Abstract
Objective
To evaluate the current knowledge on the risk factors for recurrence, efficacy of adjuvant therapy in preventing recurrence, and the optimal management of recurrence after resection of hepatocellular carcinoma (HCC).
Summary Background Data
The long-term prognosis after resection of HCC remains unsatisfactory as a result of a high incidence of recurrence. Prevention and effective management of recurrence are the most important strategies to improve the long-term survival results.
Methods
A review of relevant English articles was undertaken based on a Medline search from January 1980 to July 1999.
Results
Pathologic factors indicative of tumor invasiveness such as venous invasion, presence of satellite nodules, large tumor size, and advanced pTNM stage, are the best-established risk factors for recurrence. Active hepatitis activity in the nontumorous liver and perioperative transfusion also appear to enhance recurrence. Recent molecular research has identified tumor biologic factors such as the proliferative and angiogenic activities of the tumor as new risk factors for recurrence. There is a lack of convincing evidence for the efficacy of neoadjuvant or adjuvant therapy in preventing recurrence. Retrospective studies suggested that postoperative hepatic arterial chemotherapy might improve disease-free survival, but results were conflicting. For the management of postoperative recurrence, studies have consistently indicated that surgical resection should be the treatment of choice for localized recurrence, be it in the liver remnant or extrahepatic organs. Transarterial chemoembolization and percutaneous ethanol injection are widely used to prolong survival in patients with unresectable intrahepatic recurrence, and combined therapy with these two modalities may offer additional benefit.
Conclusions
Knowledge of the risk factors for postoperative recurrence provides a basis for logical approaches to prevention. Minimal surgical manipulation of tumors to prevent tumor cell dissemination, avoidance of perioperative blood transfusion, and suppression of chronic hepatitis activity in the liver remnant are strategies that may be useful in preventing recurrence. The efficacy of postoperative adjuvant regional chemotherapy deserves further evaluation. New concepts on the influence of tumor biologic factors such as angiogenic activity on recurrence of HCC suggest a potential role of novel approaches such as antiangiogenesis for adjuvant therapy in the future. Currently, the most realistic approach in prolonging survival after resection of HCC is early detection and aggressive management of recurrence. Randomized trials are needed to define the roles of various treatment modalities for recurrence and the benefit of multimodality therapy.
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. Although liver transplantation has provided an alternative option of surgical management for HCC, 1 partial hepatic resection remains the mainstay of treatment offering a hope of cure. With advances in surgical techniques and perioperative care, immediate results of hepatic resection for HCC have greatly improved. In recent years, surgeons in experienced centers have achieved a hospital death rate close to zero. 2,3 However, the long-term prognosis after resection of HCC remains unsatisfactory, and improvement of the long-term survival is the next target of surgeons. A 5-year survival rate of 40% to 50% has been reported in recent series from Eastern and Western centers, 4–10 but a high incidence of postoperative recurrence is universal and continues to be the main cause of late deaths. The cumulative 5-year recurrence rate was in the range of 75% to 100% from most centers. 11–17 Recurrence occurs in the liver remnant in 78% to 96% of cases, as a result of either intrahepatic metastasis from the primary tumor or multicentric occurrence. 2,7,8,11,15,17
The high incidence of intrahepatic recurrence after resection of HCC has prompted some authors to suggest liver transplantation as an alternative. 11 In recent years, transplantation has been established as a treatment for small (<5 cm) HCC associated with cirrhosis, for which the disease-free survival rate after transplantation is superior to resection, but transplantation for large HCC is generally considered contraindicated. 1 The severe shortage of organ donors has restricted the use of transplantation even for patients with small HCC. Hence, hepatic resection will continue to be the first-line treatment for most patients with resectable HCC. With improved surgical safety and earlier diagnosis of HCC afforded by better imaging modalities, it can be foreseen that more patients will undergo hepatic resection for HCC.
Prevention and effective management of recurrence are undoubtedly the most important strategies to improve the overall survival after resection of HCC. During the past two decades, tremendous efforts have been devoted to research on the risk factors for recurrence, the use of adjuvant therapy to prevent recurrence, and management of recurrence after resection of HCC. However, controversy remains in such areas as the relative importance of different risk factors, the value of adjuvant therapy, and the roles of various treatment modalities for recurrence. This review focuses on the current knowledge on the risk factors for recurrence, efficacy of adjuvant therapy, and management of postoperative recurrent HCC. Data from relevant articles in the English literature available in the Medline from January 1980 to July 1999 were evaluated. Based on the results, potential strategies that may be useful to improve the long-term prognosis after resection of HCC are discussed.
RISK FACTORS FOR POSTOPERATIVE RECURRENCE
The risk factors for postoperative recurrence after resection of HCC have been extensively studied. They can be categorized into tumor, host, and surgical factors.
Tumor Factors
The prognostic effects of conventional pathologic factors on the risk of recurrence have been most widely studied. In recent years, molecular biology research has revealed the prognostic significance of more basic tumor biologic factors.
Pathologic Factors
HCC is characterized by its propensity for vascular invasion. The presence of microscopic venous invasion is the most consistently reported risk factor for recurrence after resection of HCC. 4,6,7,10,14,18–23 In some studies, it was the single most important factor for recurrence. 6,14,18 Other authors found macroscopic portal vein involvement a major risk factor. 12,24–26 It is widely accepted that intrahepatic metastasis by the portal venous system is an important mechanism for intrahepatic recurrence. 18,25,27
Satellite nodules around the main tumor are considered to arise from intrahepatic metastasis, and the presence of satellite nodules is another indicator of tumor invasiveness. Increased incidence of postoperative recurrence in association with satellite nodules has been well documented. 14,17,18,20,21,23,28,29 Other authors reported an increased risk of recurrence associated with multiple tumors. 7,12,13,30,31 Multiple tumors could be due to either intrahepatic metastasis or multicentric occurrence, both of which could contribute to recurrence in the liver remnant.
Several studies showed that a large tumor size, especially >5 cm, incurred a significantly higher risk of recurrence. 4,7,11,12,21,29,31–33 The influence of tumor size is attributed to increased invasiveness associated with a larger tumor, as demonstrated by a higher incidence of intrahepatic metastasis and portal venous invasion with tumors >5 cm. 4,34 Although a few studies have failed to find a significant effect of tumor size on recurrence rate, 13,14,18,19 the overall evidence suggests that large tumor size is an important factor for recurrence after resection of HCC.
The International Union Against Cancer (UICC) pathologic tumor-node-metastasis (pTNM) staging incorporates tumor size, number of tumor nodules, and vascular invasion in its tumor (T) classification. 35 Conceivably, it should be useful in stratifying patients according to the risk of recurrence. However, it was often ignored in previous studies on the risk factors for recurrence. Three recent reports, including one from our institution, have shown that pTNM staging provides an accurate prognostic classification of the long-term survival after resection of HCC. 7,8,36 In another study, a modified pTNM staging was suggested to be more useful. 26
The effects of other tumor pathologic features such as tumor encapsulation and histologic differentiation on the risk of recurrence are less conclusive. The presence of a tumor capsule has been associated with a lower incidence of recurrence in some studies, 14,32,37–39 but not in others. 18,20,22 In two histopathologic studies, tumor encapsulation was found to be associated with a reduced incidence of local venous invasion and direct invasion into the surrounding liver, which could account for the better prognosis with encapsulated tumors. 40,41 Paradoxically, in another study, the presence of tumor capsule was found to be a strong predictor of portal venous invasion, attributed to a high incidence of tumor invasion of blood vessels in the capsule. 34 Some authors have looked more specifically into the prognostic significance of capsular invasion by tumor cells and demonstrated an associated higher recurrence rate. 12,22
The prognostic significance of histologic grading of HCC on the risk of recurrence has also been debated. One study demonstrated a significantly higher recurrence rate with poorly differentiated HCC, 13 and another study showed that high-grade tumor was a strong independent predictor of portal vein invasion. 34 However, tumor differentiation was not found to have a significant impact on the risk of recurrence in many other studies. 12,18–20,22,29,42,43 Shirabe et al 42 showed that well-differentiated HCC had a similar incidence of intrahepatic metastasis and portal vein invasion as less differentiated tumors, and concluded that well-differentiated HCC was not clinically early cancer.
Biologic Factors
Several tumor biologic factors related to the growth and invasiveness of HCC have been evaluated in recent years as new risk factors for recurrence. The nuclear DNA content of tumor cells has been known to reflect the malignant potential of various tumors. Ezaki et al 44 first evaluated the prognostic significance of DNA ploidy in HCC and found that DNA aneuploidy was associated with large and high-grade HCC, but they did not find a significant correlation between DNA ploidy and prognosis after resection of HCC. Subsequently, a few studies using flow cytometric analysis revealed that DNA aneuploidy was an independent risk factor for recurrence. 19,29,45 However, other authors could not demonstrate a correlation between DNA ploidy and risk of recurrence or survival. 46–48
The proliferative activity of tumor cells is directly related to tumor growth and is another potential prognostic indicator. In addition, tumor cell proliferation can lead to the development of clonal subpopulations with an increased capacity for invasion and metastasis. Proliferating cell nuclear antigen (PCNA) is an auxiliary protein for DNA polymerase-delta. Its expression is related to DNA synthesis and cell replication, and it is a marker for G1/S phase in the cell cycle. It can be evaluated by immunohistochemical study and is a commonly used index of tumor proliferative activity. A high PCNA expression has been shown to be an independent predictor of recurrence, especially for small HCC. 49–51 A high PCNA index is associated with a higher incidence of venous invasion and direct liver invasion, 49,50 suggesting that it is related not only to the growth but also to the invasiveness of the tumor.
Telomerase is another nuclear protein that has been associated with tumor cell proliferation. It is a ribonucleoprotein enzyme that stabilizes the ends of chromosomes, or telomeres. In most normal dividing somatic cells, progressive shortening of the ends of the chromosomes has been observed; this eventually leads to cell senescence. Most malignant tumors have been found to express reactivated telomerase, which allows continued proliferation of the tumor cells. Two recent studies have suggested that a high telomerase activity in HCC could be a predictor of early postoperative recurrence. 52,53
The expression of androgen receptor has also been proposed to be related to the growth of HCC. Nagasue et al 54 found that expression of androgen receptors in HCC was strongly associated with increased risk of intrahepatic recurrence. In a subsequent study, the presence of androgen receptors in the tumor was not found to have a significant effect on postoperative recurrence, whereas the presence of androgen receptor in the surrounding nontumorous liver predicted a higher incidence of recurrence. 55 The exact significance of androgen receptor expression in HCC has not been clarified.
Similarly, conflicting results have been reported regarding the significance of mutation of the p53 gene, a tumor suppressor gene, on the risk of recurrence in HCC. 56,57 This gene encodes a nuclear protein that controls cell cycle, apoptosis, and cellular differentiation. Mutations of p53 gene are commonly observed in various cancers and could result in loss of its normal growth regulatory functions. In a Japanese study, p53 gene mutation was an independent risk factor for recurrence, 56 but a study from our institution failed to find such a correlation. 57
Boix et al 58 have studied another gene, the nm23-A1 gene, in relation to recurrence after resection of HCC. This gene encodes a protein involved in preserving normal tissue architecture and cell-to-cell relations and is thought to act as a metastasis suppressor gene. Their study demonstrated that overexpression of this gene was associated with a lower recurrence rate after surgical resection of HCC.
Angiogenesis, the process by which tumors develop new blood vessels, is now recognized to play a central role in tumor growth and metastasis. HCC is a hypervascular tumor characterized by neovascularization. It has been shown that the invasiveness of HCC is related to its angiogenic activity. 59,60 Tumor expression of mediators of angiogenesis such as vascular endothelial growth factor and basic fibroblast growth factor has been associated with invasive features such as portal vein infiltration and capsular tumor invasion. 59,60 Two recent studies showed that a high tumor microvessel density, an index of angiogenic activity, was associated with an increased risk of recurrence and a shorter recurrence time after resection of HCC. 61,62
Another new concept regarding cancer invasion and metastasis relates to the intercellular adhesiveness between tumor cells. Reduced expression of intercellular adhesion molecules such as cadherins has been found to be correlated with vascular or capsular invasion in HCC. 63 A recent study suggested that reduced E-cadherin expression might have some contribution to early recurrence of HCC after resection. 64
The inconsistent results obtained in some of these studies of biologic risk factors for recurrence in HCC may be related in part to different patient populations among studies. However, the discrepancy probably also reflects a lack of consistent and objective methodology. Many of these studies were based on semiquantitative techniques such as immunohistochemistry, the accuracy of which could be influenced by errors in interpretation and tumor specimen sampling. The elucidation of biologic factors related to recurrence is pertinent to the understanding of the mechanisms of recurrence after resection of HCC. Further studies using standardized, precise, and objective methodology are needed to clarify the significance of various biologic factors.
Host Factors
It was a common finding among studies that the age and sex of patients had no independent influence on the risk of recurrence. Women with HCC appear to have a better prognosis than men, but this is related to the more favorable pathologic tumor features such as a lower incidence of vascular invasion and a higher incidence of tumor encapsulation in women. 65
Most studies have not found the cause of the tumor to be a significant factor influencing the risk of recurrence. However, some authors have reported that the recurrence rate after resection of HCC in patients with hepatitis C virus infection was higher than those with hepatitis B virus infection, and this was attributed to a higher risk of multicentric occurrence in chronic hepatitis C cirrhosis. 66,67 In another study, alcohol abuse was found to be associated with an increased risk of recurrence of HCC. 68
The effect of underlying cirrhosis in the nontumorous liver on the risk of recurrence after resection of HCC is a controversial issue. Cirrhosis was reported in some studies to be a significant risk factor for recurrence in the liver remnant, presumably as a result of a predisposition to multicentric hepatocarcinogenesis. 14,18,69 Conversely, no correlation between cirrhosis and recurrence was indicated in other studies, and it has been suggested that the risk of developing a new HCC is probably limited in early cirrhosis. 15,19,22,29,38
There is more consistent evidence that active inflammatory activity in the nontumorous liver, as indicated by the serum transaminase levels or histologic assessment of hepatitis activity, is an independent risk factor for intrahepatic recurrence. 43,70–72 Active inflammation of the nontumorous liver has been associated with a higher proliferative activity, as measured by the PCNA index. 43 Others have reported that the proliferative capacity of the nontumorous liver, measured by DNA flow cytometric analysis or the bromodeoxyuridine labeling index, was a predictive factor for intrahepatic recurrence of HCC. 73,74 It has been hypothesized that increased hepatocyte proliferation in the liver remnant after hepatectomy may enhance multicentric hepatocarcinogenesis. 70,72 However, Shirabe et al 71,75 showed that multicentric occurrence constituted only 25% of intrahepatic recurrences by histologic comparison of primary and recurrent tumors, and their group suggested that active hepatitis activity in the liver remnant might enhance the development of metastasis by upregulating the expression of vascular adhesion molecules. Ueno et al 76 also demonstrated that moderate hepatitis activity enhanced postoperative recurrence of HCC by increasing the incidence of intrahepatic metastasis rather than multicentric occurrence. Interestingly, in this study severe hepatitis was not associated with an increased risk of recurrence compared with patients with low hepatitis activity, the exact reason for which was not clear. Further research is needed to clarify the mechanism by which hepatitis activity enhances recurrence.
Surgical Factors
The extent of resection, resection margin, and perioperative transfusion are the three surgical factors that have been most widely examined for their effects on postoperative recurrence. Most studies found that the extent of resection, whether major or minor, and whether anatomical or nonanatomical, had no significant influence on the risk of recurrence. 7,8,15,17,19,23,43 Yamamoto et al 18 advocated wide anatomical rather than nonanatomical resection according to their postulation of portal venous system-based segment-by-segment intrahepatic spread, but they showed no significant influence of the extent of surgical resection on postoperative recurrence. Ko et al, 70 however, demonstrated that recurrence-free survival after major hepatectomy was significantly lower than after minor hepatectomy, especially in patients with chronic active hepatitis. It was hypothesized that the increased hepatocyte regeneration after major hepatectomy might enhance hepatocarcinogenesis in the liver remnant.
A wide resection margin to ensure histologic clearance is a general principle of surgical oncology, but its significance for HCC has remained controversial despite extensive studies. A macroscopic margin of 1 cm or more has been shown to be associated with a reduced postoperative recurrence rate and better long-term survival in some studies, and hence a “curative” resection for HCC is frequently defined by a tumor-free margin of more than 1 cm. 15,36,77–80 Chau et al 80 found that a resection margin of less than 1 cm was associated with a higher incidence of recurrence in the same or an adjacent segment after resection of HCC, and suggested that a wide resection margin would provide a chance of histologic clearance of early metastasis in the vicinity of the primary lesion. Tang et al 81 advocated an even larger resection margin of 2 to 3 cm for all small tumors. In contrast, Yoshida et al 82 found no significant effect of the width of the resection margin on postoperative recurrence, and in their study 83% of intrahepatic recurrences occurred in a segment distant from the resected tumor. In accordance, other authors have demonstrated that only a small proportion of intrahepatic recurrences occurred near the resection margin. 11,25 These findings were supported by a pathologic study from our department using multiple histologic sections to survey the resection margins, which demonstrated that microsatellites and histologic vascular invasion beyond 1 cm were very common with both small and large HCC, and hence a margin of 1 cm could not ensure complete disease clearance. 83 Ochiai et al 84 focused on the relation between surgical margin and recurrence at the stump in the liver remnant, and they failed to find a correlation between the width of the margin and stump recurrence. Numerous other studies have demonstrated no correlation between the width of the surgical margin and the incidence of recurrence. 4,8,18,22,23,26,29,43,70 Although tumor clearance at the resection margin may be helpful in preventing local recurrence at the resection margin, given that most intrahepatic recurrences arise from either portal venous dissemination or multicentric carcinogenesis, it is understandable that a wide resection margin may not have a significant impact on the risk of recurrence of HCC. In patients with limited liver function reserve undergoing resection of HCC, preservation of liver parenchyma may be a more important consideration than a wide resection margin.
Relatively few studies have investigated the effect of intraoperative blood loss or perioperative transfusion on the risk of recurrence after resection of HCC, but all have demonstrated an enhancing effect of perioperative transfusion on postoperative recurrence. 18,85–87 Interestingly, Matsumata et al 85 found that the adverse effect of transfusion was recognized only in patients without intrahepatic metastasis. Yamamoto et al 86 also showed that the recurrence-promoting effect of transfusion was not observed in patients with vascular invasion, but was significant in patients with a cirrhotic liver. Asahara et al 87 reported that transfusion was a significant prognostic indicator of recurrence in stage I-II patients, but not in stage III-IV patients. These studies seem to indicate that transfusion may enhance multicentric occurrence in the liver remnant rather than intrahepatic metastasis. Perioperative transfusion is thought to promote postoperative recurrence by suppressing the host’s antitumor immune mechanism. It has been postulated that transfusion may have its greatest effect on the early stage of HCC development. 86 The exact mechanism by which perioperative transfusion promotes the development of recurrence after resection of HCC needs further investigation.
Intraoperative manipulation of HCC, especially large bulky tumors, has been regarded by some authors as an important factor leading to tumor cell dissemination by the portal venous system, which could account for the early widespread intrahepatic recurrence frequently seen after resection of advanced HCC. 15,25 This hypothesis was supported by the recovery of tumor cells from portal venous blood during resection of large tumors or tumors with portal venous invasion. 88 Matsumata et al 89 attempted to prevent intraoperative dissemination of tumor cells by temporary portal vein embolization with starch microspheres in eight patients, and no recurrence was observed after 6 to 24 months. However, the exact significance of surgical manipulation as a risk factor for postoperative recurrence has not been clearly evaluated, probably because of the difficulty in quantifying tumor manipulation during surgery.
PREVENTION OF RECURRENCE BY ADJUVANT THERAPY
The conventional approach to adjuvant therapy in surgical oncology is to use chemotherapy before or after surgery. Because most of the recurrent tumors occur in the liver remnant, studies on adjuvant therapy for HCC have focused on the use of regional chemotherapy, either before surgery in combination with selective embolization of the tumor, or after surgery with or without systemic chemotherapy.
Preoperative Transarterial Chemoembolization
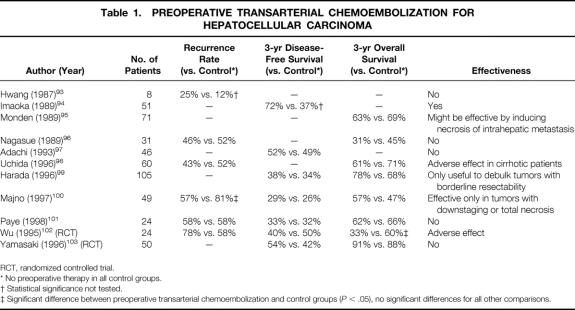
Transarterial chemoembolization (TACE) is a procedure involving the injection of lipiodol and a chemotherapeutic agent (usually doxorubicin or cisplatin) into the hepatic artery supplying the tumor, followed by embolization with gelatin or Gelfoam particles. It was introduced in the late 1970s, initially for the treatment of unresectable HCC. Since the beginning of 1980s, TACE has been used as a neoadjuvant therapy in an attempt to reduce the postoperative recurrence rate and improve long-term survival. 90–92 The intent of preoperative TACE is to reduce tumor size, induce tumor necrosis, and prevent tumor cell dissemination during surgery. Histopathologic studies of resected specimens after TACE have demonstrated that it is effective in inducing partial and sometimes complete necrosis of HCC. 90–92 However, it remains uncertain whether TACE is effective in reducing the incidence of postoperative recurrence and prolonging survival. Results of studies on the effect of preoperative TACE on postoperative recurrence and long-term survival after resection of HCC are summarized in Table 1.
Table 1. PREOPERATIVE TRANSARTERIAL CHEMOEMBOLIZATION FOR HEPATOCELLULAR CARCINOMA
RCT, randomized controlled trial.
* No preoperative therapy in all control groups.
† Statistical significance not tested.
‡ Significant difference between preoperative transarterial chemoembolization and control groups (P < .05), no significant differences for all other comparisons.
Hwang et al 93 found that preoperative TACE did not reduce the incidence of local or metastatic recurrence, but the number of patients was not sufficient to draw a valid conclusion. Imaoka et al 94 recommended preoperative TACE based on a retrospective study, without establishing a matched control group. Monden et al 95 observed that preoperative TACE induced necrosis of intrahepatic metastatic lesions in some patients, resulting in improved survival in these patients compared with those with viable intrahepatic metastasis. Hence, they suggested that preoperative TACE might be useful, but they did not obtain an overall improved survival rate. Several other retrospective studies have failed to demonstrate a positive effect of preoperative TACE. 96–101 Adachi et al 97 analyzed the efficacy of preoperative TACE according to the degree of tumor necrosis induced. Their study found that preoperative TACE resulted in better disease-free survival rates if it produced complete tumor necrosis, but actually increased the risk of recurrence if only partial necrosis was induced, probably by encouraging dislodgement of residual tumor cells during surgical manipulation. Majno et al 100 demonstrated downstaging or total necrosis of tumors after preoperative TACE in 62% of patients, with improved disease-free survival in this subgroup, but they failed to demonstrate an overall improvement of survival with preoperative TACE.
Only two prospective randomized trials on preoperative TACE have been reported, one specifically for large HCC (>10 cm 102) and the other for small HCC (2 to 5 cm 103). Both studies showed no significant differences in rates of surgical complications, death, and disease-free survival between TACE treatment and the untreated control group. Yamasaki et al 103 found no difference in the overall survival rates, regardless of the degree of necrosis of HCC induced by TACE. Wu et al 102 showed a worse overall survival result and a higher incidence of extrahepatic recurrence with preoperative TACE, the latter being attributed to easier tumor cell dislodgement during surgery.
A strong argument against the use of preoperative TACE is a high incidence of associated complications. Significant complications such as severe adhesions, gallbladder gangrene, bile duct necrosis, liver infarction, abscess, and acute pancreatitis have been encountered in 37% to 57% of patients treated with preoperative TACE, rendering the liver resection more difficult. 96,99,101 The risk of complications appears to increase with repetition of TACE. 101,104 Preoperative TACE may also damage the liver, especially in cirrhotic patients, resulting in an increased incidence of liver failure and gastrointestinal bleeding. 98 Other potential disadvantages of preoperative TACE include a delay in surgery and difficulty in further TACE for recurrent lesions as a result of the development of collateral tumor feeding vessels after embolization of the proper hepatic artery. 102
Based on the currently available evidence, preoperative TACE cannot be recommended as a routine procedure before hepatectomy for a resectable HCC, and it may be considered contraindicated in patients with cirrhosis. However, a few studies have shown that preoperative TACE may downstage initially unresectable tumors, allowing resection that might improve the prognosis of these patients. 99,100,104 Subject to further studies, this may turn out to be the most appropriate indication for preoperative TACE.
Postoperative Regional Chemotherapy
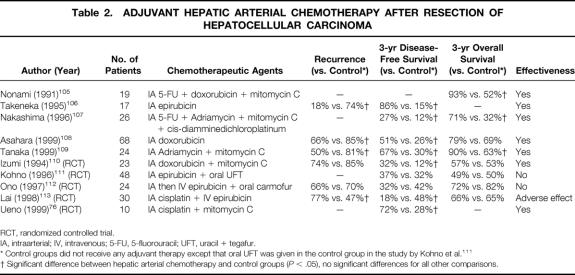
Postoperative regional chemotherapy involves the delivery of chemotherapeutic agents mixed with lipiodol to the hepatic artery by means of a catheter either placed during surgery or inserted percutaneously through the femoral artery. The results of published studies on postoperative hepatic arterial chemotherapy (HAC) are summarized in Table 2.
Table 2. ADJUVANT HEPATIC ARTERIAL CHEMOTHERAPY AFTER RESECTION OF HEPATOCELLULAR CARCINOMA
RCT, randomized controlled trial.
IA, intraarterial; IV, intravenous; 5-FU, 5-fluorouracil; UFT, uracil + tegafur.
* Control groups did not receive any adjuvant therapy except that oral UFT was given in the control group in the study by Kohno et al. 111
† Significant difference between hepatic arterial chemotherapy and control groups (P < .05), no significant differences for all other comparisons.
Nonami et al 105 first reported the use of postoperative HAC in 1991 and demonstrated a beneficial survival effect in patients with any one of four risk factors for recurrence: surgical margin <1 cm, intrahepatic metastasis, tumor embolus in the portal vein, and lack of capsule formation. Subsequently, other retrospective studies have demonstrated a better disease-free or overall survival rate with adjuvant HAC. 106–109 However, randomized controlled trials have yielded conflicting results. Two randomized trials using HAC alone found superior disease-free survival results compared with untreated patients, 76,110 whereas three other trials using HAC combined with systemic or oral chemotherapy failed to demonstrate a beneficial effect. 111–113 In a trial conducted by our department, combined postoperative adjuvant arterial and systemic chemotherapy in fact resulted in a higher incidence of extrahepatic metastasis and a compromised disease-free survival compared with the untreated group. 113 The reason was uncertain, but one possible mechanism was suppression of host immune surveillance, especially by the systemic chemotherapy. A similar explanation was given in another study to explain the worse disease-free survival rate among patients who received adjuvant HAC combined with systemic chemotherapy. 112 Hence, it appears that the addition of systemic chemotherapy to postoperative HAC may have an adverse rather than a beneficial effect. Because postoperative recurrence occurs most commonly in the liver remnant, regional therapy alone may be a more useful approach. Apart from the differences in chemotherapy regimens, there was also a notable difference in the target patients recruited in these studies. In the two trials with a positive outcome, only patients with a high risk of intrahepatic metastasis were recruited. Izumi et al 110 recruited only patients with invasive HCC (i.e., those with vascular invasion or intrahepatic metastasis), and Ueno et al 76 included only patients with moderate hepatitis activity, which was shown to be a risk factor for intrahepatic metastasis. In contrast, the patients recruited in the other three trials were unselected in terms of the risk for recurrence. 111–113 It has been suggested that HAC would be more effective in suppressing intrahepatic metastasis, which receives its blood supply mainly from the artery, rather than new multicentric occurrence, which is usually hypovascular, with its blood supply derived predominantly from the portal venous system. 108,109
Compared with preoperative chemoembolization, postoperative HAC appears to be better tolerated. Serious side effects were rarely observed, even in patients with cirrhosis. 105–107,109 Based on the currently available data, further trials on postoperative regional chemotherapy are warranted, particularly on HAC alone in selected patients with a high risk for intrahepatic metastasis.
Postoperative Oral Chemotherapy
A few authors have investigated the effectiveness of adjuvant oral chemotherapy with 5-fluorouracil or its derivative 1-hexylcarbamoyl-5-fluorouracil. 106,114,115 Two uncontrolled studies showed no preventive effect, 106,114 but a multiinstitutional randomized trial demonstrated better disease-free and overall survival rates with 1-hexylcarbamoyl-5-fluorouracil. 115 The favorable results of this trial, however, are questionable because chemotherapy was suspended as a result of side effects in 12 (44%) of the 27 patients. No further data are available yet to support the routine use of oral chemotherapy after resection of HCC.
Other Approaches in Adjuvant Therapy
Recent studies have investigated the efficacy of targeted immunotherapy combined with chemotherapy as a new approach to adjuvant therapy. Kawata et al 116 compared the effect of postoperative arterial doxorubicin, interleukin-2, and lymphokine-activated killer cells with arterial doxorubicin alone in a randomized trial in 24 patients, but they failed to show a definite benefit of the former regimen on disease-free survival. In contrast, Lygidakis and Tsiliakos 117 showed that pre- and postoperative locoregional combined immunotherapy (interferon-γ and interleukin-2) and chemotherapy (mitomycin, carboplatin, and mitoxantrone) reduced the incidence of postoperative recurrence and improved overall survival results in a prospective randomized trial involving 91 patients. This appears to be a promising approach that deserves further study.
Another novel adjuvant therapy for HCC recently reported is the use of polyprenoic acid, an acyclic retinoid. In a multicenter randomized controlled trial, Muto et al 118,119 showed that oral polyprenoic acid for 12 months after resection of HCC was effective in preventing multicentric occurrence and improving long-term survival results, but it did not reduce the risk of intrahepatic metastasis. It was suggested that acyclic retinoid could induce clonal deletion of premalignant and latent malignant cells in the liver remnant. 119
MANAGEMENT OF POSTOPERATIVE RECURRENCE
Intrahepatic Recurrence
Studies on the management of postoperative recurrence have largely focused on recurrence in the liver remnant. The therapeutic modalities commonly used for primary tumors, including surgical resection, TACE, and percutaneous ethanol injection therapy (PEIT), have been used to treat recurrent tumors in the liver remnant.
Surgical Resection
Repeat hepatic resection has been widely accepted as the best treatment modality for recurrent intrahepatic HCC, although no prospective randomized trials have been performed to compare its efficacy with nonsurgical treatments. In 1986, Nagasue et al 120 reported the first series of second hepatic resection for recurrent HCC in nine patients with no surgical deaths, and they demonstrated a better survival rate among these patients compared with 22 patients with recurrent disease managed by nonsurgical means. Around the same period, other authors also reported their experience of repeat resection of recurrent HCC in the liver remnant, without a detailed analysis of the long-term survival rate. 114,121
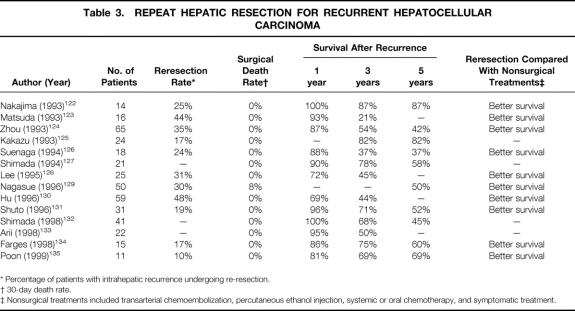
In the 1990s, the long-term survival results after repeat resection for recurrent HCC have been documented in several larger series, some of which included a comparison with nonsurgical treatments (Table 3). The reresection rate among patients with intrahepatic recurrence in these studies ranged from 10% to 48%. Reresection was generally offered as the treatment of choice to patients with a solitary recurrence or a limited number of recurrences confined to the liver, provided the liver function reserve was satisfactory and the recurrent tumors were anatomically resectable. In these studies, wedge excision, subsegmentectomy, and segmentectomy were the most frequently performed procedures in the second operation. The reresection rate depended on the extent of first resection, the underlying liver status, and the pattern of recurrence among patients in different studies. Studies with a high reresection rate were characterized by a high proportion of minor resections during the first operation, 123,126,129 whereas series with a higher percentage of major resections during the first hepatectomy tended to have a lower reresection rate. 134,135 Reresection has been proven to be a safe treatment: only one study found any surgical deaths. Nagasue et al observed an 8% death rate among 50 patients who underwent a second hepatectomy, and the postoperative deaths were mostly ascribed to poor liver function reserve in patients with Child’s B or C cirrhosis; this should probably be considered a contraindication to a repeat hepatectomy. 129
Table 3. REPEAT HEPATIC RESECTION FOR RECURRENT HEPATOCELLULAR CARCINOMA
* Percentage of patients with intrahepatic recurrence undergoing re-resection.
† 30-day death rate.
‡ Nonsurgical treatments included transarterial chemoembolization, percutaneous ethanol injection, systemic or oral chemotherapy, and symptomatic treatment.
The survival results after reresection for recurrent HCC in these studies were generally favorable, although the reported 5-year survival rate ranged from 37% to 87%. In two studies, including one from our institution, it was shown that the overall survival rates calculated from the first hepatectomy were similar among patients with recurrence managed by repeat resection and those without recurrence, suggesting that this is an effective treatment in prolonging survival. 126,135 Several studies have demonstrated prolonged disease-free survival in patients after reresection of intrahepatic recurrence. 123,128,130,135 All of the studies that compared reresection with nonsurgical treatment modalities such as TACE, PEIT, or systemic chemotherapy showed a superior survival result with reresection. However, in only three of these studies was the control group matched with the reresection group. 128,131,134 In the other studies, patients treated with nonoperative measures were those ineligible for reresection. Without a prospective randomized trial, it is difficult to prove that the better outcome of patients with reresection was not simply a function of selection bias. The wide range of survival rates obtained in different series probably reflects a wide variation in the background of patients selected for reresection. The best candidates for repeat hepatectomy have not been established. A recent study analyzing the prognostic factors of survival after repeat resection found that the only independent adverse prognostic factor was portal vein invasion in the first hepatectomy, which was considered to predispose to intrahepatic metastasis. 132 Another study showed a better survival among patients with recurrent tumors considered to be multicentric occurrence than those with intrahepatic metastasis after reresection. 133 These studies suggested that patients with a new multicentric tumor may be more favorable candidates for reresection. Adjuvant therapy may be appropriate for those with intrahepatic metastasis even after reresection.
Transarterial Chemoembolization
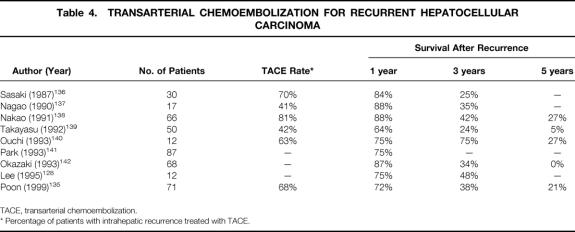
TACE is the most widely used treatment modality for intrahepatic recurrent tumors that are unresectable because of a multifocal pattern or inadequate liver function reserve. Several retrospective series have reported a favorable 1-year survival rate of 64% to 88%, but the 5-year survival rate was only in the range of 0% to 27% (Table 4). Although the survival results appear modest compared with reresection, TACE does have an important role in the overall management strategy for recurrent HCC, because it is applicable to most patients with intrahepatic recurrence. However, the role of TACE even for primary unresectable HCC has not been fully established, and it is impossible to prove its role for recurrent HCC without prospective randomized trials.
Table 4. TRANSARTERIAL CHEMOEMBOLIZATION FOR RECURRENT HEPATOCELLULAR CARCINOMA
TACE, transarterial chemoembolization.
* Percentage of patients with intrahepatic recurrence treated with TACE.
Percutaneous Ethanol Injection Therapy
PEIT is another modality used for local control of HCC <5 cm, by inducing tumor necrosis. Its use for recurrent HCC has been reported in only a few studies. 134,135–144 In one study, the survival result of patients with PEIT after recurrence was not significantly different from that of patients treated with repeat hepatectomy. 143 However, its use is limited to patients with a solitary tumor or fewer than three small recurrent tumors. Combined TACE and PEIT has been reported to yield better results than TACE or PEIT alone for HCC. 145 Tumor necrosis by TACE may enhance the diffusion of ethanol into the entire tumor. Alternatively, tumor tissue refractory to TACE may be necrotized by PEIT. Recent retrospective studies have also suggested that combined TACE and PEIT treatment may produce better results for intrahepatic recurrent HCC. 146,147 Randomized studies are required to confirm the benefit of such a combined approach.
New Treatment Modalities
Percutaneous acetic acid injection may be superior to PEIT in the local ablation of small HCC, 148 but its effect for postoperative recurrence has not been documented. Acetic acid has a higher penetrating power than ethanol, resulting in a more homogeneous diffusion in the tumor. 148 Percutaneous radiofrequency thermal ablation is another new technique being evaluated for the local control of HCC. Its use for recurrent HCC was documented in a recent case report. 149 In our institution, cryotherapy has been used in selected patients with recurrent HCC not suitable for resection, and our preliminary experience showed that it was a safe therapy with a reasonable survival outcome. 150 These new modalities of local therapy for recurrent HCC demand further evaluation.
Extrahepatic Recurrence
Few studies in the literature have addressed the management of extrahepatic recurrence after resection of HCC. This is probably related to the much lower incidence of extrahepatic than intrahepatic recurrence, but the presence of extrahepatic metastasis is also generally assumed to represent advanced systemic disease. As a result, patients with extrahepatic recurrence were often offered either systemic chemotherapy or supportive treatment only. 114,128,143 The response rate of HCC to systemic chemotherapy is generally low, and results with systemic chemotherapy for recurrent HCC were disappointing. 114,135 Recent studies have indicated that aggressive surgical treatment may be of benefit in selected patients with solitary extrahepatic recurrence.
Two studies from our department have demonstrated the value of resection for extrahepatic recurrence. 151,152 In one study, surgical resection of solitary lesions in the lung or abdomen in 12 patients resulted in a mean survival of 20 months, with 6 patients surviving disease-free for more than 1 year. 151 Lung is the most common site of extrahepatic recurrence of HCC. In another study, we found that resection of a solitary lung metastasis in nine patients resulted in a 5-year survival rate of 67% after recurrence. 152 Zhou et al 124 also reported long-term survival ranging from 33 to 168 months after resection of solitary lung metastasis from HCC in six patients. Recent studies by other authors have documented prolonged survival after surgical resection in selected patients with solitary recurrence in the adrenal gland or peritoneal cavity. 153,154 It appears from these studies that surgical resection is an effective option for patients with isolated extrahepatic recurrence, although it is difficult to prove that surgery really prolongs survival rather than simply selects out patients with a more favorable natural course of disease.
DISCUSSION
An updated knowledge on the risk factors of recurrence, efficacy of adjuvant therapy, and management of postoperative recurrence is important for clinicians in designing a strategy to optimize the chance of long-term survival in patients undergoing hepatic resection for HCC. Numerous studies have been performed in these areas, but inconsistent or even contradictory data have been frequently reported. This review is the first attempt to provide a comprehensive summary of data from a large number of relevant studies performed during the past two decades. In particular, we have highlighted areas of controversy that need to be clarified by further studies, and also new developments in several aspects that are worth pursuing. A critical evaluation of the available data in the literature also provides insights into potential strategies that may be useful in the prevention or management of recurrent HCC.
An important but unresolved question pertaining to intrahepatic recurrence after resection of HCC is the relative importance of intrahepatic metastasis and multicentric occurrence. This is of relevance in determining preventive and therapeutic strategies for recurrence. In most studies conducted so far, factors related to tumor invasiveness were the most dominant risk factors for recurrence, suggesting that intrahepatic metastasis may account for the majority of intrahepatic recurrences. Recent studies on the prognostic significance of tumor biologic factors such as expression of adhesion molecules and angiogenic activity have also indicated the importance of intrahepatic metastasis in the mechanism of recurrence. Some studies have attempted to differentiate between intrahepatic metastasis and multicentric occurrence based on histologic criteria, 66,132 but the most accurate method of discriminating the two is by analyzing the DNA clonal origin of the recurrent and primary tumors. Limited studies based on clonal analysis have confirmed that both intrahepatic metastasis and multicentric occurrence contribute to postoperative recurrence, 155,156 but a study in a large number of patients with intrahepatic recurrence is not yet available to clarify the exact role of each. Nonetheless, it seems reasonable to consider prevention of intrahepatic metastasis a strategy of prime importance in attempts to curtail postoperative recurrence.
Intrahepatic metastasis might have occurred before surgical resection of the tumor, but dissemination of tumor cells through the portal venous system could also occur during surgery. 88 In this regard, surgeons may play an active role by using surgical techniques that minimize the chance of tumor cell spread to the liver remnant. Matsumata et al 89 demonstrated that temporary intraoperative portal venous embolization might prevent intrahepatic recurrence. Another possible technical refinement that may help prevent tumor cell dissemination is to minimize intraoperative manipulation of the tumor. Excessive manipulation is particularly likely during mobilization of a large right-lobe tumor. An anterior approach to resection for right-lobe tumors, in which liver transection was completed before mobilization of the right lobe, may avoid dissemination of tumor cells. 157 An analysis of 54 patients with large right-lobe HCC >5 cm resected by the anterior approach compared with 106 patients with similar tumors resected by the conventional approach in our department between 1989 and 1997 revealed significantly better 5-year disease-free (32% vs. 13%) and overall survival (46% vs. 28%) rates with the former approach, 158 suggesting that it may be effective in reducing the risk of intrahepatic metastasis. We are contemplating a prospective randomized trial to compare these two approaches for patients with large right-lobe HCC.
A second strategy by which surgeons could reduce postoperative recurrence is to minimize intraoperative blood loss and avoid transfusion. Although the exact mechanism remains to be elucidated, there is convincing evidence that perioperative blood transfusion promotes the development of postoperative recurrence. Meticulous surgical techniques and the appropriate use of special maneuvers such as portal triad clamping and reduction of central venous pressure during hepatic transection are essential to minimize intraoperative blood loss. 3 Thus, one way that hepatic surgeons can improve the long-term prognosis of patients undergoing resection for HCC is to optimize their own surgical performance.
Active hepatitis activity of the liver remnant also appears to enhance intrahepatic recurrence, although there is controversy regarding whether it promotes intrahepatic metastasis or multicentric hepatocarcinogenesis. Whatever the mechanism, suppression of chronic inflammatory activity in the liver remnant may be a novel approach to preventing recurrence. New antiviral therapy such as nucleoside analog, which suppresses chronic hepatitis activity from hepatitis B infection, is now available. 159 For patients with cirrhosis from chronic hepatitis C viral infection, a prospective randomized trial has shown that interferon-α may suppress the inflammatory activity and development of HCC. 160 The efficacy of these antiviral therapies in preventing recurrence after resection of HCC has not been studied, and this may be an area of future research interest.
To date, convincing evidence supporting the effectiveness of adjuvant therapy in preventing recurrence is lacking. The validity of the information in the literature is limited by a paucity of randomized studies. Even for the few randomized trials reported, a meaningful metaanalysis is impossible because of the different therapeutic regimens used and the lack of stratification of patients according to the risk of recurrence. It appears from the data available that postoperative adjuvant HAC rather than preoperative TACE is more likely to be of benefit in preventing recurrence. Further randomized trials should concentrate on the use of postoperative HAC in patients at high risk of intrahepatic metastasis, and such trials should include a sufficient number of patients and a long enough follow-up. However, preoperative TACE may have a role in increasing the resectability rate of HCC by downstaging tumors with initially borderline resectability, and this demands further study. Further investigations are also needed to delineate the roles of new adjuvant therapies such as immunotherapy and polyprenoic acid. The elucidation of the influence of biologic factors such as angiogenesis on recurrence of HCC may open up novel approaches to adjuvant therapy, such as antiangiogenic therapy, in the near future. Antiangiogenic therapy is a new anticancer treatment targeted at tumor neovessels, and several antiangiogenic agents have already entered clinical trials. 161 Experimental evidence also suggests that antiangiogenic therapy may suppress the growth and metastasis of HCC. 162
Until effective adjuvant therapy is available, aggressive management of postoperative recurrence remains the most important strategy in prolonging the survival of patients after resection of HCC. The importance of early detection of recurrence by close postoperative surveillance cannot be overemphasized. Surgical resection is considered the treatment of choice for selected patients with localized disease in the liver remnant or extrahepatic locations. However, nonsurgical approaches such as TACE or PEIT appear to be important in prolonging survival for most patients with unresectable recurrence. New therapeutic modalities such as percutaneous acetic acid injection and radiofrequency thermal ablation may expand the armamentarium for managing unresectable intrahepatic recurrence. Further studies, properly conducted in a prospective randomized manner, are required to clarify the role of each treatment modality and also to evaluate the benefit of multimodality therapy. In the new century, one of the greatest challenges facing liver surgeons is to establish the optimal strategies in preventing and managing postoperative recurrent HCC by a combination of surgical and nonsurgical measures.
Footnotes
Correspondence: Ronnie T.P. Poon, MS, FRCS(Edin), Dept. of Surgery, Queen Mary Hospital, 102 Pokfulam Rd., Hong Kong, China.
E-mail: poontp@hkucc.hku.hk
Accepted for publication October 25, 1999.
References
- 1.Mor E, Kaspa RT, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med 1998; 129:643–653. [DOI] [PubMed] [Google Scholar]
- 2.Makuuchi M, Takayama T, Kubota K, et al. Hepatic resection for hepatocellular carcinoma: Japanese experience. Hepatogastroenterology 1998; 45:1267–1274. [PubMed] [Google Scholar]
- 3.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999; 229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosuge T, Makuuchi M, Takayama T, et al. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology 1993; 40:328–332. [PubMed] [Google Scholar]
- 5.Bismuth H, Chiche L, Castaing D. Surgical treatment of hepatocellular carcinomas in noncirrhotic liver: experience with 68 liver resections. World J Surg 1995; 19:35–41. [DOI] [PubMed] [Google Scholar]
- 6.Vauthey JN, Klimstra D, Franceschi D, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg 1995; 169:28–35. [DOI] [PubMed] [Google Scholar]
- 7.Takenaka K, Kawahara N, Yamamoto K, et al. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg 1996; 131:71–76. [DOI] [PubMed] [Google Scholar]
- 8.Lau H, Fan ST, Ng IOL, Wong J. Long-term prognosis after hepatectomy for hepatocellular carcinoma: a survival analysis of 204 consecutive patients. Cancer 1998; 83:2302–2311. [PubMed] [Google Scholar]
- 9.Lise M, Bacchetti S, Da Pian P, et al. Prognostic factors affecting long-term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer 1998; 82:1028–1036. [DOI] [PubMed] [Google Scholar]
- 10.Mazziotti A, Grazi GL, Cavellari A. Surgical treatment of hepatocellular carcinoma on cirrhosis: a Western experience. Hepatogastroenterology 1998; 45:1281–1287. [PubMed] [Google Scholar]
- 11.Belghiti J, Panis Y, Farges O, et al. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg 1991; 214:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arii S, Tanaka J, Yamozoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda K, Saitoh S, Tsubota A, et al. Risk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinoma. Cancer 1993; 71:19–25. [DOI] [PubMed] [Google Scholar]
- 14.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494. [DOI] [PubMed] [Google Scholar]
- 15.Chen MF, Hwang TL, Jeng LB, et al. Postoperative recurrence of hepatocellular carcinoma. Two hundred five consecutive patients who underwent hepatic resection in 15 years. Arch Surg 1994; 129:738–742. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto T, Nakayama S, Fukkuhara T, et al. The state of recurrence and the role of hepatectomy in hepatocellular carcinoma. Hepatogastroenterology 1994; 41:144–149. [PubMed] [Google Scholar]
- 17.Balsells J, Charco R, Lazaro JL, et al. Resection of hepatocellular carcinoma in patients with cirrhosis. Br J Surg 1996; 83:758–761. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto J, Kosuge T, Takayama T, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg 1996; 83:1219–1222. [PubMed] [Google Scholar]
- 19.Okada S, Shimada K, Yamamoto J, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology 1994; 106:1618–1624. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki S, Makuuchi M, Miyagawa S, et al. Results of hepatic resection for hepatocellular carcinoma. World J Surg 1995; 19:31–34. [DOI] [PubMed] [Google Scholar]
- 21.Di Carlo V, Ferrari G, Castoldi R, et al. Surgical treatment and prognostic variables of hepatocellular carcinoma in 122 cirrhotics. Hepatogastroenterology 1995; 42:222–229. [PubMed] [Google Scholar]
- 22.Shirabe K, Kanematsu T, Matsumata T, et al. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology 1991; 14:802–805. [DOI] [PubMed] [Google Scholar]
- 23.Fuster J, Garcia-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg 1996; 223:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu HC, Wu TT, Wu MZ, et al. Tumor invasiveness and prognosis in resected hepatocellular carcinoma. Clinical and pathogenetic implications. Cancer 1988; 61:2095–2099. [DOI] [PubMed] [Google Scholar]
- 25.Matsumata T, Kanematsu T, Takenaka K, et al. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology 1989; 9:457–460. [DOI] [PubMed] [Google Scholar]
- 26.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994; 106:720–727. [DOI] [PubMed] [Google Scholar]
- 27.Toyosaka A, Okamoto E, Mitsunobu M, et al. Pathologic and radiographic studies of intrahepatic metastasis in hepatocellular carcinoma: the role of efferent vessels. HBP Surgery 1996; 10:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda Y, Kajiyama K, Adachi E, et al. Early recurrence after surgery of hepatocellular carcinoma. Hepatogastroenterology 1995; 42:469–472. [PubMed] [Google Scholar]
- 29.Jwo SC, Chiu JH, Chau GY, et al. Risk factors linked to tumor recurrence of human hepatocellular carcinoma after hepatic resection. Hepatology 1992; 16:1367–1371. [DOI] [PubMed] [Google Scholar]
- 30.Harada T, Shigemura T, Kodama S, et al. Hepatic resection is not enough for hepatocellular carcinoma. A follow-up study of 92 patients. J Clin Gastroenterol 1992; 14:245–250. [DOI] [PubMed] [Google Scholar]
- 31.Suenaga M, Nakao A, Harada A, et al. Hepatic resection for hepatocellular carcinoma. World J Surg 1992; 16:97–104. [DOI] [PubMed] [Google Scholar]
- 32.Nagao T, Inoue S, Goto S, et al. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg 1987; 205:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otto G, Heuschen U, Hofmann WJ, et al. Survival and recurrence after liver transplantation versus resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg 1998; 227:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adachi E, Maeda T, Kajiyama K, et al. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer 1996; 77:2022–2031. [DOI] [PubMed] [Google Scholar]
- 35.Sobin LH, Wittekind Ch, eds. TNM classification of malignant tumors. 5th ed. New York: John Wiley; 1997.
- 36.Nonami T, Harada A, Kurokawa T, et al. Hepatic resection for hepatocellular carcinoma. Am J Surg 1997; 173:288–291. [DOI] [PubMed] [Google Scholar]
- 37.Lai ECS, Ng IOL, Ng MMT, et al. Long-term results of resection for large hepatocellular carcinoma: a multivariate analysis of clinicopathological features. Hepatology 1990; 11:815–818. [DOI] [PubMed] [Google Scholar]
- 38.Ng IOL, Lai ECS, Fan ST, et al. Prognostic significance of pathologic features of hepatocellular carcinoma. A multivariate analysis of 278 patients. Cancer 1995; 76:2443–2448. [DOI] [PubMed] [Google Scholar]
- 39.Franco D, Capussotti L, Smadja C, et al. Resection of hepatocellular carcinoma. Results in 72 European patients with cirrhosis. Gastroenterology 1990; 98:733–738. [PubMed] [Google Scholar]
- 40.Ng IOL, Lai ECS, Ng MMT, Fan ST. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer 1992; 70:45–49. [DOI] [PubMed] [Google Scholar]
- 41.Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma in cirrhotic and noncirrhotic livers. A clinico-histopathologic study of 804 North American patients. Am J Clin Pathol 1996; 105:65–75. [DOI] [PubMed] [Google Scholar]
- 42.Shirabe K, Matsumata T, Adachi E, et al. Prognosis of well differentiated small hepatocellular carcinoma—is well-differentiated hepatocellular carcinoma clinically early cancer? Hepatogastroenterology 1995; 42:923–930. [PubMed] [Google Scholar]
- 43.Adachi E, Maeda T, Matsumata T, et al. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology 1995; 108:768–775. [DOI] [PubMed] [Google Scholar]
- 44.Ezaki T, Kanematsu T, Okamura T, et al. DNA analysis of hepatocellular carcinoma and clinicopathologic implications. Cancer 1988; 61:106–109. [DOI] [PubMed] [Google Scholar]
- 45.Chiu JH, Kao HL, Wu LH, et al. Prediction of relapse or survival after resection in human hepatomas by DNA flow cytometry. J Clin Invest 1992; 89:539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen MF, Hwang TL, Tsao KC, et al. Flow cytometric DNA analysis of hepatocellular carcinoma: preliminary report. Surgery 1991; 109:455–458. [PubMed] [Google Scholar]
- 47.McEntee GP, Batts KA, Katzmann JA, et al. Relationship of nuclear DNA content to clinical and pathologic findings in patients with primary hepatic malignancy. Surgery 1992; 111:376–379. [PubMed] [Google Scholar]
- 48.Nagasue N, Yamanoi A, Takemoto Y, et al. Comparison between diploid and aneuploid hepatocellular carcinoma. Br J Surg 1992; 79:667–670. [DOI] [PubMed] [Google Scholar]
- 49.Kitamoto M, Nakanishi T, Kira S, et al. The assessment of proliferating cell nuclear antigen immunohistochemical staining in small hepatocellular carcinoma and its relationship to histologic characteristics and prognosis. Cancer 1993; 72:1859–1865. [DOI] [PubMed] [Google Scholar]
- 50.Suehiro T, Matsumata T, Itasaka H, et al. Clinicopathologic features and prognosis of resected hepatocellular carcinomas of varied sizes with special reference to proliferating cell nuclear antigen. Cancer 1995; 76:399–405. [DOI] [PubMed] [Google Scholar]
- 51.Ng IOL, Lai ECS, Fan ST, et al. Prognostic significance of proliferating cell nuclear antigen expression in hepatocellular carcinoma. Cancer 1994; 73:2268–2274. [DOI] [PubMed] [Google Scholar]
- 52.Ohta K, Kanamaru T, Morita Y, et al. Telomerase activity in hepatocellular carcinoma as a predictor of postoperative recurrence. J Gastroenterol 1997; 32:791–796. [DOI] [PubMed] [Google Scholar]
- 53.Suda T, Isokawa O, Aoyagi Y, et al. Quantitation of telomerase activity in hepatocellular carcinoma: a possible aid for a prediction of recurrent diseases in the remnant liver. Hepatology 1998; 27:402–406. [DOI] [PubMed] [Google Scholar]
- 54.Nagasue N, Yu L, Yukaya H, et al. Androgen and oestrogen receptors in hepatocellular carcinoma and surrounding liver parenchyma: impact on intrahepatic recurrence after hepatic resection. Br J Surg 1995; 82:542–547. [DOI] [PubMed] [Google Scholar]
- 55.Boix L, Castells A, Bruix J, et al. Androgen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resection. J Hepatol 1995; 22:616–622. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi H, Sugio K, Matsumata T, et al. The clinical significance of p53 gene mutation in hepatocellular carcinoma from Japan. Hepatology 1995; 22:1702–1707. [PubMed] [Google Scholar]
- 57.Ng IOL, Lai ECS, Chan AS, So MKP. Overexpression of p53 in hepatocellular carcinomas: a clinicopathological and prognostic correlation. J Gastroenterol Hepatol 1995; 10:250–255. [DOI] [PubMed] [Google Scholar]
- 58.Boix L, Bruix J, Campo E, Sole M, Castells A, Fuster J, et al. nm23–A1 expression and disease recurrence after surgical resection of small hepatocellular carcinoma. Gastroenterology 1994; 107:486–491. [DOI] [PubMed] [Google Scholar]
- 59.Mise M, Arii S, Higashituji H, et al. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology 1996; 23:455–464. [DOI] [PubMed] [Google Scholar]
- 60.Chow NH, Hsu PI, Lin XZ, et al. Expression of vascular endothelial growth factor in normal liver and hepatocellular carcinoma: an immunohistochemical study. Hum Pathol 1997; 28:698–703. [DOI] [PubMed] [Google Scholar]
- 61.El-Assal ON, Yamanoi A, Soda Y, et al. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology 1998; 27:1554–1562. [DOI] [PubMed] [Google Scholar]
- 62.Ker CG, Chen HY, Juan CC, et al. Role of angiogenesis in hepatitis and hepatocellular carcinoma. Hepatogastroenterology 1999; 46:646–650. [PubMed] [Google Scholar]
- 63.Kozyraki R, Scoazec JY, Flejou JF, et al. Expression of cadherins and alpha-catenin in primary epithelial tumors of the liver. Gastroenterology 1996; 110:1137–1149. [DOI] [PubMed] [Google Scholar]
- 64.Huang GT, Lee HS, Chen CH, et al. Correlation of E-cadherin expression and recurrence of hepatocellular carcinoma. Hepatogastroenterology 1999; 46:1923–1927. [PubMed] [Google Scholar]
- 65.Ng IOL, Ng MMT, Lai ECS, Fan ST. Better survival in female patients with hepatocellular carcinoma. Possible causes from a pathologic approach. Cancer 1995; 75:18–22. [DOI] [PubMed] [Google Scholar]
- 66.Kumada T, Nakano S, Takeda I, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology 1997; 25:87–92. [DOI] [PubMed] [Google Scholar]
- 67.Yamanaka N, Tanaka T, Tanaka W, et al. Correlation of hepatitis virus serologic status with clinicopathologic features in patients undergoing hepatectomy for hepatocellular carcinoma. Cancer 1997; 79:1509–1515. [DOI] [PubMed] [Google Scholar]
- 68.Okada S, Ishii H, Nose H, et al. Influence of alcohol abuse on recurrence after curative resection of hepatocellular carcinoma. Hepatogastroenterology 1995; 42:944–999. [PubMed] [Google Scholar]
- 69.Sasaki Y, Imaoka S, Matsutani S, et al. Influence of coexisting cirrhosis on long-term prognosis after surgery in patients with hepatocellular carcinoma. Surgery 1992; 112:515–521. [PubMed] [Google Scholar]
- 70.Ko S, Nakajima Y, Kanehiro H, et al. Significant influence of accompanying chronic hepatitis status on recurrence of hepatocellular carcinoma after hepatectomy. Results of multivariate analysis. Ann Surg 1996; 224:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shirabe K, Takenaka K, Taketomi A, et al. Postoperative hepatitis status as a significant risk factor for recurrence in cirrhotic patients with small hepatocellular carcinoma. Cancer 1996; 77:1050–1055. [PubMed] [Google Scholar]
- 72.Tarao K, Takemiya S, Tamai S, et al. Relationship between the recurrence of hepatocellular carcinoma (HCC) and serum alanine aminotransferase levels in hepatectomized patients with hepatitis C virus-associated cirrhosis and HCC. Cancer 1997; 79:688–694. [DOI] [PubMed] [Google Scholar]
- 73.Chiu JH, Wu LH, Kao HL, et al. Can determination of the proliferative capacity of the nontumor portion predict the risk of tumor recurrence in the liver remnant after resection of human hepatocellular carcinoma? Hepatology 1993; 18:96–102. [PubMed] [Google Scholar]
- 74.Tarao K, Hoshino H, Shimizu A, et al. Role of increased DNA synthesis activity of hepatocytes in multicentric hepatocarcinogenesis in residual liver of hepatectomized cirrhotic patients with hepatocellular carcinoma. Jpn J Cancer Res 1994; 85:1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taketomi A, Takenaka K, Matsumata T, et al. Circulating intercellular adhesion molecule-1 in patients with hepatocellular carcinoma before and after hepatic resection. Hepatogastroenterology 1997; 44:477–483. [PubMed] [Google Scholar]
- 76.Ueno S, Tanabe G, Yoshida A, et al. Postoperative prediction of and strategy for metastatic recurrent hepatocellular carcinoma according to histologic activity of hepatitis. Cancer 1999; 86:248–254. [PubMed] [Google Scholar]
- 77.Okamoto E, Tanaka N, Yamanaka N, Toyosaka A. Results of surgical treatments of primary hepatocellular carcinoma: some aspects to improve long-term survival. World J Surg 1984; 8:360–366. [DOI] [PubMed] [Google Scholar]
- 78.Lee CS, Sung JL, Hwang LY, et al. Surgical treatment of 109 patients with symptomatic and asymptomatic hepatocellular carcinoma. Surgery 1986; 99:481–490. [PubMed] [Google Scholar]
- 79.Sugioka A, Tsuzuki T, Kanai T. Postresection prognosis of patients with hepatocellular carcinoma. Surgery 1993; 113:612–618. [PubMed] [Google Scholar]
- 80.Chau GY, Lui WY, Tsay SH, et al. Prognostic significance of surgical margin in hepatocellular carcinoma resection: an analysis of 165 Child’s A patients. J Surg Oncol 1997; 66:122–126. [DOI] [PubMed] [Google Scholar]
- 81.Tang ZY, Yu YQ, Zhou XD, et al. Surgery of small hepatocellular carcinoma. Analysis of 144 cases. Cancer 1989; 64:536–541. [DOI] [PubMed] [Google Scholar]
- 82.Yoshida Y, Kanematsu T, Matsumata T, et al. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis. Further evaluation of limited hepatic resection. Ann Surg 1989; 209:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lai ECS, You KT, Ng IOL, Shek TWH. The pathological basis of resection margin for hepatocellular carcinoma. World J Surg 1993; 17:786–791. [DOI] [PubMed] [Google Scholar]
- 84.Ochiai T, Takayama T, Inoue K, et al. Hepatic resection with and without surgical margins for hepatocellular carcinoma in patients with impaired liver function. Hepatogastroenterology 1999; 46:1885–1889. [PubMed] [Google Scholar]
- 85.Matsumata T, Ikeda Y, Hayashi H, et al. The association between transfusion and cancer-free survival after curative resection for hepatocellular carcinoma. Cancer 1993; 72:1866–1871. [DOI] [PubMed] [Google Scholar]
- 86.Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery 1994; 115:303–309. [PubMed] [Google Scholar]
- 87.Asahara T, Katayama K, Itamoto T, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg 1999; 23:676–680. [DOI] [PubMed] [Google Scholar]
- 88.Yamanaka N, Okamoto E, Fujihara S, et al. Do the tumor cells of hepatocellular carcinomas dislodge into the portal venous stream during hepatic resection? Cancer 1992; 70:2263–2267. [DOI] [PubMed] [Google Scholar]
- 89.Matsumata T, Kanematsu T, Takenaka K, Sugimachi K. Lack of intrahepatic recurrence of hepatocellular carcinoma by temporary portal venous embolization with starch microspheres. Surgery 1989; 105:188–191. [PubMed] [Google Scholar]
- 90.Okamura J, Horikawa S, Fujiyama T, et al. An appraisal of transcatheter arterial embolization combined with transcatheter arterial infusion of chemotherapeutic agent for hepatic malignancies. World J Surg 1982; 6:352–357. [DOI] [PubMed] [Google Scholar]
- 91.Nakamura H, Tanaka T, Hori S, et al. Transcatheter embolization of hepatocellular carcinoma: assessment of efficacy in cases of resection following embolization. Radiology 1983; 147:401–405. [DOI] [PubMed] [Google Scholar]
- 92.Sakurai M, Okamura J, Kuroda C. Transcatheter chemo-embolization effective for treating hepatocellular carcinoma. A histopathologic study. Cancer 1984; 54:387–392. [DOI] [PubMed] [Google Scholar]
- 93.Hwang TL, Chen MF, Lee TY, et al. Resection of hepatocellular carcinoma after transarterial embolization. Reevaluation of the advantages and disadvantages of preoperative embolization. Arch Surg 1987; 122:756–759. [DOI] [PubMed] [Google Scholar]
- 94.Imaoka S, Sasaki Y, Shibata T, et al. A pre-operative chemoembolization therapy using lipiodol, cisplatin and gelatin sponge for hepatocellular carcinoma. Cancer Chemother Pharmacol 1989; 23:S126–S128. [DOI] [PubMed] [Google Scholar]
- 95.Monden M, Okamura J, Sakon M, et al. Significance of transcatheter chemoembolization combined with surgical resection for hepatocellular carcinomas. Cancer Chemother Pharmacol 1989; 23:S90–S95. [DOI] [PubMed] [Google Scholar]
- 96.Nagasue N, Galizia G, Kohno H, et al. Adverse effects of preoperative hepatic artery chemoembolization for resectable hepatocellular carcinoma: a retrospective comparison of 138 liver resections. Surgery 1989; 106:81–86. [PubMed] [Google Scholar]
- 97.Adachi E, Matsumata T, Nishizaki T, et al. Effects of preoperative transcatheter hepatic arterial chemoembolization for hepatocellular carcinoma. The relationship between postoperative course and tumor necrosis. Cancer 1993; 72:3593–3598. [DOI] [PubMed] [Google Scholar]
- 98.Uchida M, Kohno H, Kubota H, et al. Role of preoperative transcatheter arterial oily chemoembolization for resectable hepatocellular carcinoma. World J Surg 1996; 20:326–331. [DOI] [PubMed] [Google Scholar]
- 99.Harada T, Matsuno K, Inoue T, et al. Is preoperative hepatic arterial chemoembolization safe and effective for hepatocellular carcinoma? Ann Surg 1996; 224:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg 1997; 226:688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paye F, Jagot P, Vilgrain V, et al. Preoperative chemoembolization of hepatocellular carcinoma: a comparative study. Arch Surg 1998; 133:767–772. [DOI] [PubMed] [Google Scholar]
- 102.Wu CC, Ho YZ, Ho WL, et al. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg 1995; 82:122–126. [DOI] [PubMed] [Google Scholar]
- 103.Yamasaki S, Hasegawa H, Kinoshita H, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res 1996; 87:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu YQ, Xu DB, Zhou XD, et al. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer 1993; 71:62–65. [DOI] [PubMed] [Google Scholar]
- 105.Nonami T, Isshiki K, Katoh H, et al. The potential role of postoperative hepatic artery chemotherapy in patients with high-risk hepatomas. Ann Surg 1991; 213:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takenaka K, Yoshida K, Nishizaki T, et al. Postoperative prophylactic lipiodolization reduces the intrahepatic recurrence of hepatocellular carcinoma. Am J Surg 1995; 169:400–405. [DOI] [PubMed] [Google Scholar]
- 107.Nakashima K, Kitano S, Kim YI, et al. Postoperative adjuvant arterial infusion chemotherapy for patients with hepatocellular carcinoma. Hepatogastroenterology 1996; 43:1410–1414. [PubMed] [Google Scholar]
- 108.Asahara T, Itamoto T, Katayama K, et al. Adjuvant hepatic arterial infusion chemotherapy after radical hepatectomy for hepatocellular carcinoma—results of long-term follow-up. Hepatogastroenterology 1999; 46:1042–1048. [PubMed] [Google Scholar]
- 109.Tanaka K, Shimada H, Togo S, et al. Use of transcatheter arterial infusion of anticancer agents with lipiodol to prevent recurrence of hepatocellular carcinoma after hepatic resection. Hepatogastroenterology 1999; 46:1083–1088. [PubMed] [Google Scholar]
- 110.Izumi R, Shimizu K, Iyobe T, et al. Postoperative adjuvant hepatic arterial infusion of lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology 1994; 20:295–301. [PubMed] [Google Scholar]
- 111.Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409. [PubMed] [Google Scholar]
- 112.Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(Suppl 6):S6–18–S6–25. [PubMed] [Google Scholar]
- 113.Lai ECS, Lo CM, Fan ST, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg 1998; 133:183–188. [DOI] [PubMed] [Google Scholar]
- 114.Kanematsu T, Matsumata T, Takenaka K, et al. Clinical management of recurrent hepatocellular carcinoma after primary resection. Br J Surg 1988; 75:203–206. [DOI] [PubMed] [Google Scholar]
- 115.Yamamoto M, Arii S, Sugahara K, Tobe T. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. Br J Surg 1996; 83:336–340. [DOI] [PubMed] [Google Scholar]
- 116.Kawata A, Une Y, Hosokawa M, et al. Adjuvant chemoimmunotherapy for hepatocellular carcinoma patients. Adriamycin, interleukin-2, and lymphokine-activated killer cells versus Adriamycin alone. Am J Clin Oncol 1995; 18:257–262. [DOI] [PubMed] [Google Scholar]
- 117.Lygidakis NJ, Tsiliakos S. Multidisciplinary management of hepatocellular carcinoma. Hepatogastroenterology 1996; 43:1611–1619. [PubMed] [Google Scholar]
- 118.Muto Y, Moriwaki H, Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. N Engl J Med 1996; 334:1561–1567. [DOI] [PubMed] [Google Scholar]
- 119.Muto Y, Moriwaki H, Saito A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med 1999; 340:1046–1047. [DOI] [PubMed] [Google Scholar]
- 120.Nagasue N, Yukaya H, Ogawa Y, et al. Second hepatic resection for recurrent hepatocellular carcinoma. Br J Surg 1986; 73:434–438. [DOI] [PubMed] [Google Scholar]
- 121.Lange JF, Leese T, Castaing D, Bismuth H. Repeat hepatectomy for recurrent malignant tumors of the liver. Surg Gynecol Obstet 1989; 169:119–126. [PubMed] [Google Scholar]
- 122.Nakajima Y, Ohmura T, Kimura J, et al. Role of surgical treatment for recurrent hepatocellular carcinoma after hepatic resection. World J Surg 1993; 17:792–795. [DOI] [PubMed] [Google Scholar]
- 123.Matsuda Y, Ito T, Oguchi Y, et al. Rationale of surgical management for recurrent hepatocellular carcinoma. Ann Surg 1993; 217:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou XD, Yu YQ, Tang ZY, et al. Surgical treatment of recurrent hepatocellular carcinoma. Hepatogastroenterology 1993; 40:333–336. [PubMed] [Google Scholar]
- 125.Kakazu T, Makuuchi M, Kawasaki S, et al. Repeat hepatic resection for recurrent hepatocellular carcinoma. Hepatogastroenterology 1993; 40:337–341. [PubMed] [Google Scholar]
- 126.Suenaga M, Sugiura H, Kokuba Y, et al. Repeated hepatic resection for recurrent hepatocellular carcinoma in eighteen cases. Surgery 1994; 115:452–457. [PubMed] [Google Scholar]
- 127.Shimada M, Matsumata T, Taketomi A, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery 1994; 115:703–706. [PubMed] [Google Scholar]
- 128.Lee PH, Lin WJ, Tsang YM, et al. Clinical management of recurrent hepatocellular carcinoma. Ann Surg 1995; 222:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nagasue N, Kohno T, Hayashi M, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma. Br J Surg 1996; 83:127–131. [DOI] [PubMed] [Google Scholar]
- 130.Hu RH, Lee PH, Yu SC, et al. Surgical resection for recurrent hepatocellular carcinoma: prognosis and analysis of risk factors. Surgery 1996; 120:23–29. [DOI] [PubMed] [Google Scholar]
- 131.Shuto T, Kinoshita H, Hirohashi K, et al. Indications for, and effectiveness of, a second hepatic resection for recurrent hepatocellular carcinoma. Hepatogastroenterology 1996; 43:932–937. [PubMed] [Google Scholar]
- 132.Shimada M, Takenaka K, Taguchi K, et al. Prognostic factors after repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg 1998; 227:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arii S, Monden K, Niwano M, et al. J Results of surgical treatment for recurrent hepatocellular carcinoma: comparison of outcome among patients with multicentric carcinogenesis, intrahepatic metastasis, and extrahepatic recurrence. J Hepatobiliary Pancreat Surg 1998; 5:86–92. [DOI] [PubMed] [Google Scholar]
- 134.Farges O, Regimbeau JM, Belghiti J. Aggressive management of recurrence following surgical resection of hepatocellular carcinoma. Hepatogastroenterology 1998; 45:1275–1280. [PubMed] [Google Scholar]
- 135.Poon RTP, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sasaki Y, Imaoka S, Fujita M, et al. Regional therapy in the management of intrahepatic recurrence after surgery for hepatoma. Ann Surg 1987; 206:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nagao T, Inoue S, Yoshimi F, et al. Postoperative recurrence of hepatocellular carcinoma. Ann Surg 1990; 211:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nakao N, Kamino K, Miura K, et al. Recurrent hepatocellular carcinoma after partial hepatectomy: value of treatment with transcatheter arterial chemoembolization. AJR Am J Roentgenol 1991; 156:1177–1179. [DOI] [PubMed] [Google Scholar]
- 139.Takayasu K, Wakao F, Moriyama N, et al. Postresection recurrence of hepatocellular carcinoma treated by arterial embolization: analysis of prognostic factors. Hepatology 1992; 16:906–911. [DOI] [PubMed] [Google Scholar]
- 140.Ouchi K, Matsubara S, Fukuhara K, et al. Recurrence of hepatocellular carcinoma in the liver remnant after hepatic resection. Am J Surg 1993; 166:270–273. [DOI] [PubMed] [Google Scholar]
- 141.Park JH, Han JK, Chung JW, et al. Postoperative recurrence of hepatocellular carcinoma: results of transcatheter arterial chemoembolization. Cardiovasc Intervent Radiol 1993; 16:21–24. [DOI] [PubMed] [Google Scholar]
- 142.Okazaki M, Yamasaki S, Ono H, et al. Chemoembolotherapy for recurrent hepatocellular carcinoma in the residual liver after hepatectomy. Hepatogastroenterology 1993; 40:320–323. [PubMed] [Google Scholar]
- 143.Shimada M, Takenaka K, Gion T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology 1996; 111:720–726. [DOI] [PubMed] [Google Scholar]
- 144.Ezaki T, Koyanagi N, Yamagata M, et al. Postoperative recurrence of solitary small hepatocellular carcinoma. J Surg Oncol 1996; 62:115–122. [DOI] [PubMed] [Google Scholar]
- 145.Tanaka K, Okazaki H, Nakamura S, et al. Hepatocellular carcinoma: treatment with a combination therapy of transcatheter arterial embolization and percutaneous ethanol injection. Radiology 1991; 179:713–717. [DOI] [PubMed] [Google Scholar]
- 146.Ishii H, Okada S, Sato T, et al. Effect of percutaneous ethanol injection for postoperative recurrence of hepatocellular carcinoma in combination with transcatheter arterial embolization. Hepatogastroenterology 1996; 43:644–650. [PubMed] [Google Scholar]
- 147.Sato M, Watanabe Y, Iseki N, et al. Chemoembolization and percutaneous ethanol injection for intrahepatic recurrence of hepatocellular carcinoma after hepatic resection. Hepatogastroenterology 1996; 43:1421–1426. [PubMed] [Google Scholar]
- 148.Ohnishi K, Yoshioka H, Ito S, Fujiwara K. Prospective randomized controlled trial comparing percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology 1998; 27:67–72. [DOI] [PubMed] [Google Scholar]
- 149.Kainuma O, Asano T, Aoyama H, Shinohara Y. Recurrent hepatocellular carcinoma successfully treated with radiofrequency thermal ablation. J Hepatobiliary Pancreat Surg 1999; 6:190–194. [DOI] [PubMed] [Google Scholar]
- 150.Lam CM, Yuen WK, Fan ST. Hepatic cryosurgery for recurrent hepatocellular carcinoma after hepatectomy: a preliminary report. J Surg Oncol 1998; 68:104–106. [DOI] [PubMed] [Google Scholar]
- 151.Lo CM, Lai ECS, Fan ST, et al. Resection for extrahepatic recurrence of hepatocellular carcinoma. Br J Surg 1994; 81:1019–1021. [DOI] [PubMed] [Google Scholar]
- 152.Lam CM, Lo CM, Yuen WK, et al. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg 1998; 85:1198–1200. [DOI] [PubMed] [Google Scholar]
- 153.Sakamoto Y, Kubota K, Mori M, et al. Surgical management for adrenal gland metastasis of hepatocellular carcinoma. Hepatogastroenterology 1999; 46:1036–1041. [PubMed] [Google Scholar]
- 154.Nakayama H, Takayama T, Makuuchi M, et al. Resection of peritoneal metastases from hepatocellular carcinoma. Hepatogastroenterology 1999; 46:1049–1052. [PubMed] [Google Scholar]
- 155.Chen PJ, Chen DS, Lai MY, et al. Clonal origin of recurrent hepatocellular carcinoma. Gastroenterology 1989; 96:527–529. [DOI] [PubMed] [Google Scholar]
- 156.Yamamoto T, Kajino K, Kudo M, et al. Determination of the clonal origin of multiple human hepatocellular carcinomas by cloning and polymerase chain reaction of the integrated hepatitis B virus DNA. Hepatology 1999; 29:1446–1452. [DOI] [PubMed] [Google Scholar]
- 157.Lai ECS, Fan ST, Lo CM, et al. Anterior approach for difficult major right hepatectomy. World J Surg 1996; 20:314–318. [DOI] [PubMed] [Google Scholar]
- 158.Liu CL, Fan ST, Lo CM, et al. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg 2000; 232:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lai CL, Ching CK, Tung AKM, et al. Lamivudine is effective in suppressing hepatitis B virus DNA in Chinese hepatitis B surface antigen carriers: a placebo-controlled trial. Hepatology 1997; 25:241–244. [DOI] [PubMed] [Google Scholar]
- 160.Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-α on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet 1995; 346:1051–1055. [DOI] [PubMed] [Google Scholar]
- 161.Kuiper RA, Schellens JH, Blijiham GH, et al. Clinical research on antiangiogenic therapy. Pharmacol Res 1998; 37:11–16. [DOI] [PubMed] [Google Scholar]
- 162.Xia JL, Yang BH, Tang ZY, et al. Inhibitory effect of the angiogenesis inhibitor TNP-470 on tumor growth and metastasis in nude mice bearing human hepatocellular carcinoma. J Cancer Res Clin Oncol 1997; 123:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]