Abstract
Objective
To assess the quality of life (QOL) and functional outcome of patients after pancreaticoduodenectomy.
Summary Background Data
Pancreaticoduodenectomy is gaining acceptance and is being performed in increasing numbers for various malignant and benign diseases of the pancreas and periampullary region. There is a general impression that pancreaticoduodenectomy can severely impair QOL and alter normal activities. Only a few small studies have evaluated QOL after pancreaticoduodenectomy.
Methods
A standard QOL questionnaire was sent to 323 patients surviving pancreaticoduodenectomy who had undergone surgery at The Johns Hopkins Hospital between 1981 and 1997. Thirty items on a visual analog scale were categorized into three domains: physical (15 items), psychological (10 items), and social (5 items). Scores are reported as a percentile, with 100% being the highest possible score. The same QOL questionnaire was also sent to laparoscopic cholecystectomy patients and healthy controls. A separate component of the questionnaire asked about functional outcomes and disabilities.
Results
Overall QOL scores for the 192 responding pancreaticoduodenectomy patients in the three domains (physical, psychological, social) were 78%, 79%, and 81%, respectively. These QOL scores were comparable to those of the 37 laparoscopic cholecystectomy patients and the 31 healthy controls. The pancreaticoduodenectomy patients were subgrouped into chronic pancreatitis, other benign disease, pancreatic adenocarcinoma, and other cancers. Patients who underwent resection for chronic pancreatitis and pancreatic adenocarcinoma had significantly lower QOL scores in the physical and psychological domains compared with the laparoscopic cholecystectomy patients and the healthy controls. Common problems after pancreaticoduodenectomy were weight loss, abdominal pain, fatigue, foul stools, and diabetes.
Conclusions
This is the largest single-institution experience assessing QOL after pancreaticoduodenectomy. These data demonstrate that as a group, patients who survive pancreaticoduodenectomy have near-normal QOL scores. Many patients report weight loss and symptoms consistent with pancreatic exocrine and endocrine insufficiency. Most patients have QOL scores comparable to those of control patients and can function independently in daily activities.
Pancreaticoduodenectomy is gaining acceptance as an appropriate procedure for various malignant 1–7 and benign diseases 8,9 of the pancreas and periampullary region. In many tertiary referral centers, pancreaticoduodenectomy is now performed with complication rates less than 40% and with death rates of 5% or lower. 4,10–14 As experience with pancreaticoduodenectomy grows, there are increasing numbers of pancreaticoduodenectomy survivors who have recovered from the procedure and who live with the requisite altered upper gastrointestinal anatomy. These survivors have not been adequately studied in terms of their postprocedure quality of life (QOL) and outcomes such as pain, stool habits, activity levels, and other parameters.
The concept of QOL has its origins in the theoretical works of ancient philosophers and ethicists who dealt with issues of life’s worth, meaning, and value. Although QOL is a concept that is easily understood intuitively, it has proven difficult to define and measure. In a simple sense, as it relates to surgical patients after a procedure for a disease process, health-related QOL seeks to measure the impact of the disease process on the physical, psychological, and social aspects of the person’s life and feeling of well-being. 15
Only a few studies have evaluated QOL in patients who have undergone pancreaticoduodenectomy. For example, Patti et al 16 and Fink et al 17 evaluated gastric emptying and gastrointestinal function; they found many patients with mild gastrointestinal symptoms. McLeod et al 18 compared 25 patients after pancreaticoduodenectomy with 25 age- and sex-matched patients who had undergone cholecystectomy using six QOL instruments; they found no significant differences. Similarly, Patel et al 19 found little deficit in overall function when they analyzed 23 pancreaticoduodenectomy patients with the Memorial Sloan-Kettering Pain Assessment Card. Melvin et al 20 reported QOL assessment using the Short Form-36 health survey (SF-36), as well as nutritional parameters and pancreatic exocrine evaluation in 45 pancreaticoduodenectomy patients, comparing 21 pylorus-preserved patients and 24 standard resection patients with age-matched controls. Their results appeared to favor pylorus preservation over standard resection, although the differences were significant in only three of eight QOL domains. Further, a recent study by Kokoska et al 21 used self-reported Karnofsky performance status as a QOL index; the authors found that patients who underwent resection for localized pancreatic cancer had improved QOL and survival compared with patients who did not undergo resection. Finally, a recent review by Schmier et al 22 addressed the state of the literature on health-related QOL assessment in patients with pancreatic cancer.
The current study was designed to assess the QOL of patients after pancreaticoduodenectomy and to provide a comparison with laparoscopic cholecystectomy patients and healthy controls. A separate component of this study involved a questionnaire that asked about functional capabilities and disabilities after surgery.
METHODS
The data collection and methodology for this study were approved by the Joint Committee on Clinical Investigation of The Johns Hopkins Hospital. A standard QOL survey instrument 23 was sent by U.S. mail to 323 patients surviving pancreaticoduodenectomy who had undergone surgery between 1981 and 1997, inclusive, at The Johns Hopkins Hospital. All patients had undergone the surgery at least 6 months previously. The instrument comprised 30 items categorized into three domains: physical (15 items), psychological (10 items), and social (5 items). Scores are reported as a percentile, with 100% being the highest possible QOL score.
The survey tool was a minor modification of the City of Hope Medical Center Quality of Life Survey, which evolved from work by Padilla et al 24 and Present et al. 25 Each question in this multidimensional survey instrument consists of a 100-mm visual analogue scale with word extremes as anchors at each end. This survey instrument (or minor modifications of it) has been used to assess the QOL of cancer patients receiving radiation or chemotherapy, patients receiving bone marrow transplants, cancer patients with pain, long-term survivors of breast and other cancers, and pancreaticoduodenectomy survivors. 23,26–29 The survey instrument has been subjected to psychometric analysis using 686 subjects from the National Coalition for Cancer Survivorship, with assessment of both reliability and validity. 27 Two measures of reliability included test–retest (r = 0.89) and internal consistency (r = 0.93). Five measures of validity (content, predictive, concurrent, construct, and discriminate) were used to determine the extent to which the instrument measured the concept of QOL. The findings demonstrated that this survey instrument adequately measured QOL in this group of cancer survivors.
The same QOL survey instrument was also sent to 100 age- and sex-matched laparoscopic cholecystectomy patients who had undergone the procedure at least 6 months previously, and to a group of 100 age- and sex-matched healthy controls (hospital personnel at The Johns Hopkins Hospital). Laparoscopic cholecystectomy patients were chosen as the comparable control group for statistical purposes because a cholecystectomy is performed as part of a pancreaticoduodenectomy, allowing better assessment of the effect of pancreaticoduodenal resection alone.
A separate component of the survey instrument that was sent to pancreaticoduodenectomy and laparoscopic cholecystectomy patients inquired about functional outcomes. This questionnaire specifically asked about the following items before and after surgery: changes in weight, bowel habits, self-care capabilities, social habits, exercise tolerance, and specific conditions such as diabetes, foul stool, abdominal pain, thirst, frequent urination, and fatigue. The healthy control group did not complete this functional outcome survey because they did not undergo surgery.
Comparisons between groups were performed with the Student t test and chi-square statistics. Results are reported as mean ± standard error. Significance was accepted at the 5% level.
RESULTS
Study Populations
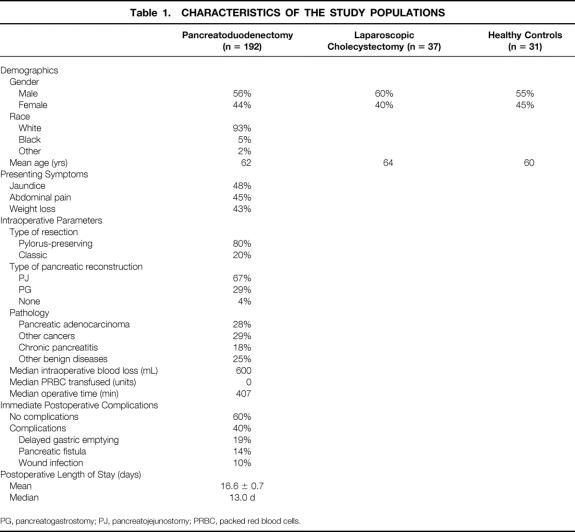
Completed questionnaires were returned from 192 pancreaticoduodenectomy patients (59%), 37 laparoscopic cholecystectomy patients (37%), and 31 healthy controls (31%). The characteristics of the study populations are depicted in Table 1. The three groups were comparable with respect to sex and age. The pancreaticoduodenectomy population was 56% male and 93% white, with a mean age of 62.2 ± 0.9 years and a mean follow-up of 47 months (median = 41 months) after surgical resection. The most common presenting symptoms were jaundice, abdominal pain, and weight loss. Eighty percent of the patients underwent pylorus-preserving pancreaticoduodenectomy and 67% had pancreaticojejunostomy performed as the method of pancreatic-enteric reconstruction. Fifty-seven percent of the resection specimens revealed malignant pathology, with 28% of all specimens revealing pancreatic adenocarcinoma.
Table 1. CHARACTERISTICS OF THE STUDY POPULATIONS
PG, pancreatogastrostomy; PJ, pancreatojejunostomy; PRBC, packed red blood cells.
Intraoperative parameters in the pancreaticoduodenectomy group included a median intraoperative blood loss of 600 mL, with the median units of packed red cells transfused being zero. The most common immediate postoperative complications were delayed gastric emptying, pancreatic fistula, and wound infection. The median postoperative length of hospital stay was 13 days.
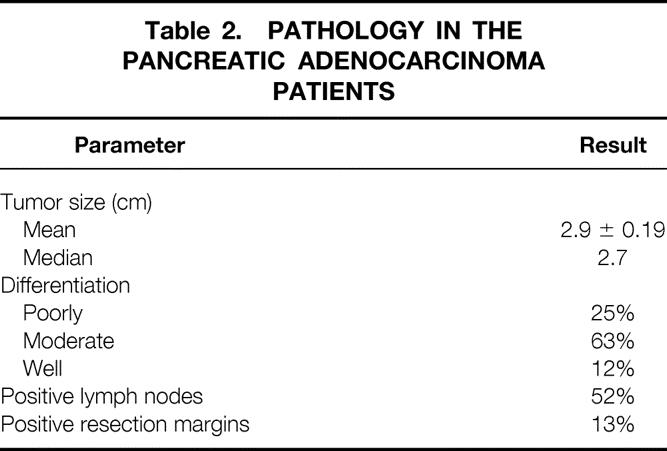
In the subgroup of patients who underwent pancreaticoduodenectomy for pancreatic adenocarcinoma (n = 54), the pathologic details of the surgical specimen are shown in Table 2. The median tumor size was 2.7 cm, 63% of the tumors were moderately differentiated, 52% of the specimens were positive for metastatic tumor in specimen lymph nodes, and 13% of the specimens had positive resection margins.
Table 2. PATHOLOGY IN THE PANCREATIC ADENOCARCINOMA PATIENTS
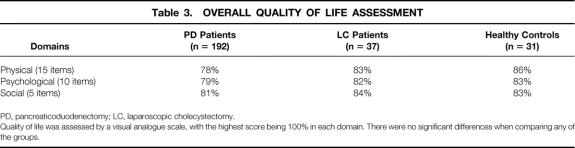
The overall QOL assessment scores for the pancreaticoduodenectomy patients, laparoscopic cholecystectomy patients, and healthy controls are given in Table 3. For pancreaticoduodenectomy patients, the QOL scores in the physical, psychological, and social domains were 78%, 79%, and 81%, respectively. These scores were not significantly different than the scores of 83%, 82%, and 84%, respectively, seen in the laparoscopic cholecystectomy patients or the scores of 86%, 83%, and 83%, respectively, seen in healthy controls.
Table 3. OVERALL QUALITY OF LIFE ASSESSMENT
PD, pancreaticoduodenectomy; LC, laparoscopic cholecystectomy.
Quality of life was assessed by a visual analogue scale, with the highest score being 100% in each domain. There were no significant differences when comparing any of the groups.
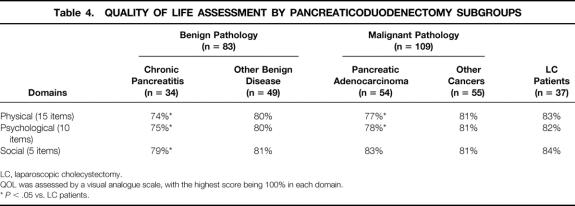
The QOL data for several subgroups of pancreaticoduodenectomy patients, comparing the subgroups with the laparoscopic cholecystectomy patients, are shown in Table 4. The pancreaticoduodenectomy patients were subgrouped into benign (n = 83) and malignant (n = 109) pathology and further subdivided into chronic pancreatitis (n = 34), other benign disease (n = 49), pancreatic adenocarcinoma (n = 54), and other cancers (n = 55). For chronic pancreatitis patients, the QOL scores in the physical, psychological, and social domains (74%, 75%, and 79%, respectively) were all significantly lower than the scores seen in the laparoscopic cholecystectomy patients (83%, 82%, and 84%, respectively; all P < .05). For pancreatic adenocarcinoma patients, the QOL scores in the physical and psychological domains (77% and 78%, respectively) were significantly lower than the scores seen in the laparoscopic cholecystectomy patients (both P < .05). There were no significant differences in the QOL scores in any domain when comparing either the other benign disease patients or the other periampullary cancer patients with the laparoscopic cholecystectomy patients.
Table 4. QUALITY OF LIFE ASSESSMENT BY PANCREATICODUODENECTOMY SUBGROUPS
LC, laparoscopic cholecystectomy.
QOL was assessed by a visual analogue scale, with the highest score being 100% in each domain.
*P < .05 vs. LC patients.
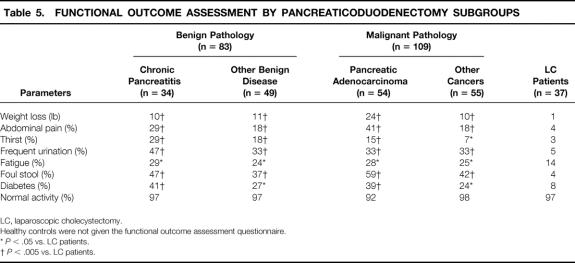
The results of the functional outcome assessment comparing the pancreaticoduodenectomy patients with the laparoscopic cholecystectomy patients are shown in Table 5. For all subgroups, the pancreaticoduodenectomy patients reported significantly more weight loss, abdominal pain, thirst, frequent urination, fatigue, foul stool, and diabetes than the laparoscopic cholecystectomy patients. Normal physical activity was reported by 92% or more of the patients in all groups. The highest percentages of thirst (29%), frequent urination (47%), and diabetes (41%) were reported by the chronic pancreatitis subgroup. In contrast, the greatest weight loss (24 lb) and the highest percentages of abdominal pain (41%) and foul stool (59%) were reported by the pancreatic adenocarcinoma subgroup.
Table 5. FUNCTIONAL OUTCOME ASSESSMENT BY PANCREATICODUODENECTOMY SUBGROUPS
LC, laparoscopic cholecystectomy.
Healthy controls were not given the functional outcome assessment questionnaire.
*P < .05 vs. LC patients.
†P < .005 vs. LC patients.
Table 6 shows the data from a univariate analysis performed to determine whether QOL scores varied when comparing several dichotomized parameters. This analysis considered the following parameters: age (younger than 65 or 65 or older), type of resection (pylorus-preserving vs. classic), presence or absence of postoperative complications, presence or absence of pancreatic fistula, presence or absence of delayed gastric emptying, and postoperative length of stay (<13 vs. ≥13 days). There were no significant differences in the QOL scores in any domain in this analysis.
Table 6. IMPACT OF SEVERAL PARAMETERS ON QUALITY OF LIFE
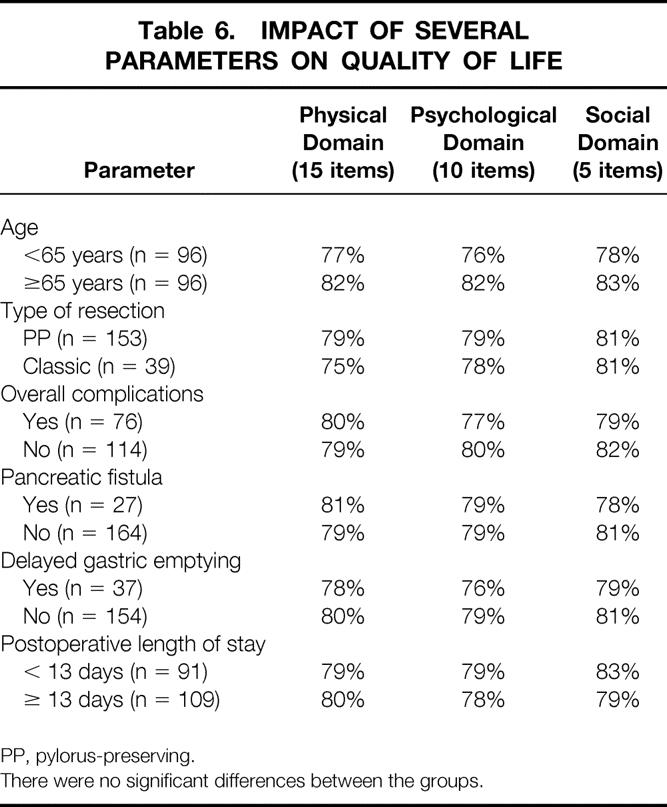
PP, pylorus-preserving.
There were no significant differences between the groups.
DISCUSSION
Several types of studies have addressed the outcomes of patients who have undergone pancreaticoduodenectomy. Reports from three statewide databases have assessed outcomes in terms of postoperative complications, death, and hospital charges, 30–32 largely focusing on the issue of surgical volume and its relation to outcome. Other reports have presented large single-institution experiences with pancreaticoduodenectomy. 1,4,10–12 Few studies have addressed the concept of QOL in patients surviving pancreaticoduodenectomy. 18–22
The two most comprehensive QOL studies published to date are those of McLeod et al 18 and Melvin et al. 20 McLeod et al 18 performed a cross-sectional survey of 25 pancreaticoduodenectomy patients, comparing them with 25 age- and sex-matched cholecystectomy patients. QOL was assessed using six instruments: Time Trade-off Technique, Direct Questioning of Objectives, Gastrointestinal Quality of Life Index, Sickness Impact Profile, Physician Global Assessment, and the Visick Scale. All six QOL assessments indicated near-normal well-being, and no instrument revealed significant differences between the pancreaticoduodenectomy patients and the control patients. An additional aspect of this study found normal nutritional status and a return to preoperative body weight in the pancreaticoduodenectomy patients. Further, Melvin et al 20 studied QOL in 45 patients who had previously undergone either pylorus-preserving pancreaticoduodenectomy or standard resection. QOL was assessed by the SF-36 in the two surgical groups and compared with normal standards of age-matched control subjects from the U.S. population. When the eight domains of the SF-36 were analyzed (spanning physical and mental health areas), there were no differences in the area of mental health between the groups. However, in the area of physical health, there were significantly lower QOL scores in the standard group versus the pylorus-preserving group and age-matched controls. No differences were seen in patients who had undergone resection for a benign process versus those with a malignant disease. An additional aspect of this study found that 42% of the patients regularly took pancreatic enzyme supplements to combat steatorrhea.
The cohort of 192 patients in the current study represents the largest number of such patients studied with respect to QOL reported to date. This cohort is representative of the current population of patients undergoing pancreaticoduodenectomy: the mean age was 62 years, there was roughly equal sex distribution, 80% underwent pylorus preservation, and 57% underwent resection for malignant pathology (see Table 1). Although the usual distribution of malignant to benign pathology in our pancreaticoduodenectomy population averages 70% malignant to 30% benign, 8 the relatively long follow-up of this cohort would tend to increase the percentage of patients with benign pathology, who are less likely to die of their disease during follow-up. Nonetheless, our analysis included four subgroups: patients with pancreatic adenocarcinoma (28% of the patients), other cancers (29%), chronic pancreatitis (18%), and other benign diseases (e.g., cystic neoplasms, endocrine tumors; 25%). Patients included in the resected pancreatic adenocarcinoma subgroup had pathology findings comparable to the usual resection population at our institution: the tumor diameter approached 3 cm, most patients were stage III (node-positive), and a minority (13%) had positive resection margins (see Table 2).
Because most of the patients in our study carried a malignant diagnosis, it appears appropriate that we used a self-reported 30-item QOL survey instrument that has been subjected to reliability and validity testing using 686 subjects from the National Coalition for Cancer Survivorship. 27 When the entire cohort of 192 pancreaticoduodenectomy survivors was compared with 37 age- and sex-matched laparoscopic cholecystectomy patients, there were no differences in the physical, psychological, or social QOL domains (see Table 3). However, when the pancreaticoduodenectomy patients were subgrouped and compared with the laparoscopic cholecystectomy patients, significant differences appeared (see Table 4). Patients with a pathologic diagnosis of chronic pancreatitis had significantly lower scores in all three QOL domains, whereas patients with pancreatic adenocarcinoma had significantly lower scores in both the physical and psychological domains.
The explanation for the lower QOL scores in the chronic pancreatitis patients may be partly explained by the chronicity of their disease, past history of alcohol abuse, and severity of disease that led to resection for chronic pancreatitis. A recent analysis of QOL in chronic pancreatitis patients at our institution has indicated that self-reported QOL scores improve after pancreaticoduodenectomy, 33 and that these patients appear to benefit from surgical resection.
The explanation for the lower scores in the pancreatic adenocarcinoma patients is uncertain. Our analysis did not provide serial QOL assessment scores for each patient at different time intervals after surgery; therefore, we cannot determine whether QOL scores in these patients are truly lower than patients in other subgroups, or if we are instead studying a group of patients with a higher burden of persistent or recurrent malignant disease. Because patients with resected pancreatic adenocarcinoma have the lowest median and 5-year survival rates compared with other periampullary cancer patients, 1 we can speculate that the lower QOL scores in these pancreatic adenocarcinoma patients may reflect the higher burden of malignant disease at the time QOL was assessed.
The functional outcome assessment performed for the four pancreaticoduodenectomy subgroups provided a self-reported measure of outcomes in these patient subgroups (see Table 5). Without exception, all subgroups reported findings that differed significantly from laparoscopic cholecystectomy patients. Weight loss averaged at least 10 lb from preoperative levels, and abdominal pain was present in at least 18% of patients. Diabetes and findings possibly related to endocrine insufficiency (e.g., thirst, frequent urination) were commonly reported. Foul stools were reported by no less than 37% of patients, suggesting pancreatic exocrine insufficiency. In all, this functional outcome assessment suggests that pancreaticoduodenectomy survivors should be carefully followed up for evidence of pancreatic endocrine and exocrine insufficiency. Past studies have reported substantial rates of diabetes and steatorrhea after pancreaticoduodenectomy, 18,20 and this current study suggests that the prevalence of these entities may be higher than previously reported.
In summary, the current study substantially adds to the literature on QOL after pancreaticoduodenectomy. QOL assessment in pancreatic diseases, both malignant and benign, is still in the early stages of data retrieval and evaluation, and there are many opportunities for future research and development. 34 Additional studies incorporating both preoperative and serial postoperative QOL assessment are needed. Studies are also needed to evaluate QOL for the various nonsurgical management strategies in pancreatic disease. Based on the data in the present study, it appears that pancreaticoduodenectomy survivors compare favorably with age- and sex-matched laparoscopic cholecystectomy patients in the broad area of global QOL assessment. More substantial differences exist in the functional outcomes assessment, where pancreaticoduodenectomy survivors reported considerably more abnormal findings than controls.
Discussion
Dr. R. Scott Jones (Charlottesville, Virginia): We all recognize that cancer of the pancreas remains one of the biggest killers that we contend with. And I realize that there were a number of patients in this study who didn’t have pancreatic cancer, but the majority of the patients did have either pancreatic or periampullary cancer.
As recently as perhaps 15 or 20 years ago, some leaders in surgery took a fairly nihilistic view for cancer, adenocarcinoma of the head of the pancreas, saying that most patients best be bypassed because of the futility of doing resections. But we all know that since that time there has been a dramatic improvement in operative mortality—or I should say, operative survival. There has been marked improvement in the application of adjuvant therapy. And although peripancreatic malignancies continue to be fairly lethal, we are seeing across the country and the world increasing numbers of survivors and long-term survivors.
The reason I review all of that and take your time with that is that now we are here today actually analyzing outcomes and quality of life after resection of pancreatic cancer and other periampullary malignancies. And I think this, the opportunity for Dr. Yeo to present this paper to us today, really simply depends upon the work and the diligence and the persistence of people who have come before.
I think it is highly probable, as we go into the next millennium, that we are going to have even more survivors because of improved techniques and treatments and recognition and understanding of the disease. So it is important now that we begin to focus attention on quality of life, as he and his colleagues are doing.
I wanted to ask a question and make another comment. And the question is to ask Dr. Yeo to elaborate a little bit—he mentioned this with the pancreatitis patients, but I would like for him to elaborate, if he can—about to what extent the progression and the recurrence of the disease in the pancreatic cancer group and the periampullar cancer group contributed to the decrement in the quality of life that they observed in those patients over time.
The other comment I wanted to make is that some of the things they recognized in this study, such as quality of stools, for example, and diabetes, are treatable, and it is important for us to recognize that. I think in our own practice we have gotten more sensitive about listening to patients and talking to them, and many of those patients can have further improvement in their quality of life with treatment of steatorrhea, which is readily available. Some of the consequences of diabetes can be controlled. Even if they have diabetes, quality of life can be controlled further with careful management of that problem.
I want to close by thanking Dr. Yeo and his colleagues for this study. I think that the fact that they had this large number of patients that have survived for a long time is really sort of a bonus to the information that is provided in this talk.
Dr. John H. Pemberton (Rochester, Minnesota): I rise, not as an expert in this operation, but rather with an interest in quality of life measures, particularly in our area of rectal cancer, colon cancer, and ileoanal anastomosis. I did enjoy the presentation very much; it is a wonderful experience. And it is interesting that your team is now asking questions about how the patients are doing, not just whether or not they are alive.
I have a couple of questions. We found it to be very important in quality of life surveys that the operating surgeon not be involved at any point in the questioning, because the patients feel that they have to respond positively. They have invested a lot of time and effort, they have watched the surgeon do the same, and there is a tendency to always try to please the surgeon. Was the operating surgeon in any way involved in determining these quality of life results?
It is important, too, I think, to characterize the group of patients who did not respond to the questionnaire. The 60% response rate in the group of patients with pancreaticoduodenectomy is really pretty good, but the 37% rate in the lap/chole and the 31% rate in the control group is pretty low. There could be some degree of misinterpretation of the data with these poor response rates.
I ask about characterizing the patients who didn’t respond because they really should be no different from the patients who did respond, in terms of diagnosis, outcome, and functional results. If they are different, then the assumption is that you selected out either the best or, alternately, the worst of the patients who responded to your questionnaire, and thus, the data would be suspect.
Finally, you pointed out that the outcome seemed to be based on diagnosis. Interestingly, the pancreaticoduodenectomy patients all had kinds of questionable function after the operation, yet those with chronic pancreatic and adenocarcinoma had worse quality of life scores than those with benign disease or good prognosis cancer. In this milieu of different types of subgroups, would an MMPI or personality index assessment of the patient groups help you interpret your data further?
Dr. Dana K. Andersen (New Haven, Connecticut): Although the Whipple operation can be regarded as safe and the results good, the early and late morbidity that accompanies the operation, and the incidence of new diabetes in particular, prompted me to explore alternative procedures for benign disease of the pancreatic head. For the past 2 years, I have used the duodenum-preserving pancreatic head resection, or Beger procedure, and the extended lateral pancreaticojejunostomy with excavation of the pancreatic head, or Frey procedure, in 16 patients with chronic pancreatitis or benign tumors. I compared my results with 11 Whipple operations, six distal pancreatic resections, and five pancreatic duct sphincterplasties done during this same time period.
The operative costs and risks of the procedures are defined here as mean operative time, mean intraoperative blood loss, hospital length of stay, and major complications. The outcomes are indicated as the incidence of new diabetes mellitus in the postoperative period, exocrine insufficiency, persistent analgesia need, and full functional recovery.
My initial experience indicates that the Beger and Frey procedures require fewer operative and hospital resources in terms of surgical operative time, intraoperative blood loss, and hospital length of stay.
I encountered one pancreatic leak in the Beger procedure and one pancreatic leak in the Whipple operation, both of which were managed with percutaneous drainage. The occurrence of new diabetes was seen in one Whipple patient, and one additional Whipple patient had a worsening of diabetes from noninsulin-dependent diabetes to insulin-dependent diabetes. Of 12 chronic pancreatitis patients who underwent either the Beger or Frey procedures, however, there were no new cases of diabetes postoperatively. And in two of five patients with preoperative diabetes, the degree of glucose intolerance actually improved, either with freedom from insulin dependency or from drug therapy.
Exocrine insufficiency and the need for pancreatic enzyme replacement was seen in half of the patients with chronic pancreatitis, and persistent analgesia need and failure to return to full employment or activity was seen only in patients with severe or familial pancreatitis.
Based on this experience, I believe that the duodenum-preserving pancreatic head resection and the Frey procedure both offer better alternatives than the Whipple operation for patients with benign disease. And so my questions for Dr. Yeo are, have you begun to offer these newer alternative operations to patients with benign disease, and if so, what determines which operation you perform?
Dr. J. Bradley Aust (San Antonio, Texas): I think these are great studies, and there is going to be a need for more of these outcome studies. As we evaluate all our forms of surgical therapy, there is this question: did we do any good?
I would like to suggest that you should have preoperative evaluation on these patients to prove that you have changed their situation for the better. This reminds me of the old story about the patient who went to see the doctor and asked him the question, “Will I be able to play the piano when we get through with this surgery?” And the doctor says, “Why, of course, you will.” He says, “That’s wonderful; I never could play before.”
Dr. Charles J. Yeo (Closing Discussion): I thank all the discussants for their excellent questions and admit that I am not a pianist. I really want to thank the Southern for having this paper on the program and bringing up this field. I think it is very important that we evaluate these outcomes now that we do have a substantial cohort.
Dr. Jones’ remarks were right on target. I think there is no question that what we are providing here is a snapshot of a group of people who happen to have survived a major operation and are now alive. Clearly, the people that responded to us are doing pretty well. They feel fairly strongly about Hopkins and are willing to respond to a questionnaire that’s mailed to them, so they have an intrinsic motivation and thanks. And I think that’s why the PD group, getting back to Dr. Pemberton’s question, responded to us so highly.
I think it is also very true that there may be some patients who received this questionnaire, but didn’t respond because they were doing poorly. There is no question that some people don’t respond because they are ill and sickly and declining.
We do have some longitudinal data that one of my coauthors, JoAnn Coleman, looked up in very preliminary fashion, sending serial quality of life assessments to pancreas cancer patients. These do show, as you would anticipate, a decline in the quality of life assessment because the disease remains troublesome with only about 15% to 20% 5-year survivors. So if you follow them long enough, I think you will definitely see a decrement, and that’s something we need to work on—how can we improve not only the quality of life, but also how can we improve the overall survivorship?
Dr. Pemberton asked about surgeon involvement. The individual attending surgeon’s names did not appear on the form that went out to the patients. The form was actually sent out as a form letter stating that we were interested in having their answer to these two simple questionnaires.
I bring up the word “simple” there because there have been a number of studies looking at quality of life. Many people in the room are more expert than I in this, but I think it’s safe to say that the assessment needs to be simple, and it needs to not be time-consuming. And getting back to Dr. Pemberton’s question, I think there may definitely be room to do further analyses of some of these subgroups, particularly with MMPI testing and other assessment tools, but you have to weigh how arduous that is for the patients and how time-consuming it is versus the simplicity in the response rates.
Dr. Andersen brings up a very important point, and that is benign disease. I thank you, Dana, for sharing your data with us. There are certainly many treatment options for chronic pancreatitis. They have expanded in recent years. There is the Whipple, there is the duodenum-sparing procedure, a Puestow-type variant, distal resections. We certainly favor nonresectional options in patients with dilated main pancreatic ducts. We note, however, that several studies have indicated that these patients are not free of ultimate exocrine or endocrine insufficiencies. So when the patients get followed long enough, because of the nature of their chronic pancreatitis as a progressive parenchymal fibrotic process, they do actually deteriorate.
Getting to your question about quality of life in this benign group, we at the SSAT meeting in May 1999 presented a quality of life assessment in over 200 patients with chronic pancreatitis, subgrouping them into the Whipple, the Puestow and Puestow-type variants including the Frey, distal resections, and ampullary procedures, and found that quality of life improved in all patients in all subgroups when the starting denominator was patients with chronic pancreatitis. So surgery in appropriately selected chronic pancreatitis patients certainly improves their quality of life.
About Dr. Aust’s question, I think preoperative evaluations are important. We have learned as we have done some of this, that it is important to know where they are starting from. In the pancreatic cancer patients, I think if you were to give them a quality of life assessment 1 week before a Whipple operation, it would be pretty dramatic. They would be absolutely scared to death. Many of them are itching, they have terrible abdominal pain, and they are absolutely socially, psychologically, and physically debilitated and just tortured by this diagnosis. So I think that would probably only more vividly show the differences in quality of life when you compare patients scared to death with a tumor, to patients who have had a successful resection and are survivors.
Footnotes
Correspondence: Charles J. Yeo, MD, Dept. of Surgery, Johns Hopkins Hospital, Blalock 606, 600 N. Wolfe St., Baltimore, MD 21287-4606.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
A portion of this work was presented at the American College of Surgeons Surgical Forum, Oct. 11, 1999, in San Francisco, CA, and has been published in abstract form (Surg Forum 1999; 50:649–650).
Supported in part by grants from the National Institutes of Health (R01-CA56130 and P50-CA62924).
E-mail: cyeo@jhmi.edu
Accepted for publication December 1999.
References
- 1.Yeo CJ, Sohn TA, Cameron JL, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg 1998; 227:821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedrazzoli S, DiCarlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreaticoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas. A multicenter, prospective, randomized study. Ann Surg 1998; 228:508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: Comparison of morbidity and mortality and short-term outcome. Ann Surg 1999; 229:613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996; 223:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talamini MA, Moesinger RC, Pitt HA, et al. Adenocarcinoma of the ampulla of Vater: a 28-year experience. Ann Surg 1997; 225:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirata K, Sato T, Mukaiya M, et al. Results of 1001 pancreatic resections for invasive ductal adenocarcinoma of the pancreas. Arch Surg 1997; 132:771–776. [DOI] [PubMed] [Google Scholar]
- 7.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar and distal tumors. Ann Surg 1996; 224:463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan GQ, Yeo CJ, Cameron JL, et al. Pancreaticoduodenectomy for selected periampullary neuroendocrine tumors: fifty patients. Surgery 1997; 122:989–997. [DOI] [PubMed] [Google Scholar]
- 9.Barnes SA, Lillemoe KD, Kaufman HS, et al. Pancreaticoduodenectomy for benign disease. Am J Surg 1996; 171:131–135. [DOI] [PubMed] [Google Scholar]
- 10.Trede M, Schwall G, Saeger H-D. Survival after pancreaticoduodenectomy: 118 consecutive resections without an operative mortality. Ann Surg 1990; 221:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, outcomes. Ann Surg 1997; 226:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995; 221:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; 165:68–73. [DOI] [PubMed] [Google Scholar]
- 14.Strasberg SM, Drebin JA, Soper NJ. Evolution and current status of the Whipple procedure: an update for gastroenterologists. Gastroenterology 1997; 113:983–994. [DOI] [PubMed] [Google Scholar]
- 15.Velanovich V. Using quality-of-life instruments to assess surgical outcomes. Surgery 1999; 126:1–4. [DOI] [PubMed] [Google Scholar]
- 16.Patti MG, Pellegrini CA, Way LW. Gastric emptying and small bowel transit of solid food after pylorus-preserving pancreaticoduodenectomy. Arch Surg 1987; 122:528–532. [DOI] [PubMed] [Google Scholar]
- 17.Fink AS, DeSouza LR, Mayer EA, et al. Long-term evaluation of pylorus preservation during pancreaticoduodenectomy. World J Surg 1988; 12:663–670. [DOI] [PubMed] [Google Scholar]
- 18.McLeod RS, Taylor BR, O’Connor BI, et al. Quality of life, nutritional status, and gastrointestinal hormone profile following the Whipple procedure. Am J Surg 1995; 169:179–185. [DOI] [PubMed] [Google Scholar]
- 19.Patel AG, Toyoma MT, Kusske AM, et al. Pylorus-preserving Whipple resection for pancreatic cancer. Is it any better? Arch Surg 1995; 130:838–842. [DOI] [PubMed] [Google Scholar]
- 20.Melvin WS, Buekers KS, Muscarella P, et al. Outcome analysis of long-term survivors following pancreaticoduodenectomy. J Gastrointest Surg 1998; 2:72–78. [DOI] [PubMed] [Google Scholar]
- 21.Kokoska ER, Stapleton DR, Virgo KS, Johnson FE, Wade TP. Quality of life measurements do not support palliative pancreatic cancer treatments. Int J Oncol 1998; 13:1323–1329. [DOI] [PubMed] [Google Scholar]
- 22.Schmier J, Elixhauser A, Halpern MT. Health-related quality of life evaluations of gastric and pancreatic cancer. Hepato-Gastroenterology 1999; 46:1998–2004. [PubMed] [Google Scholar]
- 23.Ferrell BR, Wisdom C, Wenzl C. Quality of life as an outcome variable in the management of cancer pain. Cancer 1989; 63:2321–2327. [DOI] [PubMed] [Google Scholar]
- 24.Padilla G, Present C, Grant M, et al. Quality of life index for patients with cancer. Research Nurs Health 1983; 6:117–126. [DOI] [PubMed] [Google Scholar]
- 25.Present C, Klahr C, Hogan L. Evaluating quality of life in oncology patients: pilot observations. Oncol Nurs Forum 1981; 8:26–30. [PubMed] [Google Scholar]
- 26.Grant M, Ferrell B, Schmidt G, et al. Measurement of quality of life in bone marrow transplantation survivors. Quality Life Res 1992; 1:375–384. [DOI] [PubMed] [Google Scholar]
- 27.Ferrell BR, Hassey Dow K, Grant M. Measurement of the quality of life in cancer survivors. Quality Life Res 1995; 4:523–531. [DOI] [PubMed] [Google Scholar]
- 28.Hassey Dow K, Ferrell BR, Leigh S, et al. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat 1996; 39:261–273. [DOI] [PubMed] [Google Scholar]
- 29.Coleman J. Quality of life following the Whipple procedure. In: Nolan MP, Mock V, eds. Measuring Patient Outcomes. Thousand Oaks, CA: Sage Publications, Inc.; 2000:127–142.
- 30.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998; 228:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relationship of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg 1995; 222:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glasgow RE, Mulvihill SJ. Hospital volume influences outcome in patients undergoing pancreatic resection for cancer. West J Med 1996; 165:294–300. [PMC free article] [PubMed] [Google Scholar]
- 33.Sohn TA, Campbell KA, Pitt HA, et al. Quality of life and long-term survival after surgery for chronic pancreatitis. J Gastrointest Surg (in press). [DOI] [PubMed]
- 34.Fitzsimmons D, Johnson CD. Quality of life after treatment of pancreatic cancer. Langenbeck Arch Surg 1998; 383:145–151. [DOI] [PubMed] [Google Scholar]