Abstract
Objective
To review the authors’ 7-year experience with a surgical approach for pancreatic and duodenal neuroendocrine tumors (NETs) in patients with multiple endocrine neoplasia type 1 (MEN 1) designed to remove all gross tumor with limited complications, preserving pancreatic function.
Summary Background Data
MEN 1 is an autosomal dominant familial neoplasia syndrome characterized by the development of NETs of the duodenum and pancreas. Some tumors are clinically insignificant or follow a benign course, although a subset pursues a malignant, lethal natural history; the risk of surgical management must be appropriate to the disease course.
Methods
The clinical, biochemical, genetic, and pathologic data were retrospectively reviewed for 21 consecutive MEN 1 patients undergoing pancreatic resection for NETs between 1993 and 1999 at one institution. Age at operation, presenting symptoms, results of preoperative and intraoperative localization studies, major and minor complications, and pathology, including metastases, were analyzed.
Results
The surgical approach was selected based on the location and size of the tumors. Five patients required pancreaticoduodenectomy, 11 patients underwent non-Whipple pancreatic resections, and 5 underwent simple enucleation of benign NETs. The incidence of regional lymph node metastases was 33%.
Conclusions
Major pancreatic procedures can be performed safely in most patients with MEN 1 and NETs. Because NETs are the most common MEN 1-related cause of death in the authors’ kindreds, an aggressive surgical approach, including early intervention before malignant spread and major pancreatic resection where indicated, appears justified.
Parathyroid hyperplasia, enteropancreatic neuroendocrine tumors, and pituitary adenomas characterize the multiple endocrine neoplasia type 1 (MEN 1) syndrome. Depending on the method of study, 35% to 75% of genetically affected individuals develop neuroendocrine tumors (NETs) of the pancreas and duodenum, which are frequently malignant. In patients with MEN 1, NETs result in symptoms either from excess secretion of a specific hormone product or the effects of the tumoral process itself. Bronchial and thymic carcinoid tumors occur in approximately 7% of patients with MEN 1. These tumors are characterized by a high malignant potential and aggressive biologic behavior. The malignant duodenopancreatic tumors and intrathoracic tumors account for the majority of the disease-related complications and death in MEN1 gene mutation carriers. 1,2 Adenomas of the anterior pituitary occur in 15% to 30% of patients with MEN 1. In addition, affected individuals develop subcutaneous lipomas, benign thyroid nodules, adrenocortical tumors, ependymomas of the central nervous system, and cutaneous angiofibromas with increased frequency.
Multiple endocrine neoplasia type 1 is an autosomal dominant genetic disorder associated with germline mutations in the recently identified MEN1 tumor suppressor gene on chromosome 11q. 3 The MEN1 tumor suppressor gene encodes a predicted 610-amino acid product termed menin. Menin has no significant sequence similarity to other known proteins. The MEN1 gene contains 10 exons, with the first exon being untranslated. The 2.8-kilobase mRNA transcript is expressed in a wide variety of tissues, including lymphocytes, thymus, pancreas, thyroid, testis, and ovaries. The MEN1 mutations are evenly distributed throughout the coding sequence and include missense, nonsense, frameshift, and mRNA splicing defects. 3–7 To date, no significant genotype phenotype correlations have been demonstrated between specific MEN1 gene mutations and clinical outcome or the frequency of expression of specific functional tumors. Presymptomatic genetic testing for the presence of a MEN1 gene mutation associated with the disease phenotype is now possible for kindred members at genetic risk, especially when the specific MEN1 gene mutation associated with the disease phenotype in that particular family is known.
The management of the duodenopancreatic NETs that occur in patients with MEN 1 remains the most important clinical controversy. The unique aspects of cancer development associated with this hereditary syndrome include the diffuse nature of involvement, including a preneoplastic proliferation of gastroenteropancreatic rests of neuroendocrine cells (seen as islet cell hyperplasia in the pancreas), the involvement of multiple target tissues, and the characteristic development of multiple tumors. Although early neoplastic changes may be diffuse at the histopathologic level, some tumors may grow rapidly and metastasize early, affecting outcome or survival. Clearly, the adoption of a directed clinical management strategy requires an understanding of the natural history of NETs in patients with MEN 1, the factors associated with local invasion and regional or distant metastases, and the prognostic variables that affect clinical outcome and lethality from the respective components of disease.
The controversy reflects the lack of agreement on the best methods for early diagnosis of duodenopancreatic NETs in the setting of the MEN 1 syndrome, the optimal timing for intervention, and the most appropriate surgical procedure to perform once the tumors are identified. The impact of effective cancer surveillance and early intervention is underscored by the realization that the NETs associated with this hereditary syndrome frequently arise in young, otherwise healthy individuals. Because major pancreatic resection carries a significant risk of complications as well as a small but measurable risk of death, it is difficult to advocate major pancreatic surgery for small, benign, clinically insignificant tumors. However, delaying diagnosis and effective treatment of MEN 1-associated NETs until after local or distant metastasis has occurred can be life-limiting. It is obviously desirable to intervene early to prevent malignant spread, while minimizing the risk of complications and death (from either cancer or surgery).
This study reports the results of a 7-year experience with the management of duodenal and pancreatic NETs arising in the setting of the MEN 1 syndrome in a large series of patients treated at a single institution. A standardized surgical approach and decision-making algorithm were used. The results of biochemical and radiographic screening tests, pathologic findings, incidence of metastases, and surgical complications were analyzed. These findings and the recent advent of genetic testing, which has provided the ability to identify mutation carriers before the elevation of biochemical markers or clinically evident tumors, should be taken into consideration when making recommendations for the diagnosis and surgical management of NETs in patients with MEN 1.
METHODS
All patients with MEN 1 who were diagnosed with and surgically treated for NETs of the pancreas or duodenum by our group between 1993 and 1999 were included in the study. Surgery was performed by one of three endocrine surgeons at Washington University School of Medicine, St. Louis, Missouri. The clinical, biochemical, genetic, and pathologic data were retrospectively reviewed in 1999.
The members of 33 independent, extended kindreds with MEN 1 have been followed prospectively by our group for many years. An extensive database of patients with the multiple endocrine neoplasia syndromes is maintained at our institution as part of the Multiple Endocrine Neoplasia Program. Most of the patients were diagnosed as part of our annual biochemical screening program for known affected patients or persons at risk for MEN 1. Patients with an elevated tumor marker or clinical signs and symptoms suggesting the presence of a duodenal or pancreatic NETs (either functional or nonfunctional) undergo selective radiographic imaging studies. Essentially all patients have a high-resolution dual-phase computed tomography (CT) scan of the upper abdomen, including the pancreas and liver. In selected cases, magnetic resonance imaging (MRI) or transabdominal ultrasonography is obtained. For patients with a functional tumor such as gastrinoma or insulinoma, pancreatic arteriography with selective intraarterial injection of calcium gluconate or secretin is used, 8–10 with measurement of the resulting increase of hormone levels in the hepatic veins. Calcium gluconate and secretin are secretagogues for insulin and gastrin, respectively.
The patients underwent exploration through a bilateral subcostal or midline abdominal incision. Complete exposure of the pancreas and duodenum was accomplished by performing an extended Kocher maneuver, exposing the entire anterior surface of the pancreas through the lesser sac, and mobilizing the inferior border of the pancreas to allow inspection and bimanual palpation of the pancreatic parenchyma. Intraoperative ultrasonography was used routinely to identify small tumors in the pancreas or duodenal wall and to define the anatomical relationships between tumors and the major vascular structures, the intrapancreatic portion of the common bile duct, and the pancreatic duct. Although intraoperative ultrasound represents an extension of the visual and tactile tools in the surgeon’s armamentarium to identify tumors, the intraoperative images were obtained and interpreted in parallel by an attending radiologist.
Routine postoperative care for patients undergoing pancreatic resection was used. Extensive closed suction drainage of the pancreatic resection sites was continued until the drainage output decreased to less than 20 mL/day. Somatostatin analogue (octreotide) was not routinely used prophylactically. Patients with established pancreaticobiliary anastomotic leaks were treated with bowel rest, total parenteral nutrition, and octreotide.
The Mann-Whitney test for nonparametric statistics was used to determine the relation between tumor size and the frequency of metastases between groups. Significance was defined at P < .05.
RESULTS
Patient Characteristics
The 21 patients in the study group included members of 11 separate extended kindreds with MEN 1 that are followed prospectively at our institution. The gender distribution (10 men, 11 women) was essentially equal, which is in keeping with the autosomal dominant pattern of inheritance for the MEN 1 trait. At the time of resection of duodenal or pancreatic tumors, the mean age was 44.5 years (range 19–73 years). The average length of follow-up was 748 days for the patients undergoing enucleation, 1,015 days for the patients undergoing Whipple pancreaticoduodenectomy, and 542 days for the patients undergoing non-Whipple pancreatic resections.
The detection of a clinically significant NET was based on the results of systematic annual biochemical screening in patients at risk, selective radiographic localizing studies in patients with elevated tumor markers, 11 the onset of signs and symptoms related to local tumor growth, or the presence of a specific syndrome of hormone excess.
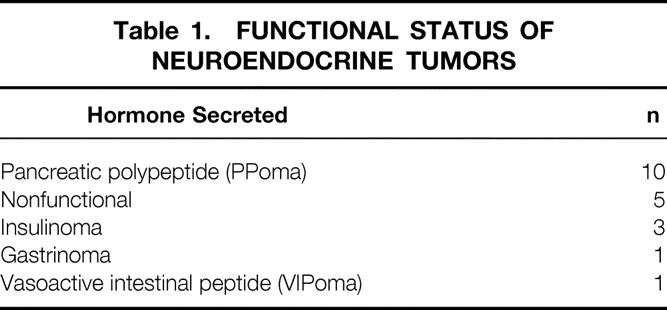
The NETs that develop in patients with MEN 1 may be nonfunctional, or they may be associated with hypersecretion of any of a variety of peptide products. The distribution of different functional NETs of the duodenum or pancreas in this cohort of 21 MEN 1 patients is shown in Table 1. Diffuse neoplastic change with the development of multiple pancreatic NETs is characteristic of patients with MEN 1. The most clinically significant or dominant functional tumor present in each patient is indicated in the table. Pancreatic polypeptide-producing tumors and nonfunctional tumors accounted for 15 (75%) of the 20 most clinically important tumors. Insulinoma, gastrinoma, and vasoactive intestinal peptide-producing NETs were less common.
Table 1. FUNCTIONAL STATUS OF NEUROENDOCRINE TUMORS
Preoperative Radiographic Localizing Studies
After a clinical or biochemical diagnosis of NET is established in patients with MEN 1, most patients undergo preoperative noninvasive or invasive localizing studies. A high-resolution cross-sectional radiographic study such as CT or MRI should be obtained before surgery in essentially all patients to define the anatomical relations of larger pancreatic tumors and to detect enlarged regional lymph nodes or hepatic metastases. The presence of metastatic spread beyond the primary site significantly affects the surgical management of NETs in patients with MEN 1.
Invasive radiographic imaging studies may be used in selected patients. These are particularly useful when a patient with MEN 1 has a biochemical syndrome of hormone excess and the offending functional tumor is occult on noninvasive imaging studies. The goal of these invasive studies is usually to provide regional localization of the functional NET. The characteristic occurrence of multiple NETs in the setting of the familial MEN 1 syndrome underscores the importance of identifying or regionally localizing the functional tumor. Unfortunately, insulinomas and gastrinomas in patients with MEN 1 often are not successfully localized even with invasive localizing studies in up to 50% of patients. 12
The accuracy of preoperative invasive and noninvasive imaging studies in this study is summarized in Table 2. The selection of localizing studies was based on clinical indications, and therefore not all studies were performed in all patients. Transabdominal ultrasonography and CT were relatively insensitive in detecting the most clinically important NETs in patients with MEN 1. The frequency of true-positive CT scans, defined as scans that accurately detected the largest or most clinically significant NET, was 10 (59%) of 17 studies. Only one patient underwent MRI scanning, with the specific purpose of evaluating a suspicious mass lesion in the liver.
Table 2. RESULTS OF IMAGING TESTS FOR LOCALIZATION OF NEUROENDOCRINE TUMORS
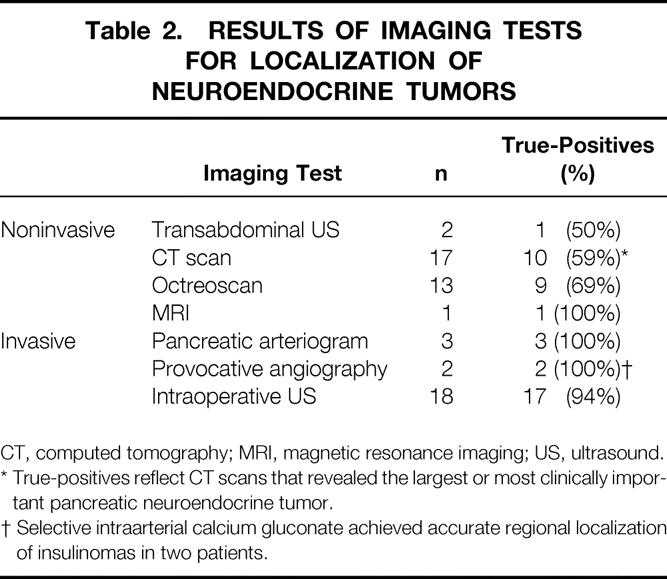
CT, computed tomography; MRI, magnetic resonance imaging; US, ultrasound.
* True-positives reflect CT scans that revealed the largest or most clinically important pancreatic neuroendocrine tumor.
† Selective intraarterial calcium gluconate achieved accurate regional localization of insulinomas in two patients.
The somatostatin receptor scintigraphy scan (SRS), or octreoscan, localizes NETs based on the presence of type II cell surface receptors for somatostatin. In this study and others, 13 SRS provides increased sensitivity for the detection of NETs compared with conventional CT scanning, but it does not localize small tumors or tumors that characteristically lack a high cell surface concentration of somatostatin receptors (e.g., insulinomas). SRS may be indicated in MEN 1 with clear biochemical evidence of a functional NET but no evidence of mass lesion on conventional noninvasive imaging such as CT. The frequency of true-positive SRSs in this study was 9 (69%) of 13 studies.
Invasive localizing studies were used in the subgroup of patients in whom preoperative definitive or regional localization of a functional NET was desirable. This study and previous reports 8–10 confirmed the increased sensitivity of selective provocative angiography in the localization of occult, functional NETs in patients with MEN 1. Intraoperative ultrasound was used in 18 patients, including all patients in the latter portion of the series and identified all NETs that were resected. One patient underwent enucleation of a mass lesion from the uncinate process that was identified by preoperative CT scanning, SRS, and intraoperative ultrasound, although no evidence of NET was revealed by pathology. The incidence of true-positive results from intraoperative ultrasound was therefore 17 (94%) of 18.
Surgical Findings and Outcome
The surgical approach was based on the size and location of the tumors in the pancreas or duodenal wall. The broad surgical goals were to resect functional tumors and tumors with a significant risk of malignancy while preserving pancreatic function. A prerequisite to the effective surgical management of NETs in patients with MEN 1 is an understanding that the enteropancreatic neuroendocrine cells are affected with a diffuse preneoplastic hyperplasia and a predisposition to the formation of multiple tumors. Small, circumscribed benign tumors, such as the insulinoma resected from a 19-year-old woman in this series, are adequately treated by enucleation. Major pancreatic resection, such as distal pancreatectomy, was used when multiple tumors were detected in the body and tail of the pancreas. Enucleation of additional tumors from the pancreatic head was combined with distal pancreatectomy where appropriate. Pancreaticoduodenectomy was performed for large tumors of the pancreatic head and uncinate process, especially when evidence of metastatic neuroendocrine carcinoma was present in regional lymph nodes. Pancreaticoduodenectomy was performed in 5 patients, and a non-Whipple major pancreatic resection, usually distal subtotal pancreatectomy, was performed in an additional 11 patients. In either group, enucleation of additional NETs from the remaining pancreatic parenchyma was performed when appropriate. One distal pancreatectomy was performed laparoscopically. Patients with hypergastrinemia underwent duodenotomy and exploration of the wall of the duodenum for a gastrinoma primary. A complete regional lymphadenectomy was performed for patients with Zollinger-Ellison syndrome or obvious malignant NET of the pancreas. Five patients underwent enucleation alone of small, apparently benign NETs.
The regional localization of 39 primary NETs for which a definitive description was available from review of the surgical and pathology reports is shown in Figure 1. The tumors demonstrated an essentially uniform distribution throughout the pancreatic head, uncinate process, and body and tail of the pancreas, corresponding roughly to the approximate volume of pancreatic parenchyma in each region. Two gastrinomas were identified and resected from the duodenal wall. The range of maximum diameter of the largest NET resected was 0.1 to 8.0 cm (mean 1.83 cm). One patient underwent enucleation of a mass lesion in the uncinate process that was imaged by CT and was associated with a focus of increased uptake on SRS. The final pathology revealed an intrapancreatic lymph node with no evidence of malignancy. This patient had false-positive preoperative imaging studies.
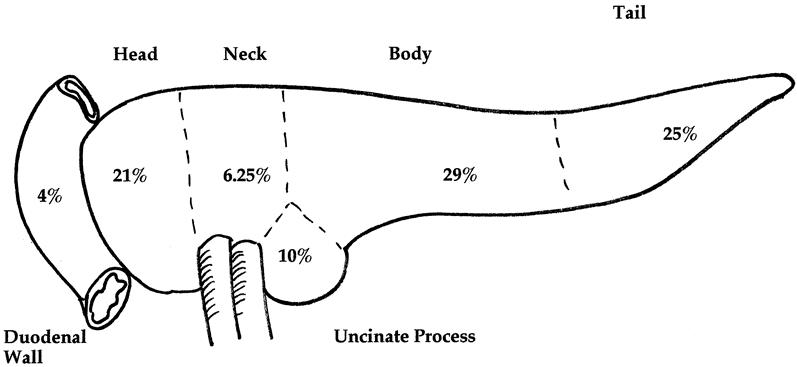
Figure 1. Distribution of primary duodenal and pancreatic neuroendocrine tumors in 21 patients with multiple endocrine neoplasia type 1. Two gastrinomas were located in the duodenal wall. The intrapancreatic tumors demonstrated an even distribution according to the approximate volume of parenchyma in each pancreatic region. Maximum diameter of largest tumor: N = 39; range 0.1–8.0 cm; mean 1.83 cm.
Table 3 depicts the incidence of regional lymph node and hepatic metastases. Regional lymph node metastases were present in one third of the patients. Most of the patients with evidence of regional lymph node metastases underwent pancreaticoduodenectomy or distal pancreatectomy. One patient in the enucleation group had a malignant gastrinoma resected from the duodenal wall and evidence of metastatic neuroendocrine carcinoma in peripancreatic lymph nodes. The incidence of detectable hepatic or distant metastases at the time of operation was approximately 10%. Wedge resection of liver metastases was performed in two patients, for pathologic diagnosis in one and potential cure in the other.
Table 3. INCIDENCE OF METASTATIC SPREAD BEYOND THE PRIMARY SITE
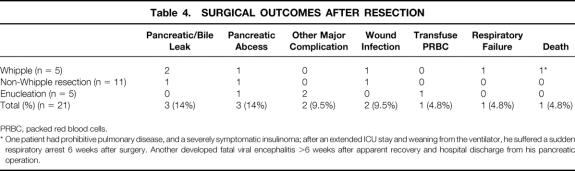
The surgical outcomes are reported in Table 4. The frequency of postoperative complications was consistent with that reported in other series of major pancreatic resections for NETs. 14,15 Three patients (14%) developed pancreatic or biliary anastomotic leaks (defined as drainage of >50 mL of amylase- or bile-rich fluid in the closed suction drain per day for 3 consecutive days, after postoperative day 7). All of the anastomotic leaks healed with conservative management, including a period of bowel rest and total parenteral nutrition with or without somatostatin analogue (octreotide) treatment. Two patients (9.5%) had other major complications. One patient had a postoperative duodenal obstruction after enucleation of a tumor from the uncinate process, requiring reoperation. A second patient with a malignant gastrinoma and Zollinger-Ellison syndrome underwent concomitant esophagogastrectomy for an adenocarcinoma of the stomach arising at the gastroesophageal junction. He developed a leak from the esophagojejunostomy that was successfully treated with transthoracic drainage and total parenteral nutrition.
Table 4. SURGICAL OUTCOMES AFTER RESECTION
PRBC, packed red blood cells.
* One patient had prohibitive pulmonary disease, and a severely symptomatic insulinoma; after an extended ICU stay and weaning from the ventilator, he suffered a sudden respiratory arrest 6 weeks after surgery. Another developed fatal viral encephalitis >6 weeks after apparent recovery and hospital discharge from his pancreatic operation.
One patient died during the perioperative period and another died after the perioperative period of causes unrelated to either MEN 1 or surgery. The former patient had prohibitive pulmonary disease (FEV1 = 700 mL) but a severely symptomatic insulinoma requiring dependence on a 10% dextrose infusion. He failed to improve with medical therapy with diazoxide. He underwent a pancreaticoduodenectomy for a large insulinoma in the pancreatic head. After an extended intensive care unit stay and eventual weaning from the ventilator, he suffered a sudden respiratory arrest in the hospital approximately 6 weeks after surgery. The latter patient had an uneventful recovery after distal pancreatectomy but developed an apparently unrelated viral encephalitis more than 6 weeks after surgery and died.
Tumor Size Versus Regional and Distant Metastasis
The relation between the maximum diameter of the largest NET and the incidence of regional lymph node or hepatic metastasis is shown in Figure 2. The scatter plot depicts the maximum diameter of the largest NET as it relates to the group assignment of no evidence of metastases, regional lymph node metastases only, and hepatic or distant metastases at the time of the initial operation. There appeared to be a trend toward an increased frequency of regional lymph node metastases in patients with tumors exceeding approximately 3 cm in maximum diameter, but there was substantial overlap of all groups. When the tumors were stratified according to the presence of metastatic spread beyond the primary site (group I = no metastases, group II = regional lymph node or distant metastases), there were no statistically significant differences between groups using the Mann-Whitney test for nonparametric statistics (P = .1135).
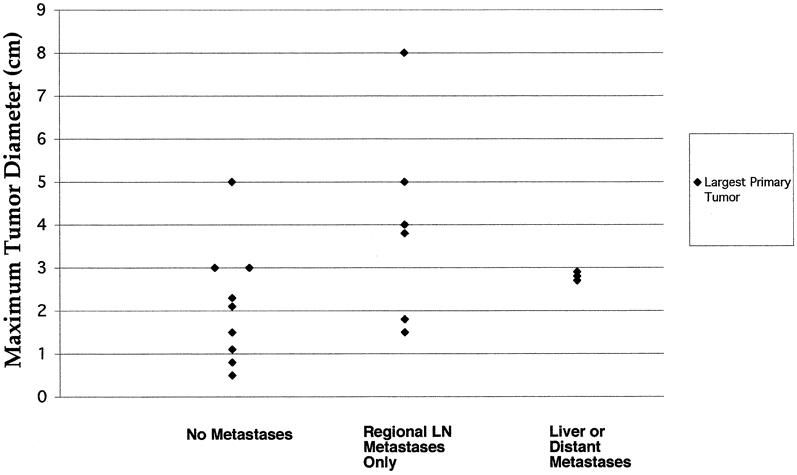
Figure 2. Scatter plot depicting the relation between the maximum diameter of the largest primary tumor and the presence of regional lymph node (LN) or distant metastases. There was significant overlap between all groups. The relation between tumor size and the presence of metastases (group I = no metastases, group II = LN metastases plus distant metastases) was not significant (P = .1135, Mann-Whitney test).
MEN1 Gene Mutations and Genotype/Phenotype Correlations
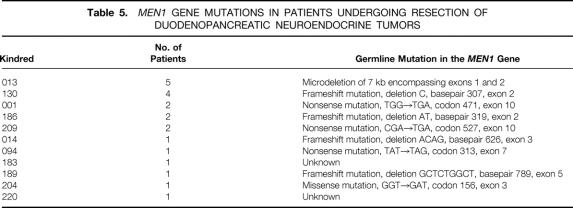
The germline mutations in the MEN1 tumor suppressor gene 7 for the patients in the study are shown in Table 5. The reported mutations in the MEN1 gene include missense, nonsense, frameshift, or mRNA splicing defects and are distributed throughout the nine coding exons or intervening intron sequences. An identical mutation is present in each affected individual in a kindred because the mutation is passed from generation to generation. The description of the germline mutations reveals that each family has a unique mutation. Except for the mutations in kindreds 013 and 204, each of the remaining mutations is predicted to result in premature termination of translation and a truncated menin protein product. There is no evident correlation between any specific mutation and the frequency of expression of a particular functional tumor (e.g., insulinoma, gastrinoma) or in the apparent biologic aggressiveness of the NET in that family, as assessed by the frequency of lymph node metastases.
Table 5. MEN1 GENE MUTATIONS IN PATIENTS UNDERGOING RESECTION OF DUODENOPANCREATIC NEUROENDOCRINE TUMORS
DISCUSSION
Widely accepted indications for the surgical removal of pancreatic and duodenal NETs in patients with MEN 1 include the presence of a functional syndrome caused by excess secretion of a specific hormone product by the tumor (with the arguable exception of Zollinger-Ellison syndrome resulting from gastrinoma) and a significant risk of malignant spread if the tumor is left untreated. However, understanding the natural history of the enteropancreatic NETs that occur in patients with MEN 1 is of paramount importance to an effective program of clinical screening and therapeutic intervention aimed at improved patient outcome. Studies of surgically resected pancreatic specimens and autopsy studies in patients with MEN 1 have confirmed the presence of preneoplastic changes such as diffuse islet cell hyperplasia and multiple microscopic or macroscopic foci of neuroendocrine carcinoma in virtually all affected patients. 16,17 Because prophylactic total pancreatectomy or major pancreatic resection for microscopic or clinically insignificant tumors cannot be advocated, many expert clinicians recommend a conservative surgical approach based on the notion that there is a relation between the size and the metastatic potential of these tumors. The best support for this rationale is provided by extensive data from Weber and coworkers 18 that found a significant correlation between tumor size and metastatic potential in gastrinomas. These data were specific to gastrin-producing tumors, included both sporadic and familial cases, and were based on a select referral group of patients treated over many years at the National Institutes of Health. This study has been used to support the use of tumor size as a criterion for surgical excision of duodenopancreatic NETs. However, these data may not necessarily be extrapolated to the multiple functional and nonfunctional enteropancreatic tumors that occur in the familial MEN 1 syndrome. In a recent retrospective report by our group, there was no correlation between the size of the primary NET and the incidence of regional or distant metastases in a group of 48 patients with MEN 1. 19
Others have recommended a more aggressive approach consisting of early surgical exploration and resection of tumors based on biochemical progression before these neoplasms could be detected radiographically. 20–23 The group in Uppsala, Sweden, has an extensive prospective experience in screening for duodenopancreatic tumors in patients with MEN 1. The objective of these authors’ approach is to detect and remove potentially malignant tumors when patients are asymptomatic, before malignant transformation and metastatic spread to local and distant sites. These authors emphasized the reported inefficiency of radiographic imaging tests in the early detection of the small, multifocal endocrine tumors that are characteristic of the multiple endocrine neoplasia syndromes. 24 Beginning as early as age 10 years, routine biochemical screening of MEN 1 family members at risk was performed by measuring endocrine pancreatic hormones after the administration of a standardized meal test. 25 Systematic biochemical testing resulted in a reduction in the average age at detection of pancreatic endocrine involvement of almost two decades 20 (mean age at diagnosis 25 years vs. 44 years). These data suggested a tendency toward a reduced death rate in patients who undergo early surgery for biochemically detected tumors, but longer follow-up is needed to determine whether such intervention will affect the disease-related death rate. 20 The potential shortcomings of this approach include the inefficiency of the available diagnostic tests, the possibility that early surgical exploration will unveil no tumors or only clinically insignificant tumors, and most importantly the potential for treatment-related complications.
Our approach to the treatment of NETs in patients with the MEN 1 syndrome has been to recommend surgery for patients with functional tumors or tumors that are large enough to be demonstrable by conventional radiographic tests (≥1–2 cm in maximum diameter). In this series of 21 patients undergoing surgery based on these criteria, the mean maximum diameter of the dominant NET resected was 1.83 cm. As expected, most patients had multiple tumors detected at the time of surgical inspection and examination of the pancreas with intraoperative ultrasound. Conventional noninvasive imaging studies, such as CT scanning and SRS, were relatively insensitive for detecting the most clinically significant NET (59% and 69% true-positive results, respectively) and infrequently displayed the multiple smaller tumors identified intraoperatively. Most significantly, when the size of the largest primary was analyzed in relation to the presence of metastases, no significant correlation was observed. The results of biochemical screening tests were frequently positive, but in this series and others the most common NET is a nonfunctional or pancreatic polypeptide-producing tumor. Biochemical and radiographic studies were relatively insensitive at depicting the multiple small tumors in patients with MEN 1.
The incidence of regional lymph node metastases was 33% and the incidence of hepatic or distant metastases was 9.5% in this series of patients undergoing surgery based on traditional criteria. This frequency of metastatic spread at the time of initial attempt at surgical control of the NETs is clearly undesirable, especially in light of the generally young age and excellent overall health of affected patients.
Major pancreatic resection or enucleation in this series was associated with complications and a low incidence of death. Patients with MEN 1 generally have soft, normal pancreas glands, making the performance of pancreatic–enteric anastomoses challenging and unforgiving. The incidence of pancreatic or biliary fistula in this series (14%) was nearly identical to that in other recent reports of pancreatic resection or enucleation of NETs. 14,15 No patient required reoperation for pancreaticobiliary fistula. The young age and overall excellent physiologic reserve are definitely assets in overcoming complications after pancreatic surgery in patients with MEN 1. Although the perioperative death in this series occurred in a 63-year-old patient with advanced preoperative pulmonary disease, it is fair to say that major pancreatic resection carries a measurable risk of operative mortality, even in otherwise healthy patients.
These clinical studies were performed before we had the ability to identify persons at genetic risk for MEN 1-associated tumors based on direct mutational testing. The recent identification of the MEN1 gene allows genetic diagnosis before the elevation of biochemical markers for neuroendocrine neoplasia, the onset of clinical signs and symptoms, or the presence of a radiographically detectable tumor mass. The data in this study support a need for aggressive cancer surveillance and screening to detect pancreatic and duodenal as well as intrathoracic NETs early, as well as appropriate surgical intervention to prevent malignant dissemination in patients who are determined to harbor a MEN1 gene mutation.
CONCLUSIONS
The genetic predisposition to the development of NETs in the setting of MEN 1 is a unique clinical cancer problem that requires critical consideration and discussion, as well as a directed and evidence-based surgical approach. The indications for surgery are not clear and must be modified for patients based on age, health status, clinical syndrome, and risk of metastatic dissemination or evidence of metastases at the time of intervention. The surgical approach must be appropriate to the disease under treatment. The current data suggest a more aggressive surgical approach as it relates to earlier intervention, before malignant dissemination. The widespread availability of genetic testing in the near future will place an emphasis on early detection and intervention for pancreatic and duodenal NETs. Major pancreatic resection may be avoided in some patients, depending on the size, location, and malignant potential, as well as other factors. The approach to NETs in patients with MEN 1 requires an aggressive cancer screening program and early intervention, as well as an oncologically sensible surgical procedure that minimizes complications and preserves pancreatic endocrine and exocrine function.
Discussion
Dr. Charles J. Yeo (Baltimore, Maryland): I congratulate Dr. Lairmore and his coauthors for a very provocative discussion of a very rare but really fascinating physiologic entity, that of MEN 1-associated neuroendocrine tumors.
They have really judiciously used surgical intervention in a group of 21 patients, attempting to impact on the natural history of this disease. We are all thankful to them that they have used the resources and data from their MEN 1 registry and the 33 kindreds to provide us with today’s data and, I think, to begin to answer some very important questions.
Dr. Lairmore, I have a few questions for you. You have given us a numerator, that is, an “N” of 21, of patients who were explored and resected. What is the denominator in this numerator? How many affected patients in the 33 kindreds are being followed? How many patients with these germline mutations, that is, the affected patients, have no biochemical abnormalities, and what is their age, range, and can we somehow classify them?
What is important, obviously, is to go back a step further, because you are operating on a group of patients of whom only two thirds are free of metastatic disease, and you have alluded to this in your conclusions. But what’s going to be the next step for determining who should be resected and who it is safe to follow?
Another question: clearly, at the heart of your topic, is what is the proper time to intervene in these patients? What is the correct marker for this localized nonmetastatic disease? Tell us a bit about what you think the future will be, and perhaps, even the role for gene therapy to correct what is a monogenetic abnormality here.
Third, in your manuscript you describe a “complete regional lymphadenectomy,” which you performed for obvious malignant neuroendocrine tumors. How extensive is your complete regional lymphadenectomy? What are the limits and the horizontal and vertical axes? The operative specimen you showed does not really, to me, qualify as a complete regional lymphadenectomy for what you stated was a malignant neuroendocrine tumor.
Lastly, your 10% postoperative mortality rate is high. This is for prophylactic intervention, if you will, because the natural history of this disease is that many people can live decades harboring malignant tumor. Now I grant you, in all fairness to you, the one patient that died was very symptomatic with an insulinoma and had no alternative to surgery, and the second one died 6 weeks postoperatively of a presumed viral illness. But nonetheless, that is a 10% mortality rate. If you use a risk-benefit analysis and plot that with the natural history curves, what does the operative mortality rate have to be to provide a true benefit to patients undergoing what really we would hope would be prophylactic duodenopancreatic resection for MEN 1?
Dr. John B. Hanks (Charlottesville, Virginia): This is a provocative paper, and when it comes out, it’s going to be very interesting reading.
I’d like to ask the authors the following: you have employed the Whipple for five patients with good results, and this may represent a real controversy, so I thought I’d give you a chance to expand, looking at those Whipple patients and giving the Society a more specific set of parameters for employing the Whipple, as Dr. Yeo has mentioned, there is significant morbidity associated with that.
To ask Charlie’s question a different way, 15 to 20 years ago, an MEN 1 patient with excess gastrin production might have gotten a total gastrectomy, leaving the primary tumor in place, and yet, those patients tended to live for decades. Is it reasonable to go back—and I am sure that Washington University has an extensive experience—and look at some of those patients that were operated on and did not—these would be historical controls—did not have resection? Can you give us some information about those patients’ final outcome?
Your comments about preoperative CT and MR sensitivity and specificity are important observations. Did you evaluate preoperative staging to the extent that you can specifically suggest one, or at least an algorithm or a primary imaging modality, which might perhaps allow some of us a clinical pathway or an algorithm for the work-up of these patients? Should we skip preoperative imaging and just go directly to intraoperative ultrasound, which you said had 94% success rate?
Finally, adding to Charlie’s question, how extensive are you with your resection? For example, would you advocate a lobe or hepatic resection in the face of pancreatectomy? If you felt that you could anatomically remove tumor, would you add something like hepatic resection?
Dr. Dana K. Andersen (New Haven, Connecticut): I want to address the specific question of surveillance for malignancy. You suggested that because of the incomplete penetrance of the gene defect and the uncertainty of the multiple types of defects involved, increased surveillance is clearly necessary. This is a problem also for the hereditary pancreatitics in the pediatric age group, where perhaps 40% of these children will develop carcinoma of the pancreas over the course of the next 40 years. The question is, what is the best surveillance study?
You didn’t mention endoscopic ultrasound as one of your preoperative imaging methods, and I am persuaded that this is in fact the best surveillance study we currently have, as it depicts 2- and 3-mm lesions, with high resolution and a high degree of accuracy, and it can identify pathologic lymph nodes in and around the duodenum and the head of the pancreas.
So my question for you is, do you routinely use EUS either in your preoperative assessment or as a routine surveillance tool in patients who carry this genetic defect and are at increased risk for carcinoma?
Dr. Terry C. Lairmore (Closing Discussion): Beginning with Dr. Yeo’s comments, he asked about the denominator, the number of patients who are currently asymptomatic. I think this question really goes to the penetrance of the disease. There are over 200 affected individuals in our kindreds, many of whom have already undergone surgery. So the number of patients in this series reflects all of the patients who were diagnosed and treated for neuroendocrine tumors during the study period.
We know that the peak incidence for the development of these tumors is approximately between age 35 and 40. Under this age, approximately half of the gene carriers in these kindreds are currently being followed and do not have any clinical indications for resection at present.
Dr. Yeo asked when is the proper time to intervene and what is the best biochemical marker. I think this really is the crux of the issue in these patients. We are not very good at picking these tumors up until they have already disseminated beyond the primary site. The traditional markers that have been used, including insulin, gastrin, and pancreatic polypeptide, are not very sensitive. There are other markers, including chromogranin A, that are under investigation, but we clearly need a better way to diagnose these tumors early.
This might be an appropriate point to comment on Dr. Andersen’s question about endoscopic ultrasound. Endoscopic ultrasound is very operator-dependent and has been used by many groups, particularly the group at the University of Michigan, which uses endoscopic ultrasound essentially as the only preoperative localizing test. We do not have an extensive experience with endoscopic ultrasound, but I do think it is a very sensitive test, and there certainly would be some rationale for screening these patients with endoscopic ultrasound.
I will address the extent of resection for malignant endocrine tumors. Both Dr. Hanks and Dr. Yeo asked questions regarding the performance of pancreaticoduodenectomy and the extent of resection. Essentially, the criteria for Whipple operation in these patients were either a very large tumor within the pancreatic head in close anatomic proximity to the pancreatic duct that was very difficult to manage otherwise, or patients who had clear evidence of regional lymph node metastases associated with a large primary tumor. Perhaps the only potential contraindication to a Whipple would be a patient with MEN 1 and the Zollinger-Ellison syndrome. Many people feel that MEN 1-associated gastrinomas cannot be cured surgically.
I would consider that a complete regional lymphadenectomy would be removal of the lymph nodes associated with the superior mesenteric vessels, the peripancreatic lymph nodes, including the nodes in the lesser omentum, and above and around the pancreas.
Dr. Hanks also asked about the risk of death associated with surgery for these tumors, and this was also alluded to by Dr. Yeo. He points out that the mortality was quite high in this series, in what essentially should be considered a prophylactic operation. I would like to emphasize that there was one perioperative death for a mortality rate of 4.8%. The other patient died of completely unrelated causes. These tumors can metastasize, and patients do die from them. We recently reviewed our experience with the lethality of MEN 1 in our series of patients, and we found that persons who are carriers of a mutation in the MEN1 gene have a shortened lifespan, with a mean age at the time of death of 46 years. This is as compared to the unaffected persons in the same kindreds. Of over 100 deaths that occurred in our affected patients, half of them were due to neoplastic progression of neuroendocrine tumors. About a third of those were intrathoracic tumors (bronchial or thymic carcinoids) and two thirds of them were pancreatic or duodenal neuroendocrine tumors. So I think that, although there is the impression that these tumors can be indolent and can be tolerated for many years, I think they certainly can progress and kill patients.
A final comment would be about the utility of preoperative imaging tests, to which Dr. Hanks and Dr. Andersen alluded. Certainly, in this study as well as others, the conventional preoperative tests were relatively insensitive in detecting the most clinically important neuroendocrine tumor. They almost never demonstrated all of the multiplicity of tumors. So I think that really intraoperative ultrasound is the final common test that should be used in all patients.
Could these patients be explored without any preoperative imaging tests? I think the answer is probably yes. The situation in which regional localization is particularly useful is for patients with MEN 1 with a functional tumor, such as insulinoma or gastrinonoma.
Footnotes
Correspondence: Terry C. Lairmore, MD, Dept. of Surgery, Section of Endocrine and Oncologic Surgery, Washington University School of Medicine, Box 8109, 660 S. Euclid Ave., St. Louis, MO 63110.
Presented at the 111th Annual Meeting of the Southern Surgical Association, December 5–8, 1999, The Homestead, Hot Springs, Virginia.
Supported by American Cancer Society Grant RPG-99–183-01-CCE, American College of Surgeons Franklin H. Martin Faculty Research Fellowship 1997–1999, GCRC grant M01 RR00036, and a Washington University Cancer Center Research Development Award.
E-mail: lairmoret@msnotes.wustl.edu
Accepted for publication December 1999.
References
- 1.Wilkinson S, Teh BT, Davey KR, et al. Cause of death in multiple endocrine neoplasia type 1. Arch Surg 1993; 128:683. [DOI] [PubMed] [Google Scholar]
- 2.Doherty GM, Olson JA, Frisella MM, et al. Lethality of multiple endocrine neoplasia type 1. World J Surg 1997; 22:581–586. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekharappa SC, Guru SC, Manickamp P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 1997; 276:404–407. [DOI] [PubMed] [Google Scholar]
- 4.Lemmens I, Van de Ven WJM, Kas K, et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. Hum Mol Gen 1997; 6:1177–1183. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal SK, Kester MB, Debelenko LV, et al. Germline mutations in the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum Mol Gen 1997; 6:1169–1175. [DOI] [PubMed] [Google Scholar]
- 6.Bassett JHD, Forbes SA, Pannett AAJ, et al. Characterization of mutations in patients with multiple endocrine neoplasia type 1. Am J Hum Gen 1998; 62:232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutch MG, Dilley WG, Sanjurjo F, et al. Germline mutations in the multiple endocrine neoplasia type 1 gene: evidence for frequent splicing defects. Hum Mutat 1999; 13:175–185. [DOI] [PubMed] [Google Scholar]
- 8.Doppman JL, Miller DL, Chang R, et al. Gastrinomas: localization by means of selective intraarterial injection of secretin. Radiology 1990; 174:25–29. [DOI] [PubMed] [Google Scholar]
- 9.Doppman JL, Miller DL, Chang R, et al. Insulinomas: localization with selective intraarterial injection of calcium. Radiology 1991; 178:237–241. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MS, Picus D, Lairmore TC, et al. Prospective study of provocative angiograms to localize functional islet cell tumors of the pancreas. Surgery 1997; 122:1091–1100. [DOI] [PubMed] [Google Scholar]
- 11.Mutch MG, Frisella MM, DeBenedetti MK, et al. Pancreatic polypeptide is a useful plasma marker for radiographically evident pancreatic islet cell tumors in patients with multiple endocrine neoplasia type 1. Surgery 1997; 122:1012–1020. [DOI] [PubMed] [Google Scholar]
- 12.Norton JA. Neuroendocrine tumors of the pancreas and duodenum. Curr Prob Surg 1994; 31:11–16. [DOI] [PubMed] [Google Scholar]
- 13.Yim JH, Siegel BA, DeBenedetti MK, et al. Prospective study of the utility of somatostatin-receptor scintigraphy in the evaluation of patients with multiple endocrine neoplasia type 1. Surgery 1998; 124:1037–1042. [DOI] [PubMed] [Google Scholar]
- 14.Phan GQ, Yeo CJ, Cameron JL, et al. Pancreaticoduodenectomy for selected periampullary neuroendocrine tumors: fifty patients. Surgery 1997; 122:989–997. [DOI] [PubMed] [Google Scholar]
- 15.Park BJ, Alexander R, Libutti SK, et al. Operative management of islet cell tumors arising in the head of the pancreas. Surgery 1998; 124:1056–1062. [DOI] [PubMed] [Google Scholar]
- 16.Thompson NW, Lloyd RV, Nishiyama RH, et al. MEN I pancreas: a histological and immunohistochemical study. World J Surg 1984; 8:561–574. [DOI] [PubMed] [Google Scholar]
- 17.Majewski JT, Wilson SD. The MEN-I syndrome: an all-or-none phenomenon. Surgery 1979; 86:475–484. [PubMed] [Google Scholar]
- 18.Weber HC, Venzon DJ, Lin J-T, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology 1995; 108:1637–1649. [DOI] [PubMed] [Google Scholar]
- 19.Lowney JK, Frisella MM, Lairmore TC, Doherty GM. Pancreatic islet cell tumor metastasis in multiple endocrine neoplasia type 1: correlation with primary tumor size. Surgery 1998; 124:1043–1049. [DOI] [PubMed] [Google Scholar]
- 20.Skogseid B, Eriksson B, Lundqvist G, et al. Multiple endocrine neoplasia type 1: a 10-year prospective screening study in four kindreds. J Clin Endocrinol Metab 1991; 73:281–287. [DOI] [PubMed] [Google Scholar]
- 21.Skogseid B, Grama D, Rastad J, et al. Operative tumour yield obviates preoperative pancreatic tumour localization in multiple endocrine neoplasia type 1. J Intern Med 1995; 238:281–288. [DOI] [PubMed] [Google Scholar]
- 22.Skogseid B, Öberg K. Experience with multiple endocrine neoplasia type 1 screening. J Intern Med 1995; 238:255–261. [DOI] [PubMed] [Google Scholar]
- 23.Skogseid B, Äberg K, Åkerström G, et al. Limited tumor involvement found at multiple endocrine neoplasia type I pancreatic exploration: can it be predicted by preoperative tumor localization. World J Surg 1998; 22:673–678. [DOI] [PubMed] [Google Scholar]
- 24.Doppman JL. Multiple endocrine neoplasia syndromes. A nightmare for the endocrinologic radiologist. Sem Roentgenol 1985; 20:7–16. [DOI] [PubMed] [Google Scholar]
- 25.Skogseid B, Öberg K, Benson L, et al. A standardized meal stimulation test of the endocrine pancreas for early detection of pancreatic endocrine tumors in multiple endocrine neoplasia type 1 syndrome: five years’ experience. J Clin Endocrinol Metab 1987; 64:1233–1240. [DOI] [PubMed] [Google Scholar]