Abstract
Objective
To analyze an institutional experience with pancreatitis in childhood to clarify the frequency of pancreas divisum in that patient population, the characteristics of pancreatitis in children with pancreas divisum, and the role of surgical management in their treatment.
Summary Background Data
The role of pancreas divisum in causing acute and relapsing pancreatitis and chronic, recurring abdominal pain is controversial. Although the anatomical abnormality is present from birth, most investigators have reported cases with onset of symptoms in adulthood. The reported pediatric experience with this disorder is small, and the natural history of pancreatitis in children with pancreas divisum has not been well elucidated.
Methods
A retrospective chart review of all children 18 years of age and younger with a discharge diagnosis of pancreatitis identified 135 patients treated in the authors’ institution from 1978 to 1998. Ten patients were found to have anatomical variants of pancreas divisum associated with recurrent or chronic pancreatitis. The medical records of these patients were reviewed for data on the presentation, diagnostic findings, imaging studies, treatment, surgical findings, and pathologic findings in these children. Chart review and telephone calls were used to assess the current state of health in nine patients available for follow-up.
Results
Pancreas divisum was identified in 7.4% of all children with pancreatitis and 19.2% of children with relapsing or chronic pancreatitis. Patients had early onset of recurrent episodic epigastric pain and vomiting, at a mean age of 6 years. Three patients had a positive family history of pancreatitis and one was proven by DNA analysis to have hereditary pancreatitis. Pancreatitis was documented by elevated amylase or lipase levels, and endoscopic retrograde cholangiopancreatography was the method of diagnosis of pancreas divisum in all patients. Eight patients had complete pancreas divisum and two had incomplete variants. Eight patients underwent surgery to improve ductal drainage. Seven underwent transduodenal sphincteroplasty of the accessory papilla, along with sphincteroplasty of the major papilla in two (plus septoplasty in one). Three patients underwent longitudinal pancreaticojejunostomy, as a primary procedure in one patient with midductal stenosis and in two because of recurring pancreatitis after sphincteroplasty. The surgical findings and histologic examination of five patients undergoing distal pancreatectomy revealed striking changes of advanced chronic pancreatitis. Patients responding to sphincteroplasty alone showed less severe histologic changes. Overall, three of seven patients had excellent results, three were improved, and one had continued disabling attacks of pancreatitis. The mean duration of follow-up was 7.3 years, and there were no deaths. No patients had endocrine or exocrine pancreatic insufficiency, and none required chronic analgesics.
Conclusions
Pancreas divisum is an important cause of recurrent pancreatitis in childhood and should be sought aggressively in children with more than one episode of pancreatitis or pancreatitis with a history of chronic recurrent abdominal pain. Surgical intervention is directed toward relief of ductal obstruction and may involve accessory duct sphincteroplasty alone or in combination with major sphincteroplasty and septoplasty. Patients with more distal ductal obstruction or ductal ectasia may benefit from pancreaticojejunostomy.
Pancreatitis has many causes in children, with trauma being the most common. The remainder include drug-induced pancreatitis, hereditary pancreatitis, those related to renal disease and renal transplantation, a variety of metabolic disorders, particularly those related to hyperlipidemia types I and V, inflammatory disorders, collagen vascular diseases, and a few miscellaneous causes. 1 Pancreatic duct obstruction is an essential feature of several entities related to pancreatitis in childhood, including congenital anomalies such as choledochal cyst and pancreas divisum, common duct stones, acquired bile duct strictures, tumors, and obstruction by parasites such as Ascaris lumbricoides.
Pancreas divisum is a common embryologic anomaly of the pancreatic ductal system. The condition is thought to arise from a failure of the dorsal and ventral ductal anlage of the fetal pancreas to fuse during the second month of development. The dorsal component of the pancreas is drained by the duct of Santorini and the ventral by the duct of Wirsung. The uncinate process is the residual of the ventral component and is drained by the duct of Wirsung. Opie 2 first described this anatomical variant in 1903 and later reported a 10% frequency in postmortem examinations. 3 The significance of this anomaly in pancreatic disease remained obscure until the introduction of endoscopic retrograde cholangiopancreatography (ERCP) in the 1970s as a tool for the investigation of pancreatic and biliary pathologic anatomy.
The role of pancreas divisum in acute and relapsing pancreatitis and chronic recurring abdominal pain is controversial. 4–7 It is unclear just how many patients with pancreas divisum eventually develop recurrent pancreatitis, because the onset of symptomatology is so variable, ranging anywhere from early childhood to persons in their 40s. Several investigators have reported epidemiologic studies showing an increased incidence of pancreas divisum in patients undergoing investigations for unexplained pancreatitis. 8–13 Warshaw et al 14 and Keith 15 have focused on the pathophysiology created by stenosis of the dorsal ductal orifice in patients with several anatomical variants of pancreas divisum and have analyzed predictors of surgical success in this group. Various forms of pancreas divisum have been described, but two common features associated with this anomaly are regularly noted: ductal stenosis either at its ampullary outlet or at a junction point in the ductal system, or localized ductal ectasia, particularly in the uncinate process, which is also usually associated with ampullary stenosis.
Although pancreas divisum is indeed a congenital anomaly, its role in the pathogenesis of pancreatitis in childhood remains unclear. Several publications have described small groups of children who experienced acute or recurrent pancreatitis associated with pancreas divisum. 13,16–21 The infrequency of reported cases may underestimate the actual contribution of pancreas divisum as a causative factor in that abdominal pain in childhood is ubiquitous, and pancreatitis is not often considered in the evaluation of children with recurring abdominal pain.
We undertook an analysis of our experience with pancreatitis in childhood to clarify the frequency of pancreas divisum in that patient population, the characteristics of pancreatitis in children with pancreas divisum, and the role of surgical management in their treatment.
METHODS
A retrospective review was performed for all children 18 years of age and younger with a discharge diagnosis of pancreatitis who were admitted to our medical center from 1978 to 1998. ICD-9 and CPT codes were used to supplement these data from the patient log of the department of pediatric surgery. Patient follow-up was done through chart review and telephone calls to determine the patient’s state of health and growth status and whether there were any indicators of recurrent pancreatitis.
RESULTS
Study Group
One hundred thirty-five patients had a diagnosis of acute, recurrent, or chronic pancreatitis during the 20-year study interval (Table 1). Fifty-two children had recurrent or chronic pancreatitis. Of this group, 10 patients had anatomical variants of pancreas divisum (8 complete, 2 incomplete). This represented 7.4% of all children with pancreatitis and 19.2% of children with relapsing or chronic pancreatitis. Six children were girls and four were boys. Follow-up data were available in nine patients (90%).
Table 1. CAUSES OF PANCREATITIS (1978–1998), VANDERBILT CHILDREN’S HOSPITAL
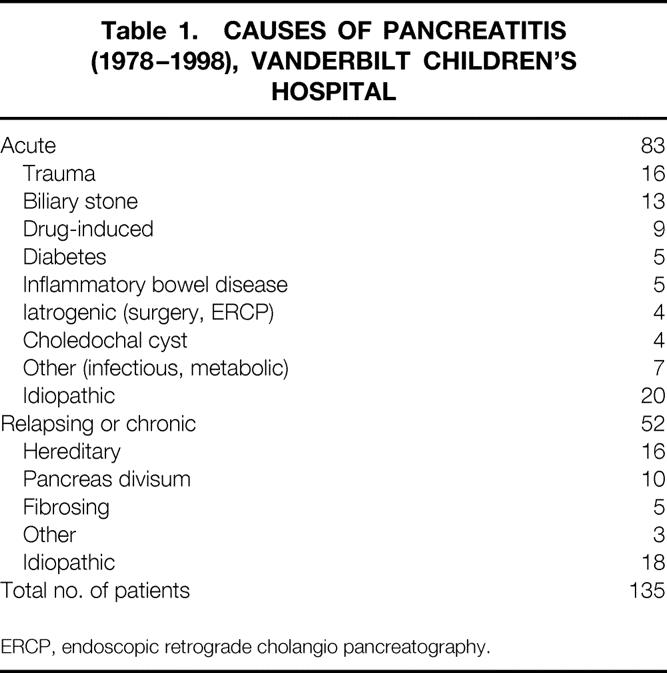
ERCP, endoscopic retrograde cholangio pancreatography.
Clinical Features
These patients consistently had recurrent bouts of acute epigastric abdominal pain associated with nausea and emesis. Episodes varied from a few days to 2 weeks in duration and were associated with anorexia and weight loss when protracted. Most families could recall fairly precisely the age of onset, with a mean age of 6 years (range 2–15 years).
All patients had recurrent bouts of abdominal pain before a definitive diagnosis was made. In all instances there was difficulty establishing the diagnosis of pancreatitis because the symptoms mimicked other conditions that are more common in childhood. Frequency of attacks varied greatly throughout the group, ranging from monthly to every 12 to 18 months. All but two patients needed repeated hospital admissions for treatment before pancreatitis was recognized as the underlying diagnosis. Patients underwent a wide variety of diagnostic tests. Five patients had abdominal ultrasonography, and the pancreas was normal in each. Six patients underwent computed tomography: the scan was normal in two and showed edema or thickening of the pancreas in two. Peripancreatic edema and ductal dilation were noted in one each. Pseudocysts developed in none of the patients.
Pancreatitis was documented by the finding of an elevated serum amylase or lipase level. The level of enzyme elevation was marked in all but two patients: amylase values were 800 to >6,000 units (normal 25–115 units) and lipase values were 2,180 to 11,560 units (normal 0–340 units). The two children with minimal enzyme elevation included a 12-year-old girl with pancreas divisum who later was determined to have hereditary pancreatitis and a 14-year-old girl with a promiscuous lifestyle suspected to have substance abuse.
Family history was positive for pancreatitis in three patients. The mother of one girl with coincidental fetal alcohol syndrome had experienced recurrent pancreatitis, reportedly since age 6, although she had a strong history of alcohol abuse. One girl’s mother had recurrent pancreatitis at age 35 and was found to have a hypertensive sphincter of Oddi, which was successfully treated with stenting and dilatation. The final patient came to our attention at age 12 years with recurrent pancreatitis and a strong family history of multiple family members requiring pancreatic surgery for relapsing pancreatitis. She was later confirmed to have hereditary pancreatitis proven by identification of the R117H mutation of the cationic trypsinogen gene when that mutational analysis became available at our institution. DNA analysis in two additional patients in this series was negative.
A thorough analysis for other possible causes of pancreatitis including hypercalcemia, hyperlipidemia, cystic fibrosis, drug exposure, and others was undertaken in all patients and was negative.
Imaging Studies
Although ultrasound and computed tomography were performed in most instances, ERCP was the primary method of diagnosis of pancreas divisum in all patients. The mean age at the time of diagnostic ERCP was 11.8 years (range 5–19 years). The mean interval between the onset of symptoms and the diagnostic ERCP was 5.7 years (range 7 weeks to 7 years) in the six patients for whom this information was available. Eight patients were noted to have complete pancreas divisum and two incomplete. Figure 1 demonstrates the anatomical variants in these patients. In all patients subsequently undergoing surgery, there was evidence of ductal obstruction either at the level of the minor ampulla or at the junction of the two ductal systems, and associated ductal ectasia in the uncinate process was noted in one patient. One 5-year-old girl had a dominant dorsal ductal system with filamentous connections between the ducts of Wirsung and Santorini. One 15-year-old boy had incomplete pancreas divisum with a stricture at the junction of the ducts of Wirsung and Santorini causing obstruction of the duct of Santorini in the body and tail of the gland (Fig. 2).
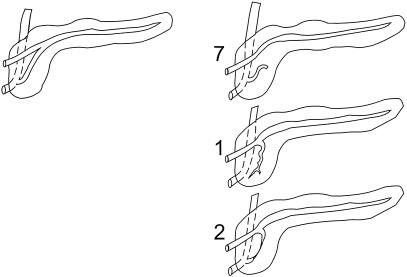
Figure 1. Variants of pancreatic ductal anatomy. (Left) Normal ductal fusion with persistent duct of Santorini. (Right) Complete and incomplete forms of pancreas divisum. Numbers of patients in this study with each anatomical variation are noted.
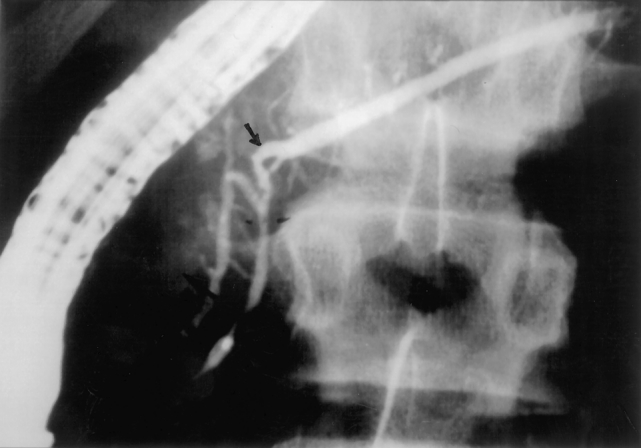
Figure 2. Endoscopic retrograde cholangiopancreatogram of a 15-year-old boy with incomplete pancreas divisum with a midductal stricture (arrow) at the junction of the ducts of Wirsung and Santorini causing obstruction of the duct of Santorini with distal ductal dilation.
The duct of Wirsung was visualized in nine patients and was judged to be normal in eight. One 14-year-old boy had dilation of the duct of Wirsung in the pancreatic head containing noncalcified filling defects (Fig. 3).
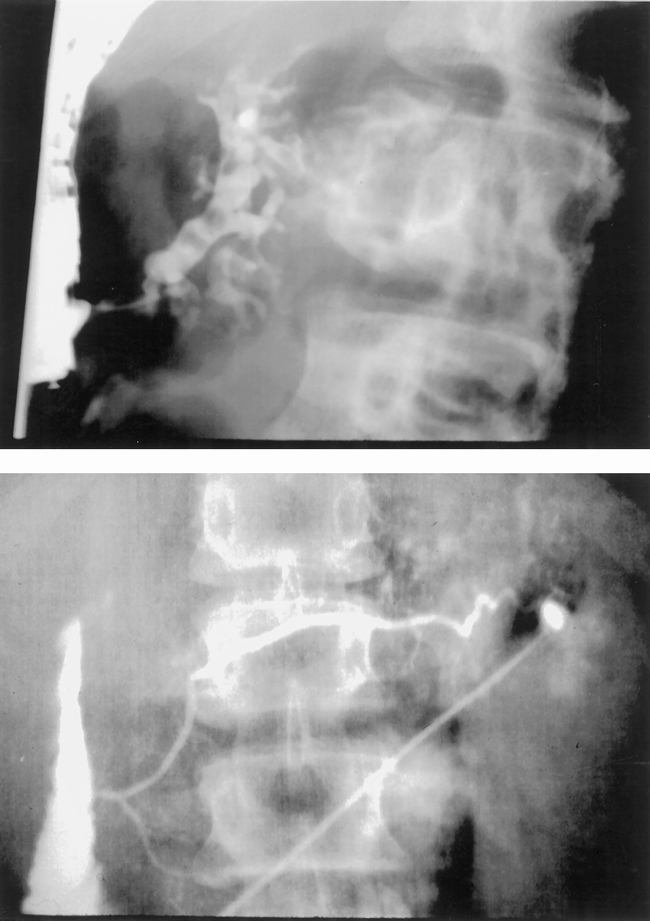
Figure 3. Endoscopic retrograde cholangiopancreatogram of a 14-year-old boy with complete divisum. Injection of the papilla of Vater (top) demonstrated marked dilation of the duct of Wirsung with ectasia and filling defects. Operative pancreatogram (bottom) revealed a normal duct of Santorini without changes of chronic pancreatitis.
The minor papilla was successfully cannulated in three of eight patients with complete pancreas divisum. Filling of the duct of Santorini was therefore successful in these three patients and two others with incomplete divisum. Ductal dilation was judged to be mild in three, prominent in one, and prominent with associated ectasia of side branches and noncalcified filling defects in the patient later proven to have hereditary pancreatitis (Fig. 4).
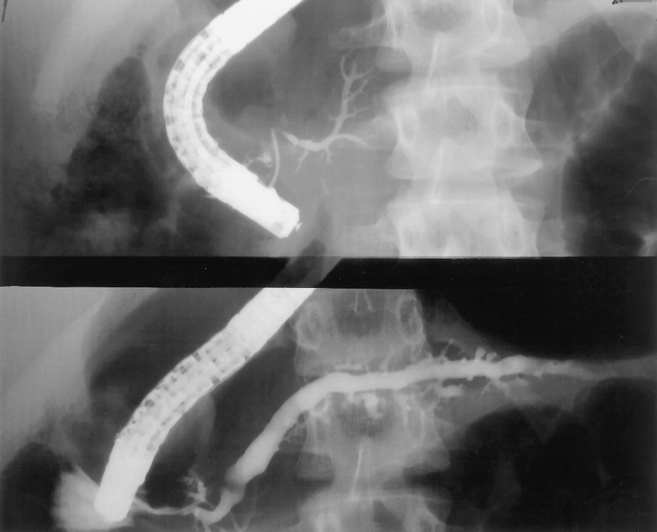
Figure 4. Endoscopic retrograde cholangiopancreatogram in a 12-year-old girl with complete pancreas divisum. Injection of the duct of Wirsung (top) revealed a prominent and foreshortened duct in the pancreatic head and uncinate process. Injection of the accessory papilla (bottom) revealed a prominent duct of Santorini with associated ectasia of side branches and noncalcified filling defects. This patient later was confirmed to have hereditary pancreatitis.
Treatment
An 18-year-old boy with poorly controlled insulin-dependent diabetes mellitus had two documented episodes of pancreatitis associated with ketoacidosis. ERCP was performed through a readily cannulated minor papilla. This patient had established pancreatic exocrine insufficiency and was managed with oral supplements of pancreatic enzymes. No obstruction was demonstrated in this patient’s ductal system or at either ampulla.
A 14-year-old girl with recurring pain and mild elevation of enzyme levels was found to have complete divisum. A small accessory papilla was successfully cannulated, revealing a duct of Santorini that was judged to be generous in size but without stricture or ectasia of side branches. Attempted endoscopic dilation and stent placement in the minor papilla was unsuccessful. She did not have further episodes of pancreatitis during a 20-month follow-up.
Eight patients underwent 10 surgical procedures to improve pancreatic ductal drainage. Seven patients underwent transduodenal sphincteroplasty of the accessory papilla. This involved identification of the accessory papilla and calibration with a small lacrimal duct probe. The stenotic orifice was opened widely overlying the probe, and the duodenal mucosa and duct were approximated with fine sutures (current preference is for synthetic monofilament absorbable sutures). Transpapillary stents were not used. Calibration of the ampulla of Vater was judged to confirm ampullary stenosis in two of the seven patients who also underwent sphincteroplasty of the sphincter of Oddi and cholecystectomy. The patient with dilation of the duct of Wirsung and debris in the duct underwent septoplasty as well. Five of the eight patients underwent coincident appendectomy, and one underwent a Meckel’s diverticulectomy.
Three patients underwent longitudinal pancreaticojejunostomy associated with limited distal pancreatectomy and splenic preservation to allow identification of the duct of Santorini. One of these three patients underwent pancreaticojejunostomy as a primary procedure because of ductal stenosis at the point of fusion of the ducts of Wirsung and Santorini. The two remaining patients underwent a Puestow procedure secondarily because of recurring bouts of pancreatitis after accessory sphincteroplasty. Both these patients underwent repeat ERCP because of their continuing symptoms. The study showed a widely patent accessory papilla in the patient with hereditary pancreatitis. The accessory papilla appeared open but could not be cannulated in a 5-year-old girl with incomplete divisum, and increasing ductal dilation was considered an indication for the Puestow procedure.
Surgical Findings and Pathology
The pancreas was noted to be firm and nodular in all but one of the eight patients undergoing surgery. In two patients the changes were greater in the head of the gland. The only patient without gross morphologic findings of chronic pancreatitis was a 5-year-old girl with a remarkably brief duration of symptoms of only 4 months before her sphincteroplasty.
The accessory papilla and ampulla of Vater were calibrated at each transduodenal procedure using lacrimal probes. In all but one instance, the accessory papilla was judged to be stenotic (opening would not accept or was snug to a 0.3-mm [0000] probe). Two patients were thought to have concomitant stenosis of the ampulla of Vater.
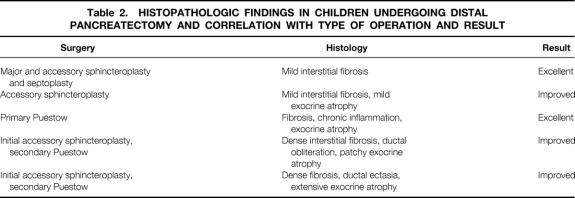
Histopathology was available on five patients who had distal pancreatectomies. Two distal pancreatectomies were performed at the time of exploration to facilitate surgical pancreatography in patients in whom the dorsal duct was not visualized during ERCP. Three patients underwent distal pancreatectomy at the time of longitudinal pancreaticojejunostomy.
Findings of chronic pancreatitis were noted in all specimens, ranging from mild interstitial fibrosis in its mildest form to dense fibrosis, chronic inflammatory cell infiltrates, ductal ectasia, and acinar cell atrophy. The degree of fibrosis and exocrine atrophy was relatively mild in two patients who improved after sphincteroplasty alone and more severe in the three patients requiring pancreaticojejunostomy (Table 2).
Table 2. HISTOPATHOLOGIC FINDINGS IN CHILDREN UNDERGOING DISTAL PANCREATECTOMY AND CORRELATION WITH TYPE OF OPERATION AND RESULT
Outcome
The postoperative hospital stay for patients undergoing transduodenal sphincteroplasty was a mean of 8.3 days. The only complication encountered was a pancreatic fistula presumed to be at the site of the distal pancreatectomy; it closed spontaneously at home after 6 weeks. The mean hospital stay for patients after pancreaticojejunostomy was 9.7 days. There were no complications in the latter group.
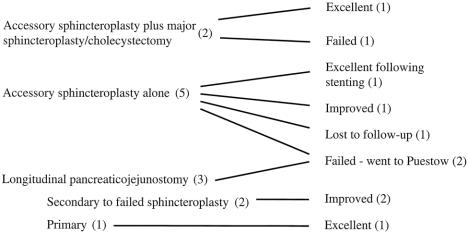
Seven of the eight patients who underwent surgery were available for follow-up. The range of follow-up was 9 months to 13 years, with a mean of 7.3 years. During this interval, there were no deaths. One patient was lost to follow-up. The results of surgery are shown in Figure 5.
Figure 5. Outcome after surgery in the eight patients in this study group.
Follow-up information is current for six patients who underwent transduodenal accessory duct sphincteroplasty. Two patients had excellent results, with no ongoing episodes of pancreatitis or pain. One of these patients had recurrent pain 3 years after sphincteroplasty and required endoscopic stent placement but had not experienced symptoms during the past 3 years. One patient improved but continues to have occasional attacks of pain and required hospital admission every 2 to 3 years for recurrences. One patient from another state continues to be incapacitated by recurrent episodes of pancreatitis requiring frequent hospital admissions; he receives his care at another medical center closer to his home and has not chosen to consider further surgical options. Two patients required Puestow procedures because of ongoing recurrent pancreatitis and were improved.
Of three patients undergoing pancreaticojejunostomy, two were markedly improved. The patient with midductal stricture was weaned off the narcotics required for preoperative pain control and is clinically well. The patient with hereditary pancreatitis has six to eight attacks of pain per year that are self-limited. She has not required hospital admission during the past 5 years since the Puestow procedure. The third patient has recently experienced troublesome bouts of recurring pain, and additional evaluation is being undertaken. Both of these latter patients have been documented to have mild amylase elevations during these attacks to confirm pancreatitis as the cause.
Overall, three of seven patients had excellent results, three were improved, and one continues to have disabling attacks of recurring pancreatitis. No patients have diabetes mellitus, and none have symptoms of pancreatic exocrine insufficiency. None require chronic analgesics.
DISCUSSION
This series of children with pancreas divisum illustrates the complex nature of this congenital anomaly and the difficulties encountered in establishing the role of pancreas divisum in the pathogenesis of pancreatitis in any individual patient. The variety of anatomical configurations necessitates accurate studies to define the pathologic anatomy precisely and allow planning of appropriate surgical treatment. Even with careful patient selection and meticulous surgical technique to accomplish relief of ductal obstruction, the response to surgery is inconsistent. In adult series, 14,22,23 approximately 75% of patients have a favorable outcome after surgery, and that is similar to our results in this series. It is also clear that it may take more than one operation to achieve a lasting satisfactory result.
Pancreatitis associated with pancreas divisum has been reported in adults by Warshaw, 9,14 Carey et al, 24 and others, 10–12 but no large series have been reported in children, usually only case reports. The youngest patient in this series was 5 years old at the time of surgery, but most patients were older. This may be because the signs of pancreatitis in patients such as these are nonspecific and other diagnoses, such as acute appendicitis or gastroenteritis, are made. Acute hemorrhagic pancreatitis is uncommon in children and is usually related only to drug-induced pancreatitis. Recurrent pancreatitis associated with pancreas divisum characteristically starts in a mild fashion and becomes severe over time. The usual beginning symptoms are anorexia, feeding problems, occasional vomiting, abdominal pain, diarrhea, or even jaundice without pain. Because pancreas divisum may be a normal variant of pancreatic anatomy and may be asymptomatic if obstruction is not present, it is also possible that obstruction may occur as a late event in some patients, accounting for the fact that most patients are older when they come to medical attention, even though pancreas divisum is a congenital malformation. When pancreatitis is suspected, serum amylase and lipase determinations are useful in confirming the diagnosis, but in approximately 20% of patients, enzyme elevation is not noted during an individual episode.
If imaging studies are performed during bouts of abdominal pain in children, pancreatic thickening, peripancreatic fluid collections, and pancreatic ductal dilatation are seen in only a few instances. Ultrasound was not informative in the patients in this series. Computed tomography scanning was more useful in confirming pancreatic inflammation but provided no specific anatomical data. At present, ERCP is the most accurate way to delineate the anatomy, but it is not always possible to cannulate the minor papilla, as was the case in five of our eight patients with complete divisum. In patients with pancreas divisum, ERCP usually demonstrates a short or absent ventral duct of Wirsung and a normal-sized dorsal duct of Santorini, when the minor papilla can be cannulated. Even in symptomatic patients, the pancreatic ducts are usually only mildly dilated, possibly because of surrounding inflammation or fibrosis. Localized ectasia in the region of the uncinate process may be seen in patients with complete pancreas divisum. Distal ductal dilatation is usually seen in patients with incomplete pancreas divisum with stenosis at the site of ductal fusion, as in one patient in this series. Warshaw et al described the use of ultrasound with secretin stimulation to assess ductal obstruction in adults with pancreatitis 25 and have found a significant correlation between outcome after surgery and ultrasound findings. 14 Other investigators have noted difficulty documenting changes with this study. 15 Secretin stimulation was not used in patients in our series, and we believe that the small ductal diameter in children may limit the effective use of secretin stimulation in this patient population.
Another imaging study that is very promising for children with pancreas divisum is magnetic resonance cholangiopancreatography (MRCP). This technology provides the ability to delineate ductal anatomy without the risks of contrast injection (which is required in ERCP). MRCP can produce high-quality images of the biliary tree and the pancreatic ducts. Ueno et al 26 recently compared MRCP with ERCP in a series of 93 patients and concluded that MRCP can demonstrate the normal pancreatic duct and various pancreatic ductal abnormalities; however, compared with ERCP, MRCP overestimated the stenosis of the main pancreatic duct and underestimated the degree of dilatation of the branches and filling defects in the pancreatic duct in patients with pancreatitis. In that study, four of the eight patients with pancreas divisum were demonstrated by MRCP. MRCP was not available in our institution at the time of initial assessment of our patient group but would currently be considered the initial imaging study of choice in the evaluation of pancreatic ductal anatomy in children with unexplained or recurrent pancreatitis.
The usual case of hereditary pancreatitis that comes to surgery is manifested by significant ectasia of the main pancreatic duct, which is usually best alleviated by longitudinal pancreaticojejunostomy. However, Muzaffar et al 27 have described a familial form of pancreas divisum. Hereditary pancreatitis is an hereditary autosomal dominant disorder with incomplete penetrance and is characterized by onset of symptoms during childhood or adolescence. The genetic defect responsible for hereditary pancreatitis has been identified by Whitcomb et al 28 to be a histidine substitution for arginine in the cationic trypsinogen gene (R117H) on chromosome 7q35. Linkage and mutation analyses have identified additional mutations in the cationic trypsinogen gene responsible for hereditary pancreatitis in other kindreds of patients. 29
Young patients with recurrent pancreatitis without other apparent causes should undergo genetic testing for hereditary pancreatitis. Three of our patients had a positive family history of pancreatitis, and in one instance genetic analysis confirmed the hereditary pancreatitis mutation.
At present, there does not appear to be a single ideal approach to treatment of patients with either complete or incomplete pancreas divisum. In patients with complete pancreas divisum who have recurrent pancreatitis, sphincteroplasty of one or both ducts accompanied by cholecystectomy has been recommended. Recently, endoscopic dilatation or sphincterotomy and stenting have been used with increasing frequency, even in children. 19 The difficulties of performing these procedures in the small ducts encountered in children have proven to be a limiting factor. In our group of patients, the minor papilla could be cannulated for pancreatography in only 38% of patients. One patient underwent attempted dilatation and stenting; this was unsuccessful and resulted in a serious episode of pancreatitis necessitating a hospital stay of several weeks. There is evidence that surgical sphincteroplasty is more effective than endoscopic papillotomy. 23 Lehman et al 30 analyzed the results of minor papilla sphincterotomy in 52 patients who had chronic pancreatic pain and pancreatitis and found that patients with acute recurrent pancreatitis benefited 76.5% of the time; those with manifestations of chronic pancreatitis benefited only 27% of the time. Stents were left in these patients after papillotomy. Ductal strictures noted in patients with incomplete pancreas divisum also respond to stenting, but the beneficial effects are usually not sustained once the stents are removed, similar to the results noted by Ashby and Lo 31 in a large series of patients with pancreatic duct strictures not related to pancreas divisum. In their patients, stents lasted up to 30 days. In one of our patients so treated during a 2-year period, a stent could be maintained for more than 2 months, but once the stent was removed the stenosis recurred. However, successful stenting did validate the fact that the stricture was the cause of the pancreatitis in that patient and correctly predicted that distal drainage would relieve his problem. This may be one of the prime advantages of stenting in patients with incomplete pancreas divisum associated with pancreatitis, particularly if the distal duct does not appear to be very dilated. One reason why patients with either form of pancreas divisum, but particularly incomplete pancreas divisum, may have intermittent bouts of acute pancreatitis may be related to the fact that these patients intermittently form protein plugs in their ductal systems that cause obstruction, as reported by Kawatomi et al. 32 Effective papillotomy or sphincteroplasty or distal drainage permits these protein plugs to be evacuated.
The role of pancreas divisum in causing chronic pancreatitis is controversial. Warshaw 14,33 has suggested that chronic pancreatitis is rarely caused by accessory papilla stenosis, and reported poor results with accessory papilla sphincteroplasty alone in patients with chronic pancreatitis. Histologic study of pancreatectomy specimens in our group of patients was informative in terms of the striking changes of chronic pancreatitis found in these children. In addition, all but one patient was found to have a fibrotic gland at the time of surgery. Correlation of the response to surgery with the degree of chronic pancreatitis seen histologically in our series suggests that patients with less advanced disease are more likely to respond to ductal sphincteroplasty, and that more advanced changes of chronic pancreatitis require pancreaticojejunostomy. Confirmation of this finding in a larger group of children would emphasize the need for earlier diagnosis and treatment in this patient population.
The question arises about how to manage patients who fail to improve after sphincteroplasty. There appears to be a difference between patients who have recurrent bouts of acute pancreatitis and those with chronic pain. The difference between these two groups of patients has not been elucidated, even when all sites of potential stenosis have been relieved. This is a problem yet to be solved. One subset of patients in the latter group can be demonstrated to have residual ductal ectasia of the ventral ductal system in the head of the pancreas. It may be that pancreatic resection is required for at least some of these patients, but strict criteria for resection have not yet been delineated in any series of patients with pancreas divisum. However, in most patients with pancreas divisum, either complete or incomplete, associated with acute pancreatitis, relief of obstruction or distal drainage can provide long-lasting relief. In our series, longitudinal pancreaticojejunostomy relieved ductal obstruction and improved recurrent pancreatitis in three patients. Because ductal dilation in these patients was modest, limited distal pancreatectomy with splenic preservation was used to identify the main pancreatic duct. Alternatively, intraoperative ultrasonography has proven useful in identifying the dilated duct during pancreaticojejunostomy.
Acknowledgments
The authors thank Majed Dasouki, MD, and Kay Washington, MD, for their contributions to this work and Linda Thornton and Linda Stewart for their tireless support.
Discussion
Dr. Charles J. Yeo (Baltimore, Maryland): I’d like to congratulate Drs. Neblett and O’Neill for presenting to us what is really a very large series in the pediatric age group of patients with pancreatic divisum, a disease that is not often recognized and treated in childhood. I have one historical footnote and four questions.
I went back to the archives in the rare book room and pulled out Dr. Opie’s book. Dr. Opie began as an instructor at Johns Hopkins and then went to the Rockefeller Institute and ended as a professor of pathology at Washington University in St. Louis. In his seminal textbook, Disease of the Pancreas, its Cause and Nature, which was published in 1903, Dr. Opie noted that he had personally dissected the pancreatic ducts in 100 specimens, that he had injected Berlin blue into the ducts then fixed and preserved them, and he found in 10 cases (10%), that there was no communication between the duct of Wirsung, which drains through the major papilla, and the duct of Santorini, which drains through the minor papilla.
In this scenario, Dr. Opie wrote, “The duct of Santorini represents the outlet for a part of gland substance and is functionally independent of the lower duct.” In other words, here in 1903, this is one of the earliest descriptions, and some people attribute it the earliest description of pancreatic divisum.
This has remained an enigma for surgeons and gastroenterologists for 100 years. As you can see from the results in this excellent series, we don’t have all the answers, because only 70% of patients are improved.
My questions are as follows:
In your pediatric age group, only three of eight patients had successful minor papilla cannulations for diagnostic ERCP. Do you have any experience with MRCP, an emerging technology, a fine, noninvasive diagnostic test that can actually visualize the pancreatic ductal anomalies? Because ERCP is often used simply for diagnosis, MRCP may be a very viable alternative.
Number two, in your group that you treated with longitudinal pancreaticojejunostomy, there were several cases where limited distal pancreatectomy was performed in order to provide access to the main pancreatic duct. This scenario was done because you didn’t have the ductal anatomy mapped out. Do you believe that such a limited resection is at all beneficial, or could you use the intraoperative ultrasound to assist with ductal identification and eliminate the need for such limited distal resection?
Number three, in the group treated by accessory papilloplasty, please describe the procedure in more detail. Do you place sutures between the duodenal mucosa and the duct of Santorini? Do you place transpapillary stents? How long do you leave the stent in place? Do you personally remove the stent postoperatively or do you allow it to drop out? These are some controversies in the minor papillary procedure.
And number four, in your overall group of 83 patients with acute pancreatitis, there were 20 children, or 24%, who were categorized as having idiopathic disease. Have these children been genetically tested for hereditary pancreatitis? Not just the R117-H mutation, but the other HP mutations that occur with lesser frequency, but which we now know contribute to several percentage cases of hereditary pancreatitis.
Dr. J. Patrick O’Leary (New Orleans, Louisiana): I’d like to rise just to ask Skip or Jim, do you believe that pancreatic divisum actually was the cause of the pancreatitis in these patients? Because it is suggested that actually about 10% of adults at least have pancreatic divisum and, of course, only a small percentage of those patients will have pancreatitis. And if in fact you believe it is related, then what is the pathophysiologic mechanism whereby the divisum is associated with pancreatitis?
Dr. Wallace W. Neblett III (Closing Discussion): Dr. Yeo, you had inquired as to whether we have any experience using MRCP in the evaluation of these patients. Actually, we have just begun to have an experience using MRCP in some of our patients with other pancreaticobiliary anomalies, but the study was not available at the time the evaluations were ongoing for this group of patients with pancreatic divisum. We are quite excited about the possibility of its use in the future, however, and in diagnosing and mapping the anatomy in this group of patients. I think it will be particularly useful in children because of the difficulty we often encounter in cannulating the minor papilla and demonstrating the ductal anatomy of the duct of Santorini. It also has a lot of application to doing serial studies in a noninvasive fashion, and the potential that it can be used in postoperative patients to assess their duct after some of the other surgical procedures, such as the Puestow procedure, because not all of these patients have a great result, even after the Puestow. It is often questioned whether there is any residual ductal obstruction that can be surgically approached.
You asked about the Puestow group and whether the distal pancreatectomy could be avoided either with other preoperative studies to outline the ductal anatomy or the possibility of using intraoperative ultrasound. I’m intrigued by the concept of using intraoperative ultrasound, which we have not used, but have just gone on and nipped off the tail of the pancreas. But as you know, that ends up being quite a dissection sometimes to lift it out of the bed of the spleen, and certainly we are interested in preserving the spleen in these patients. So I think that would be something that I would be quite interested in trying to utilize.
You asked about the technique of accessory sphincteroplasty, and we do a formal mucosa-to-mucosa anastomosis of the pancreatic duct to the duodenum. In doing our sphincteroplasty, we have not left stents in these patients, and I think the role of stenting in children, specifically with the very tiny duct, certainly needs to be explored. The problem that all of us encounter, obviously, is that we see these patients so infrequently, it’s hard to know really whether that’s going to be of benefit or not.
You asked about our group of patients with idiopathic pancreatitis. We have been quite interested in that and have begun to do DNA testing on the patients we encounter with unexplained pancreatitis. We have so far been able to screen about 10 of the patients. Three of the patients in this group were screened for hereditary pancreatitis, with the only one showing the mutation being the patient that I alluded to, the 12-year-old girl. However, we are quite interested in expanding that group of studies, and we have discovered that not only the R117 mutation is present in our group of patients in Tennessee, but also we have found another mutation that is responsible for hereditary pancreatitis.
Dr. O’Leary, you asked whether we believed that pancreas divisum was the cause of pancreatitis in this group of patients, and if so, what is the pathophysiologic mechanism of pancreatitis. I think we have all come to believe that pancreatitis is a disorder that occurs with a wide variety of mechanisms and with a kind of final common pathway. I think ductal obstruction is one of the mechanisms that can trigger pancreatitis, but it is obviously a cofactor, because patients with ductal obstruction don’t have continuous pancreatitis.
So we think that the ductal obstruction present in patients with pancreas divisum does serve as a cofactor to cause pancreatitis. The other cofactors are certainly not worked out in patients with pancreatic divisum in general nor in our group of children with pancreatitis.
Footnotes
Correspondence: Wallace W. Neblett III, MD, Dept. of Pediatric Surgery, Medical Arts Bldg., Suite 338, 1211 21st Ave. South, Nashville, TN 37212.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
E-mail: wallace.neblett@mcmail.vanderbilt.edu
Accepted for publication December 1999.
References
- 1.Miyano T. The pancreas. In: O’Neill JA, Rowe MI, Grosfeld JL, et al, eds. Pediatric Surgery, 5th ed. St. Louis: Mosby-Yearbook; 1998: 1527–1544.
- 2.Opie E. The anatomy of the pancreas. Johns Hopkins Hospital Bull 1903; 14:229–232. [Google Scholar]
- 3.Opie E. Disease of the Pancreas: Its Cause and Nature. Philadelphia: JB Lippincott; 1910:29.
- 4.Mitchell CJ, Lintott DJ, Ruddell WSJ, et al. Clinical relevance of an unfused pancreatic duct system. Gut 1979; 20:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosch W, Koch H, Schaffner O, Demling L. The clinical significance of the pancreas divisum. Gastrointest Endosc 1976; 22:206–227. [DOI] [PubMed] [Google Scholar]
- 6.Delhaye M, Engelholm L, Cremer M. Pancreas divisum: congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology 1985; 89:951–958. [DOI] [PubMed] [Google Scholar]
- 7.Sugawa C, Walt AJ, Nunez DC, et al. Pancreas divisum: is it a normal anatomic variant? Am J Surg 1987; 153:62–67. [DOI] [PubMed] [Google Scholar]
- 8.Cotton PB. Congenital anomaly of pancreas divisum as a cause of obstructive pain and pancreatitis. Gut 1980; 21:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter JM, Shapiro RH, Mulley AG, Warshaw AL. Association of pancreas divisum and pancreatitis, and its treatment by sphincteroplasty of the accessory ampulla. Gastroenterology 1981; 81:1104–1110. [PubMed] [Google Scholar]
- 10.Gregg JA. Pancreas divisum: its association with pancreatitis. Am J Surg 1977; 34:539–543. [DOI] [PubMed] [Google Scholar]
- 11.Kruse A. Pancreas divisum: a significantly higher incidence in chronic pancreatitis? [abstr] Scand J Gastroenterol 1977; 12:52. [Google Scholar]
- 12.Bernard JP, Sahel J, Giovannini M, Sarles H. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas 1990; 5:248–254. [DOI] [PubMed] [Google Scholar]
- 13.Guelrud M. The incidence of pancreas divisum in children. Gastrointest Endosc 1996; 43:83–84. [DOI] [PubMed] [Google Scholar]
- 14.Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg 1990; 159:59–64. [DOI] [PubMed] [Google Scholar]
- 15.Keith RG. Surgery for pancreas divisum. Gastrointest Endosc Clin North Am 1995; 5:171–180. [PubMed] [Google Scholar]
- 16.Yedlin ST, Dubois RS, Philippart AI. Pancreas divisum: a cause of pancreatitis in childhood. J Pediatr Surg 1984; 19:793–794. [DOI] [PubMed] [Google Scholar]
- 17.Wagner CW, Golladay ES. Pancreas divisum and pancreatitis in children. Am Surg 1988; 54:22–26. [PubMed] [Google Scholar]
- 18.Adzick NS, Shamberger RC, Winter HS, Hendren WH. Surgical treatment of pancreas divisum causing pancreatitis in children. J Pediatr Surg 1989; 24:54–58. [DOI] [PubMed] [Google Scholar]
- 19.Brown CW, Werlin SL, Geenen JE, Schmalz M. The diagnostic and therapeutic role of endoscopic retrograde cholangiopancreatography in children. J Pediatr Gastroenterol Nutr 1993; 17:19–23. [DOI] [PubMed] [Google Scholar]
- 20.Sanada Y, Yoshizawa Y, Chiba M, et al. Ventral pancreatitis in a patient with pancreas divisum. J Pediatr Surg 1995; 30:665–667. [DOI] [PubMed] [Google Scholar]
- 21.Tagge EP, Tarnasky PR, Chandler J, et al. Multidisciplinary approach to the treatment of pediatric pancreaticobiliary disorders. J Pediatr Surg 1997; 32:158–164. [DOI] [PubMed] [Google Scholar]
- 22.Bradley EL, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. J Am Coll Surg 1996; 183:65–70. [PubMed] [Google Scholar]
- 23.Lehman GA, Sherman S. Diagnosis and therapy of pancreas divisum. Gastrointest Endosc Clin North Am 1998; 8:55–77. [PubMed] [Google Scholar]
- 24.Carey LC, Fromkes JJ, Cooperman M. Pancreas divisum: late results of Santorini sphincteroplasty. Gastroenterology 1984; 87:1041A. [Google Scholar]
- 25.Warshaw AL, Simeone J, Schapiro RH, et al. Objective evaluation of ampullary stenosis with ultrasonography and pancreatic stimulation. Am J Surg 1985; 149:65–72. [DOI] [PubMed] [Google Scholar]
- 26.Ueno E, Takada Y, Yoshida I, et al. Pancreatic diseases: evaluation with MR cholangiopancreatography. Pancreas 1998; 16:418–426. [PubMed] [Google Scholar]
- 27.Muzaffar AR, Moyer MS, Dobbins J, et al. Pancreas divisum in a family with hereditary pancreatitis. J Clin Gastroenterol 1996; 22:16–20. [DOI] [PubMed] [Google Scholar]
- 28.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 1996; 14:141–145. [DOI] [PubMed] [Google Scholar]
- 29.Dasouki MJ, Cogan J, Summar ML, et al. Heterogeneity in hereditary pancreatitis. Am J Med Genet 1998; 77:47–53. [PubMed] [Google Scholar]
- 30.Lehman GA, Sherman S, Nisi R, et al. Pancreas divisum: results of minor papilla sphincterotomy. Gastrointest Endosc 1993; 39:1–8. [DOI] [PubMed] [Google Scholar]
- 31.Ashby K, Lo S. The role of pancreatic stenting in obstructive ductal disorders other than pancreas divisum. Gastrointest Endosc 1995; 42:306–311. [DOI] [PubMed] [Google Scholar]
- 32.Kawatomi T, Nagashima K, Tsuchiya H, et al. Pancreatic lithiasis associated with anomalous arrangement of pancreaticobiliary ductal system; a review of the Japanese literature. Shounigeka 1985; 17:1351–1360. [Google Scholar]
- 33.Warshaw Al, Richter JM, Schapiro RH. The cause and treatment of pancreatitis associated with pancreas divisum. Ann Surg 1983; 198:443–452. [DOI] [PMC free article] [PubMed]