Abstract
Objective
To determine whether infiltrating lobular carcinoma (ILC) is associated with high positive-margin rates for single-stage lumpectomy procedures, and to define clinical, mammographic, or histologic characteristics of ILC that might influence the positive-margin rate, thereby affecting treatment decisions.
Summary Background Data
Infiltrating lobular cancer represents approximately 10% of all invasive breast carcinomas and is often poorly defined on gross examination.
Methods
A group of 47 patients with biopsy-proven ILC undergoing breast-conservation therapy (BCT) at the University of Virginia Health Sciences Center between 1975 and 1999 was compared with a group of 150 patients with infiltrating ductal cancer undergoing BCT during the same time period. The pathology of the lumpectomy specimen was reviewed for each patient to confirm surgical margin status. Office and surgical notes as well as mammography reports were examined to determine whether the lesions were deemed palpable before and during surgery. Patients were stratified according to age, family history, tumor size, tumor location, and histologic features of the tumor.
Results
The incidence of positive margins was greater in the ILC group compared with the infiltrating ductal cancer group. Patient age, family history, and preoperative palpability of the tumor did not correlate with surgical margin status. Of the mammographic features identified, including spiculated mass, calcifications, architectural distortion, and other densities, only architectural distortion predicted positive surgical margin status. Tumor grade, tumor size, lymph node status, and receptor status were not predictive of surgical margin status.
Conclusions
For patients with ILC, BCT is feasible, but these patients are at high risk of tumor-positive resection margins (51% incidence) after the initial resection. Only the mammographic finding of architectural distortion was identified as a preoperative marker reliably identifying a subgroup of ILC patients at especially high risk for a positive surgical margin. For all patients with ILC considering BCT, careful counseling about the potential need for a second procedure to treat the positive margin should be included in the treatment discussion.
Infiltrating lobular cancer (ILC) was first described in 1865 by Cornil 1 as a diffusely infiltrative tumor composed of small, round, and regular cells that form single lines throughout a desmoplastic stroma. In 1946, Foote and Stewart 2 developed the criteria now accepted for the diagnosis of classic ILC. Using their strict definition, ILC accounted for approximately 3% to 5% of all breast cancers. In the 1970s, solid, alveolar, mixed, and pleomorphic variants of ILC were described and the definition was broadened to include these subtypes. 3–5 This modern, more broadly accepted ILC category currently represents approximately 10% of all breast cancers. 4 During the past decade, attention has focused on comparing the treatment strategies used for ILC with the treatment strategies accepted for the more common, and better understood, infiltrating ductal cancers.
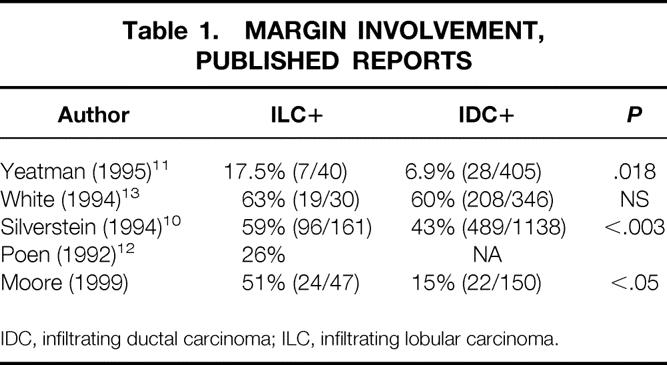
Because of its diffusely infiltrative growth pattern, ILC may fail to produce a dominant mass. Further, ILC often does not provoke a surrounding desmoplastic (fibrous) reaction, making assessment of the extent of disease difficult. 6 These features led surgeons to question whether ILC was amenable to breast-conservation therapy (BCT). Subsequently, several studies have shown that BCT produces an acceptably low recurrence rate if the lumpectomy margins are negative. 7–10 At the same time, others have highlighted the difficulty of obtaining negative margins for ILC 11–15 when attempting BCT (Table 1). Indeed, in a review of 1,200 patients, Silverstein et al 10 found a 41% success rate of obtaining negative margins for ILC, compared with 57% for the more commonly occurring infiltrating ductal cancer (IDC); the difference was significant (P = .003).
Table 1. MARGIN INVOLVEMENT, PUBLISHED REPORTS
IDC, infiltrating ductal carcinoma; ILC, infiltrating lobular carcinoma.
With the increasing use of stereotactic core biopsies for diagnosing breast cancer, single-stage lumpectomy has become feasible for many patients. 16 This evolving surgical approach places new emphasis on obtaining negative margins at a single surgical procedure. From the perspective of expeditious care of the cancer, patient satisfaction, cost, and cosmetic outcome, 16 it is desirable to avoid multiple surgical procedures to complete adequate tumor excision to negative margins. During preoperative counseling, patients considering BCT often ask about the likelihood that a single surgical procedure will be able to excise the tumor completely.
We reviewed all patients undergoing single-stage lumpectomy at the University of Virginia for ILC since 1975 to evaluate two features of the procedure: to determine whether ILC is associated with high positive-margin rates for single-stage lumpectomy procedures, and to define clinical, mammographic, or histologic characteristics of ILC that might influence the positive-margin rate, thereby affecting treatment decisions.
METHODS
Forty-seven women with biopsy-proven ILC who underwent BCT at the University of Virginia between January 1, 1975, and July 1, 1999, were reviewed. Their records were compared with those of 150 women with biopsy-proven IDC who underwent attempted BCT during the same time period. Charts were reviewed by two authors (M.M., G.B.) for clinical data including demographics, family history, clinical examination, and tumor location. Preoperative mammograms were reviewed for tumor characteristics and location. A single author (R.F.) reviewed all pathology slides and pathology reports to corroborate or redefine pathologic diagnoses and margin status. Surgical margins were deemed positive if the inked surface of the specimen contained transected tumor-filled ducts or if tumor cells were present at the perimeter. Margins were also deemed positive if tumor was present to within 2 mm of the margin and the treating surgeon had counseled the patient that she required further resection. If tumor was not transected and if the treating surgeon noted in the record that he or she viewed the margin as acceptable (e.g., tumor approaching intact fascia at the deep margin), the margin was recorded as negative. Specimens were also reviewed to ascertain whether ILC only or associated ductal carcinoma in situ (DCIS) was present at the margin. A margin with lobular carcinoma in situ only was deemed negative.
Preoperative features of patients with ILC were examined to identify variables that might influence the surgeon’s ability to obtain negative margins. Specifically, age, family history, and the treating surgeon’s preoperative assessment of tumor palpability were reviewed. Family history was deemed positive if the patient had one or more first-degree relatives with breast cancer.
Preoperative mammograms were reviewed for characteristics, including spiculation, architectural distortion, calcification, and other densities associated with the tumor. The tumor location was deemed central if it were deep to any portion of the areola based on the mammographic mapping of the lesion.
To evaluate intraoperative features that might correlate with surgical margin status, surgical reports were reviewed. The method of tumor diagnosis (needle vs. surgical biopsy) in the patients with ILC was determined. Intraoperative features analyzed included tumor location, intraoperative palpability, and frozen sections obtained. The histologic features evaluated included tumor size, estrogen and progesterone receptor status, axillary lymph node metastases, and associated noninfiltrating cancers. Tumor grade was examined; grading of the tumors was based on the scheme of Black and Speer. 16 Finally, the patient charts were analyzed to determine the total number of surgical procedures required to achieve negative margins for ILC and IDC. The extent of tumor present on subsequent surgical procedures undertaken as a result of positive surgical margins was analyzed. Patients who ultimately chose to undergo mastectomy were identified.
Pairwise comparisons for statistical significance were performed using chi-square analysis with the Bonferroni correction for multiple comparisons. Analysis was performed using SPSS-MAC (SPSS Inc., Chicago, IL). P < .05 signified statistical significance.
RESULTS
A total of 197 patients were evaluated, 150 with IDC and 47 with ILC. The incidence of positive margins was 51% in the ILC group and 15% in the IDC group (P = 2 × 10−7). Table 1 compares our results with those of other published reports.
Preoperative Features
No preoperative feature had a significant effect on margin status (Table 2). Fifty-six percent of the younger patients had positive surgical margins versus 48% of the patients older than 50. Five of eight patients (63%) with at least one first-degree relative with breast cancer had positive margins, whereas 19 of 38 (50%) with a negative family history had positive margins.
Table 2. PREOPERATIVE DATA: CLINICAL FEATURES ASSOCIATED WITH POSITIVE MARGINS
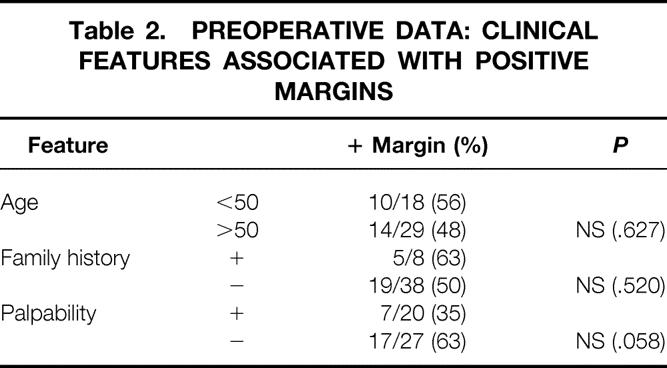
Of the 20 patients considered to have palpable tumors before surgery, 7 (35%) had positive margins, whereas 17 (63%) of the 27 patients with clinically nonpalpable tumors had positive margins after lumpectomy. This difference did not reach statistical significance (P = .058)
Intraoperative Features
Intraoperative features also had little impact on the ultimate margin status (Table 3). Tumors noted to be palpable during surgery were somewhat more likely to be excised to negative margins, but the difference was not statistically significant. In 8 (38%) of 21 palpable tumors, margins were positive, compared with 16 (61%) of 26 nonpalpable tumors. The difference was not significant by chi-square analysis.
Table 3. INTRAOPERATIVE DATA: FEATURES ASSOCIATED WITH POSITIVE MARGINS
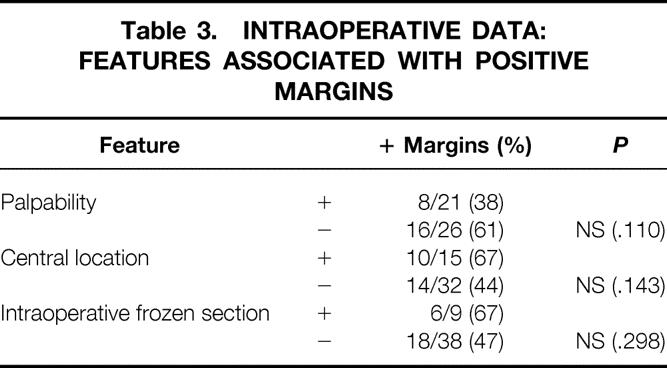
Obtaining frozen sections of the margins did not reduce the likelihood of obtaining positive margins. Of the nine tumors evaluated by frozen section, six (67%) had positive margins on final evaluation. In reviewing these six, we believe that only one patient had a false-negative frozen-section evaluation. In the remaining five, it appears likely that the surgeon obtained a frozen section of a truly negative margin. These patients, however, had positive margins that were distant from the sampled frozen-section margins.
Location of the tumor in the retroareolar or central portion of the breast was associated with positive surgical margins in 10 of 15 patients (67%) compared with 14 of 32 patients (44%) with peripherally located tumors, but this difference was not significant (P = .143).
Mammography
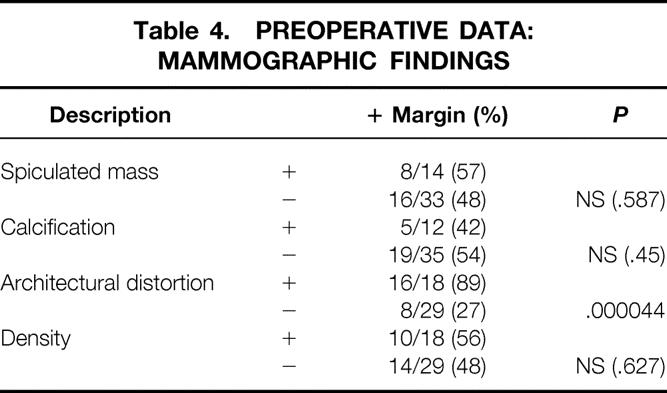
Analysis of mammographic features yielded the only positive predictor (Table 4). Architectural distortion found on the preoperative mammogram correlated with a positive-margin rate of 86%; for patients without architectural distortion, the positive-margin rate was only 14% (P = .000044). However, the mammographic finding of a spiculated mass, calcification, or other density did not correlate with margin status.
Table 4. PREOPERATIVE DATA: MAMMOGRAPHIC FINDINGS
Histology
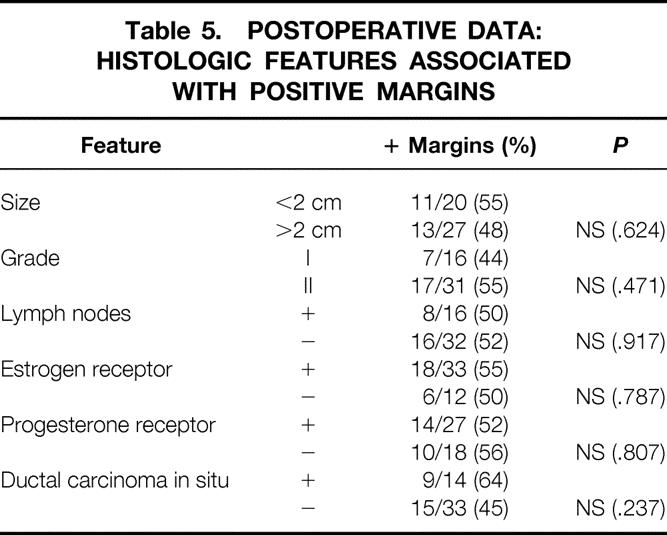
We found no correlation between tumor size and margin status (Table 5). Eleven of 20 patients (55%) with tumors of 2 cm or less (T1 lesions) had positive margins, whereas 13 of 27 patients (48%) with tumors larger than 2 cm had positive margins. Tumor grade, lymph node status, and the presence of estrogen and progesterone receptors also did not correlate with surgical margin results. The presence of DCIS increased the likelihood of positive margins: margins were positive in 9 of 14 patients (64%) with DCIS and in 15 of 33 patients (45%) without DCIS, but this difference was not significant (P = .237). In reviewing the nine specimens with DCIS present and positive margins, seven of the positive margins had DCIS present at a margin. Of these seven, DCIS alone and not ILC accounted for the positive-margin status in five cases.
Table 5. POSTOPERATIVE DATA: HISTOLOGIC FEATURES ASSOCIATED WITH POSITIVE MARGINS
As expected, the average number of surgical procedures required for patients with ILC (1.64) exceeded the average number of procedures required for patients with IDC (1.15). Twelve of the 47 patients (26%) with ILC ultimately chose to have a mastectomy, compared with 14 of the 150 patients (9%) with IDC (P < .05). Patients undergoing second procedures for IDC had no residual disease in 16 of 23 resection specimens (70%), compared with 11 of 20 (55%) of those with ILC. Although this suggests that residual disease was found more often in ILC patients, the difference was not statistically significant.
DISCUSSION
Several surgeons who have followed up patients for 3 to 10 years after BCT and radiation therapy for ILC have reported that both in terms of local recurrence and long-term survival, these patients do as well as patients with IDC treated similarly. They concluded that BCT was an acceptable alternative to mastectomy for patients with ILC. These reports stimulated a heightened acceptance of BCT for the treatment of early-stage ILC. Our results do not contradict the prior reports of the safety of BCT; rather, we highlight the difficulty of successfully performing BCT for these patients.
Our results substantiate those of previous reports 11–13 that ILC is more likely to be associated with positive margins than IDC when the surgeon attempts to perform BCT. In our series, which spans a 25-year experience at one teaching hospital, surgical margins were positive in 51% of the ILC patients undergoing BCT and 15% of the IDC patients. There is a significant range of positive-margin rates in the reports cited. White et al 13 reported a failure rate of 63% in ILC patients, compared with 26% for Poen et al. 12 Despite the discrepancy between groups, however, within any particular study surgical margins were more likely to be positive in the ILC group.
Our report stands in the middle of the cited range, although we found a greater internal discrepancy between positive-margin rates for ILC versus IDC than had previous authors. Our results may be explained by a willingness of the surgeons at our institution to offer BCT to patients with tumors that were larger or more difficult to define; these patients who may be steered toward mastectomy at other centers.
It has been the practice of our surgical group to offer patients with the diagnosis of ILC the same counseling that patients with IDC receive regarding the options of BCT and mastectomy. On review of our data, however, it is clear that patients with ILC require two or more surgical procedures to obtain negative surgical margins substantially more often than do patients with IDC. It is important for our patients to understand that multiple surgical procedures may be required to achieve adequate tumor resection, and we have instituted a change in our counseling procedures as a result of this study.
We extensively analyzed several preoperative features in the hopes of identifying characteristics that could reliably predict the surgical margin status. Only architectural distortion on mammography was statistically more likely to predict positive margins. We analyzed the histology of the tumors manifested by architectural distortion but found no association of the mammographic feature with tumor size, grade, or receptor status. However, it seems likely that tumors manifested by architectural distortion may grow in a particularly infiltrative pattern such that the margins are impossible for the surgeon to appreciate clinically. Indeed, if the tumor is diffuse and tumor cells are sparse at the edges of the specimen, standard methods of intraoperative evaluation, such as frozen-section or touch-prep histology, are also likely to be unsuccessful in determining the edge of the tumor.
Although central location and lack of palpability were not found to be predictive of positive margins in this review, our series did not have adequate power to reveal small differences. Given the percentage differences in margin status seen here between tumors that were palpable and nonpalpable at surgery, we would expect to see a significant difference in a series of 75 patients. Likewise, we see some indication in our data that central location (10/15 [67%] positive) predicts positive margins, compared with 14 of 32 (44%) positive margins seen with peripheral tumors. Once again, our study lacked the power to show a difference at this level. We hope to stimulate a larger or multicenter review to evaluate these factors.
The popularization of core biopsy has exacerbated the dichotomy in treatment pathways between patients with ILC and IDC. In previous years, when surgical excisional biopsies were commonly the first procedure on new breast masses, one could reexcise positive margins as part of BCT. Today, however, BCT is usually the first surgical procedure that the patient with breast cancer undergoes. Increased emphasis is therefore brought to bear on the surgeon’s ability to excise the tumor to negative margins while at the same time obtaining a cosmetically acceptable result in this single procedure.
CONCLUSION
We hope to stimulate the refinement of surgical, radiologic, and pathologic procedures for defining the extent of ILC. Newer techniques, such as rapid-sequence gadolinium-contrast magnetic resonance imaging and intraoperative use of ultrasound, may be cost-effective measures to define the extent of tumor. Although obtaining intraoperative frozen sections of suspicious margins did not improve the ultimate outcome of surgical margins in this series, it was clear that in our patients frozen sections were frequently obtained from suspicious but not-involved portions of the breast. Indeed, only one of nine frozen sections was a false-negative reading of a surgical margin. Based on this small sample, it may be worthwhile to consider expanding frozen-section analysis to all margins, or to obtain touch preps of all margins in those institutions where the technique is available.
Although it may be tempting for the surgeon to conclude that a wider resection is indicated for all ILCs, an undirected additional excision carries a poor cost/benefit ratio. For example, the surgeon beginning with a lumpectomy specimen 4.85 cm in diameter will double the volume of tissue resected by obtaining an additional 1-cm margin of the biopsy cavity. It is far more appealing from a cosmetic standpoint to direct the excision as precisely as possible.
We found no correlation between the size of tumor resected and the margin status and no relation between the size of the surgical specimen excised and the margin status. Undeniably, the surgeon who performs a larger resection will have an increased likelihood of randomly excising the tumor to negative margins. However, our analysis underscores the difficulty of accurately finding the subtle margins of ILC.
In the future, we must bring new techniques to bear on this problem. In the meanwhile, we will advise our patients with ILC who are considering BCT of the inherent technical challenges of the procedure.
Discussion
Dr. Edward M. Copeland III (Gainesville, Florida): Dr. Moore, congratulations to you, Dr. Hanks, and your colleagues on bringing to our attention the important observation that infiltrating lobular carcinomas are notorious for having positive surgical margins in the biopsy specimen.
I am more concerned about the positive margins that you do not find pathologically than those you do. The malignant cells of lobular carcinoma can invade singularly throughout the breast stroma or present in rows known as Indian filing. Detecting malignant cells in the surgical margin can therefore be difficult even after reexcision, and one would expect the recurrence rate to be higher for lobular carcinomas. Dr. Moore, has this been your experience?
Would it be valuable to do immunohistochemical stains on margins to detect occult malignant cells not distinguishable from normal hematopoietic cells by hematoxylin and eosin stains?
And the third question: do your radiation therapists treat patients with invasive lobular carcinoma and negative margins at the lumpectomy any different than they treat patients who have invasive ductal carcinoma with negative margins?
Dr. Marshall M. Urist (Birmingham, Alabama): I’d like to ask a question about the patient whom you know has infiltrating lobular carcinoma, and you are trying to counsel that patient. I know it’s in your data. What is the probability that that patient will be able to have breast conservation therapy compared to a patient who has invasive ductal cancer?
Some of your patients who are positive margin to begin with, even though you make your best effort, can undergo reexcision and reach negative margins. So if you are going to counsel the patient up front, simply knowing on the basis of a core needle biopsy that it is invasive lobular cancer, what’s the probability that patient will be able to have breast conservation therapy?
Dr. Courtney M. Townsend, Jr. (Galveston, Texas): I’d like to ask the grand old mammographer, what is architectural distortion? How will we know it? What is it going to look like? And does everybody know the same thing?
Dr. Marcia Moore (Closing Discussion): I’d like to acknowledge the input we have had from Dr. M. C. Wilhelm, a member of the Southern Surgical Association, who has for many years provided a great deal of support to the breast program at the University of Virginia. Many of the patients in this series—it goes back a large number of years—were Dr. Wilhelm’s patients, and he pioneered breast conservation therapy on these patients with invasive lobular carcinomas.
To answer Dr. Copeland’s question, indeed this was a predicate of mine as well, that we would find that recurrence rates were higher for patients with invasive lobular carcinomas. We do not have the kind of long-term follow-up on these patients that would enable me to answer the question of recurrence. There are a number of studies in the literature, including the work cited in the paper by Ancher and White that suggests that patients who achieve what is believed by the pathologists to be a negative margin with invasive lobular cancer do not have a higher local recurrence rate than those with infiltrating ductal cancers, which surprises me as well.
What may be the case is that as the pathologist finds the cancer, he or she is then able to follow it through sequential sections to give the surgeon an accurate assessment of the margin status. What I found in my own frozen section analysis, however, is that the surgeon obtaining frozen sections often does not know by palpation where the edge of the tumor is and is testing the wrong margin.
In response to the question about how I counsel my patients, I now counsel a patient with infiltrating lobular cancer that I am more than willing to attempt breast conservation therapy. However, I believe it is fair and prudent to inform her—especially the tumor presented with architectural distortion on a mammogram—that she may be needing two or three surgeries to obtain a negative margin, I think that if the woman is committed to undergo multiple surgeries to obtain a negative margin, then breast conservation is a good choice for her. I do think that it makes sense to counsel her up front as to what we may expect.
The average number of surgeries for patients with infiltrating lobular cancers is 1.6 compared to infiltrating ductal cancers. Those patients have 1.1 surgeries on average. It is a statistically significant difference, and I think it makes sense to counsel patients.
As to the last question about architectural distortion, I think that’s why we work closely with our mammography colleagues. Architectural distortion, as defined by Dr. Jennifer Harvey, my coauthor, is an area of disturbance of the usual pattern of Cooper’s ligaments. This is typically manifested as straight lines radiating toward a central area. Indeed, the indistinctness of the borders of an area of architectural distortion, seen mammographically, corresponds to our difficulty in finding a distinct lesion on physical examination.
Footnotes
Correspondence: Marcia M. Moore, MD, University of Virginia, Dept. of Surgery, P.O. Box 800709, Charlottesville, VA 22908-0709.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
E-mail: mmm7k@virginia.edu
Accepted for publication December 1999.
References
- 1.Cornil A. Contributions a l’histoire du development histologique des tumeur epitheliale (squirrhe, encephaloid, et). J Anatom Physiol 1865; 2:226–276. [Google Scholar]
- 2.Foote FW Jr, Stewart FW. A histologic classification of carcinoma of the breast. Surgery 1946; 19:74–99. [PubMed] [Google Scholar]
- 3.Fechner RE. Histologic variants of infiltrating lobular carcinoma of the breast. Hum Pathol 1975; 6:373–378. [DOI] [PubMed] [Google Scholar]
- 4.Martinez V, Azzopardi JG. Invasive lobular carcinoma of the breast: incidence and variants. Histopathology 1979; 3:467–488. [DOI] [PubMed] [Google Scholar]
- 5.Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW. Infiltrating lobular carcinoma of the breast. Histopathology 1982; 6:149–161. [DOI] [PubMed] [Google Scholar]
- 6.Tavassoli FA. Infiltrating lobular carcinoma. In: Pathology of the Breast. Norwalk, CT: Appleton and Lange; 1999:307–315.
- 7.Horiguchi J, Lino Y, Takei H. Surgical margin and breast recurrence after breast-conserving therapy. Oncol Report 1991; 6:135–138. [PubMed] [Google Scholar]
- 8.Schnitt SJ, Abner A, Gelman R, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patient with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer 1994; 74:1746–1751. [DOI] [PubMed] [Google Scholar]
- 9.Spivack B, Khanna MM, Tafra L, et al. Margin status and local recurrence after breast-conserving surgery. Arch Surg 1994; 129:952–957. [DOI] [PubMed] [Google Scholar]
- 10.Silverstein MJ, Lewinsky BS, Walsman JR, et al. Infiltrating lobular carcinoma: is it different from infiltrating ductal carcinoma. Cancer 1994; 73:1673–1677. [DOI] [PubMed] [Google Scholar]
- 11.Yeatman TJ, Cantor AB, Smith TJ, et al. Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg 1995; 222:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poen JC, Tran L, Juillard G, et al. Conservative therapy for invasive lobular carcinoma of the breast. Cancer 1992; 69:2789–2795. [DOI] [PubMed] [Google Scholar]
- 13.White JR, Gustafson GS, Wimbish K, et al. Conservative surgery and radiation therapy for infiltrating lobular carcinoma of the breast. Cancer 1994; 74:640–647. [DOI] [PubMed] [Google Scholar]
- 14.Anscher MS, Jones P, Prosnitz LR, et al. Local failure and margin status in early-stage breast carcinoma treated with conservation surgery and radiation therapy. Ann Surg 1993; 218:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney TJ, Morrow M. Effect of re-excision on the success of breast-conserving surgery. Ann Surg Oncol 1995; 2:303–307. [DOI] [PubMed] [Google Scholar]
- 16.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet 1957; 105:97–102. [PubMed] [Google Scholar]


