and less hypertrophic. However, it is our anecdotal experience that the areas grafted with CEA tend to contract more, which may be one more reason for the increase in reconstructive procedures in this group.
CEA took better on fascia, but this bed is not as resilient and soft as underlying fat. Indeed, there probably were dermal elements in some of the tangentially excised areas covered initially with CEA that improved the stability of these grafts in the long term, perhaps through retained keratinocytes.
Recombinant human growth hormone was used in about a third of each group. There was no effect on apparent outcomes when we compared the effects on donor site healing or length of hospital stay in these small group of patients. We can draw no conclusions from this study population on the use of growth hormone because of the lack of statistical power. We have previously shown that recombinant human growth hormone improves the expression of dermal-epidermal proteins which might conceivably improve graft take and stability, but we could not ascertain this.
Dr. Cioffi asked about late functional differences between groups, and in fact no differences were found between groups. The only difference was an increase in the number of operations for the CEA group, which could be considered a negative effect.
What was our indication for CEA? We paid for the CEA grafts out of our operating budget, which we did about once a year over the course of this study. We had about two patients per year with >90% TBSA burns, so we also treated one patient per year with >90% TBSA burn without CEA. It was simply an economic consideration.
In regard to Dr. Rue’s questions about the cost of CEA per percentage of TBSA covered, our price for the product is approximately half the cost to other centers because it was developed in the Shriners Hospital system in Boston. Therefore, your calculations of $13,000 per 1% TBSA covered for this patient group may be an overestimation. Since about 20% TBSA was closed with CEA in the end in the patients who received it, and from our cost break we paid about $100,000 per patient for the CEA, it comes out to be approximately $5000/% TBSA covered. This is still a pretty expensive outlay, however.
The culture techniques using dermal analogues as a template should clearly be done under controlled study circumstances. We do not have any experience with these techniques at this time. It is hoped that growing cells on a dermal equivalent would improve long-lasting stable take which would expand the usefulness of the technology. In addition, new techniques to shorten the culture times using growth factors and possibly gene transfection would also likely improve its utility. Our opinion would be and this study seems to support the notion that these would be a fruitful areas of investigation. We also feel that the possibility of using CEA as coverage over widely expanded grafts (9:1 or greater) is very promising and likewise needs to be studied.
Footnotes
Correspondence: Steven E. Wolf, MD, Shriners Hospitals for Children, 815 Market St., Galveston, TX 77550.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
E-mail: swolf@utmb.edu
Accepted for publication December 1999.
References
- 1.Wolf SE, Rose JK, Desai MH, et al. Mortality determinants in massive pediatric burns. An analysis of 103 children with ≥80% TBSA burns (≥70% full-thickness). Ann Surg 1997; 225:554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heimbach DM. Early burn excision and grafting. Surg Clin North Am 1987; 67:93–107. [DOI] [PubMed] [Google Scholar]
- 3.Herndon DN, Barrow RE, Rutan RL, et al. A comparison of conservative versus early excision therapies in severely burned patients. Ann Surg 1989; 209:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munster AM. Cultured skin for massive burns. A prospective, controlled trial. Ann Surg 1996; 224:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickerson WL, Compton C, Flechall S, Smith LR. Cultured epidermal autografts and allodermis combination for permanent burn wound coverage. Burns 1994; 20(Suppl 1):S52–S55. [DOI] [PubMed] [Google Scholar]
- 6.Desai MH, Mlakar JM, McCauley RL, et al. Lack of long-term durability of cultured keratinocyte burn-wound coverage: a case report. J Burn Care Rehabil 1991; 12:540–545. [DOI] [PubMed] [Google Scholar]
- 7.Herndon DN, Barrow RE, Kunkel KR, Broemeling L, Rutan RL. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg 1990; 212:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilpin DA, Barrow RE, Rutan RL, Broemeling L, Herndon DN. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg 1994; 220:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeong EK, Mann R, Engrav LH, et al. Improved burn scar assessment with use of a new scar-rating scale. J Burn Care Rehabil 1997; 18:353–355. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor NE, Mulliken JB, Banks-Schlegel S, et al. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1981; i:75–78. [PubMed] [Google Scholar]
- 11.Odessey R. Addendum: multicenter experience with cultured epidermal autograft for treatment of burns. J Burn Care Rehabil 1992; 13:174–180. [PubMed] [Google Scholar]
- 12.Munster AM, Weiner SH, Spence RJ. Cultured epidermis for the coverage of massive burn wounds. A single-center experience. Ann Surg 1990; 211:676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blight A, Mountford EM, Cheshire IM, et al. Treatment of full skin thickness burn injury using cultured epithelial grafts. Burns 1991; 17:495–498. [DOI] [PubMed] [Google Scholar]
- 14.Compton CC, Hickerson W, Nadire K, Press W. Acceleration of skin regeneration from cultured epithelial autografts by transplantation to homograft dermis. J Burn Care Rehabil 1993; 14:653–662. [DOI] [PubMed] [Google Scholar]
- 15.Hansbrough JF, Dore C, Hansbrough WB. Clinical trials of a living dermal tissue replacement placed beneath meshed, split-thickness skin grafts on excised burn wounds. J Burn Care Rehabil 1992; 13:519–529. [DOI] [PubMed] [Google Scholar]
- 16.Sheridan RL, Tompkins RG. Cultured autologous epithelium in patients with burns of ninety percent or more of the body surface. J Trauma 1995; 38:48–50. [DOI] [PubMed] [Google Scholar]
- 17.McAree KG, Klein RL, Boeckman CR. The use of cultured epithelial autografts in the wound care of severely burned patients. J Pediatr Surg 1993; 28:166–168. [DOI] [PubMed] [Google Scholar]
- 18.Rheinwald J, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975; 6:331–344. [DOI] [PubMed] [Google Scholar]
- 19.Gallico G, O’Conner N, Compton C, et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 1984; 311:448–451. [DOI] [PubMed] [Google Scholar]
- 20.Paddle-Ledinek JE, Cruickshank DG, Masterton JP. Skin replacement by cultured keratinocyte grafts: an Australian experience. Burns 1997; 23:204–211. [DOI] [PubMed] [Google Scholar]
- 21.Desai MH, Herndon DN, Broemeling L, et al. Early burn wound excision significantly reduces blood loss. Ann Surg 1990; 211:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rue LW 3rd, Cioffi WG, McManus WF, Pruitt BA Jr. Wound closure and outcome in extensively burned patients treated with cultured autologous keratinocytes. J Trauma 1993; 34:662–668. [PubMed]
- 23.Compton C, Gill J, Bradford D, et al. Skin regenerated from culture epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. Lab Invest 1989; 60:600–612. [PubMed] [Google Scholar]
- 24.Wood F, Liddiard K, Skinner A, Ballentyne J. Scar management of cultured epithelial autograft. Burns 1996; 22:451–454. [DOI] [PubMed] [Google Scholar]
- 25.Cuono C, Langdon R, Birchall N, et al. Composite autologous-allogenic skin replacement: development and clinical application. Plast Reconst Surg 1987; 80:626–637. [DOI] [PubMed] [Google Scholar]
- 26.Daniels JT, Kearney JN, Ingham E. An investigation into the potential of extracellular matrix factors for attachment and proliferation of human keratinocytes on skin substitutes. Burns 1997; 23:26–31. [DOI] [PubMed] [Google Scholar]
- 27.Boyce ST, Hansbrough JF. Biologic attachment, growth, and differentiation of cultured human epidermal keratinocytes on a graftable collagen and chondroitin-6-sulfate substrate. Surgery 1988; 103:421–431. [PubMed] [Google Scholar]
- 28.Boyce ST. Skin substitutes from cultured cells and collagen-GAG polymers. Med Biol Eng Comput 1998; 36:791–800. [DOI] [PubMed] [Google Scholar]
- 29.Pandya AN, Woodward B, Parkhouse N. The use of cultured autologous keratinocytes with Integra in the resurfacing of acute burns. Plast Reconst Surg 1998; 102:825–828. [PubMed] [Google Scholar]
- 30.Rennekampff HO, Kiessig V, Griffey S, Greenleaf G, Hansbrough JF. Acellular human dermis promotes cultured keratinocyte engraftment. J Burn Care Rehabil 1997; 18:535–544. [DOI] [PubMed] [Google Scholar]
- 31.Aihara M. Ultrastructural study of grafted autologous cultured human epithelium. Br J Plast Surg 1989; 42:35–42. [DOI] [PubMed] [Google Scholar]