Abstract
Objective
To establish criteria to evaluate performance in surgical research, and to suggest strategies to optimize research in the future.
Summary Background Data
Research is an integral component of the academic mission, focusing on important clinical problems, accounting for surgical advances, and providing training and mentoring for young surgeons. With constraints on healthcare resources, there is increasing pressure to generate clinical revenues at the expense of the time and effort devoted to surgical research. An approach that would assess the value of research would allow prioritization of projects. Further, alignment of high-priority research projects with clinical goals would optimize research gains and maximize the clinical enterprise.
Methods
The authors reviewed performance criteria applied to industrial research and modified these criteria to apply to surgical research. They reviewed several programs that align research objectives with clinical goals.
Results
Performance criteria were categorized along several dimensions: internal measures (quality, productivity, innovation, learning, and development), customer satisfaction, market share, and financial indices (cost and profitability). A “report card” was proposed to allow the assessment of research in an individual department or division.
Conclusions
The department’s business strategy can no longer be divorced from its research strategy. Alignment between research and clinical goals will maximize the department’s objectives but will create the need to modify existing hierarchical structures and reward systems. Such alignment appears to be the best way to ensure the success of surgical research in the future.
The American healthcare system is experiencing a radical transformation, driven by pressures to reduce costs. 1 This new healthcare climate has created major challenges for academic departments of surgery, and these pressures have enormous implications for surgery’s research mission. The impact of these changes on surgical research is directly related to decreasing reimbursement for surgical services and the time constraints imposed on academic surgeons.
Historically, surgical procedures have accounted for a major share of hospital profits, and academic surgical departments have often contributed money from professional revenues to medical schools (e.g., through the dean’s tax) and to support surgical research. Others, such as commercial insurers, 2 are no longer willing to compensate academic medical centers at higher rates to support research. Finally, the competition for patients has also increased; most surgical procedures, with a few exceptions, 3 can be performed equally well in community and academic hospitals.
One resolution to these pressures brought about by reduced costs and increased competition for patients is to shift the role of the university surgeon from that of a triple-threat academic to become solely a “proceduralist,” an individual who would generate as much clinical revenue as possible to subsidize academic programs in other departments, whose members are regarded by some as better equipped to participate in biomedical research. Such pressures to increase clinical productivity by increasing volume threaten to jeopardize the academic mission of surgical departments in university-owned or -affiliated hospitals. More specifically, such an approach will greatly compromise surgical research, which is the cornerstone of improvements in surgical care. However, from the standpoint of maintaining professional salaries in surgical departments, this approach is attractive.
We believe that academic departments must resist this temptation to marginalize surgical research for the sake of emphasizing clinical surgery (and income). The justification for conducting research in an academic department of surgery relates to the department’s mission. Through research, patient care is improved. If our responsibility is to train residents who can provide the best possible care to surgical patients, we must ensure that they are trained using contemporary techniques and advanced therapies. In general, individuals who work in a particular field (i.e., surgeons) are better equipped than those outside the field to identify pertinent problems and find solutions. Thus, surgical research results in improved patient care.
Moreover, we believe that effective management of the research enterprise and further investment in research will allow a department to differentiate itself from its competition and create new market opportunities. Few industries are successful without a dynamic and progressive research and development component. Likewise, the competitive edge of university-based departments of surgery will be the production of new knowledge, which will eventually evolve to improve patient care. 4
The purpose of this article is to outline an approach that will allow a surgical department to assess the contribution made by its surgical research. After such an audit, surgical leaders should align their research efforts with coexisting clinical efforts to optimize performance in both spheres. Such a task will require integration and management of both the research and clinical enterprise, but this approach is necessary to ensure a successful place for surgical research in the future.
THE RESEARCH AUDIT
Generally, research exists in academic departments of surgery, but its presence is usually independent from the clinical programs, and its activity (or size) is related to the availability of funding. Rarely is surgical research integrated philosophically or strategically into the clinical mission of the department of surgery. In other instances, superb nests of basic research are found in the department, but these units remain isolated, fragmented, and unexploited: few if any attempts are made to link the intellectual component of such a unit or its new discoveries to clinical care. On occasion, research funding from a department is used as part of a package for employment negotiation, with the intent to use this “carrot” as part of an inducement to attract or maintain a faculty member. Such an enticement may have little relation to the research capabilities of the individual or the research goals of the department. More typically, the message to the faculty is to get funded! Funding satisfies the chair’s obligation to the institution to provide money from overhead. The source of funds or the topic of the research project often seems secondary to the dollar amount obtained. In this case, the department’s performance metric is how many grants were awarded and what was their cumulative worth. A secondary consideration may be the number of papers published and the number of presentations made at national meetings.
Although there may be some merit in such quantitative measures, we believe that quality research, integrated with clinical surgery, has the best chance of succeeding in the future. To move toward this goal, the department must first perform an audit of its research. Two questions must be answered:
1. What are the strengths and weaknesses of the department’s research enterprise?
2. How can the research strengths be leveraged (and the weaknesses corrected) to support the broader mission of the department?
To answer these questions, one begins with a review of the department’s current clinical and research activities and evaluates these programs to determine if there are matches” (Table 1). In performing this audit, the evaluation should account for intangible resources. For example, the most important asset in any research effort is intellectual capital and research creativity. Drucker emphasized this when he wrote: “Core competencies are different in every organization [but represent] part of an organization’s personality. Every organization … needs one core competence: innovation. And every organization needs a way to record and appraise its innovative performance.”5
Table 1. DEPARTMENTAL ASSESSMENT: HOW WELL IS THE RESEARCH ENTERPRISE ALIGNED WITH CLINICAL PROGRAMS?
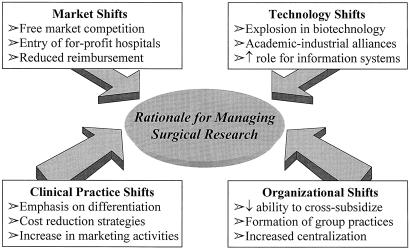
Each department is different, and the appropriate intellectual capital and research goals differ from one department to another. Some departments are limited by direct research funds but have stronger clinical interests and therefore can emphasize clinical or outcomes research. Others have a strong history of basic research programs and attract house officers or fellows with an inclination for spending several years in the laboratory or obtaining an advanced degree. Programs in health services research have recently become popular, and this will be a growing field for surgical research. Regardless of the emphasis, a strong case can be made for aligning the research mission with the clinical program and then administering the research enterprise. This process must be managed (Fig. 1).
Figure 1. Why active management of a research program is necessary. Shifts in market dynamics, organizational structure, technology, and clinical practice patterns require that academic departments focus on building their most important core competence: innovation. Successful academic departments of surgery of the future will remain committed to building a research enterprise and managing it as a strategic competitive weapon.
EVALUATING PERFORMANCE OF THE RESEARCH EFFORT
In today’s environment, merely conducting surgical research is not enough. Revenues to support unfunded research are decreasing. Outcomes assessment is as important in research as it is in clinical care. In assessing the performance of the research programs in the department, several questions must be answered: What kinds of performance measurements should be used? What is the product of our surgical research efforts?
Historically, departments have graded their research programs on the basis of numbers of papers published or numbers of grants awarded. This method of grading can be deceptive: leading performance indicators cannot be found in financial or publication data alone. Departments with a large number of grants and substantial amounts of extramural funding do not inevitably create meaningful results that enhance their business objectives.
In the pre-HMO era, this scheme would be considered a successful accomplishment. However, during periods of resource limitation, a more rigorous approach is necessary. Because of the difficulties of quantifying surgical research, assessment and judgment are often used rather than actual measurement. However, reliable tools and performance metrics are necessary if the department is to grow and accomplish more than just publishing papers and securing grants. Performance can be categorized along several dimensions 5–7 : internal measures (quality, productivity, innovation, learning and development), customer satisfaction, market share, and financial indices (cost, profitability).
Internal Measures
Quality
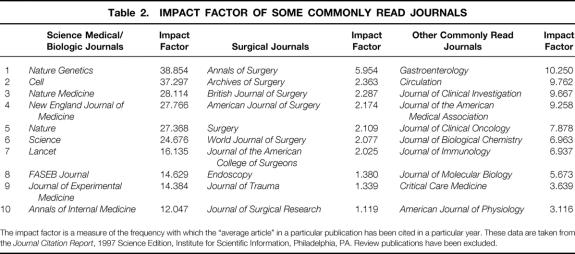
In evaluating the quality of the research program, the central question is: How good is the work? Although we recognize that quality, like beauty, is in the eye of the beholder, we tend to judge it using specific criteria, the primary one being the publication vehicle (type of journal, name of journal, reputation of journal, impact score;Table 2). This grading system has evolved in part because the journal in which the research is published influences others and can affect the author’s promotion as well as the priority score assigned by a National Institutes of Health (NIH) study section. Quality also encompasses dimensions such as laboratory reputation and number of presentations at national meetings.
Table 2. IMPACT FACTOR OF SOME COMMONLY READ JOURNALS
The impact factor is a measure of the frequency with which the “average article” in a particular publication has been cited in a particular year. These data are taken from the Journal Citation Report, 1997 Science Edition, Institute for Scientific Information, Philadelphia, PA. Review publications have been excluded.
Productivity
Productivity indices measure the quantity of a good or service that is produced during a constant period of time. This index is inherently complicated by improvements in equipment and technology. For example, it is difficult to compare the productivity of a researcher 20 years ago who made a series of blood glucose measurements using laborious enzymatic methods with that of today’s scientist, who can make the same determination in half the time using automated equipment. The issue is even more complex when one examines the number of papers published annually. Which research group is more productive, one that publishes six papers a year in surgical journals or one that publishes one paper a year in a basic science journal? Are multiple papers that describe studies of cultured cells more important that one exhaustive study in a large animal model? Despite these apparent problems, productivity is broadly assessed on the basis of total publications, amount and size of grants awarded, number of national presentations, and number of residents trained per year. Rarely is quality or innovation part of this assessment.
Innovation
This concept refers to a research laboratory’s producing important new knowledge or making breakthrough discoveries. This is in contrast to laboratories that do “contract research” or commit a large investment of time and money to replicate others’ findings. Measures of usable information are often accompanied by patents, industrial support, industrial or university collaborations, and the formation of spinoff companies.
Surgical research laboratories do more than just develop new products and services; they also design new methods to innovate a surgical department continuously. 8 Research on new, improved prototypes to develop operations and innovative care plans for clinical surgeons is just as important as research on a new genetic vector or a new receptor antagonist. An example of this concept is the work on perioperative epidural anesthesia that has culminated in successful colectomy with near-total recovery and a hospital stay of only 48 hours. 9 The need for translational research, outcomes research, epidemiologic studies, and clinical investigation has increased. As academic surgery departments struggle to remain competitive in a market economy, their foremost challenge is building the ingredients for tomorrow’s achievements while continuing to compete successfully today. In forging this future, the creation of a research enterprise that is the basis for innovation and improvements in surgical care will be critical.
Finally, an additional gain derived from an innovative surgical research laboratory is the attraction that such an environment has for bright, inquisitive, highly motivated young people. Such a work environment stimulates these individuals to think in unconventional ways, to challenge conventional dogma, and to test new concepts on their own. Bright young people are attracted to a laboratory environment that encourages this process (particularly through the interaction with other fellows), but they also expect and receive valuable counsel from its leaders. Working in such an environment is exciting. Activity continues throughout the night and on weekends. Fellows can’t wait to share their new data with others; they revel in discoveries and plan ahead to test new hypotheses. Finally, trainees from such environments are optimistic about their future, whether it be in research or in clinical surgery.
Development of Young Investigators
The training of young investigators is a critical factor that cannot be overlooked in assessing laboratory worth. 10,11 Mentoring is essential if young people are to discover their latent talents and abilities. In a competitive environment where time is one of our most valuable commodities, departments must develop mentoring skills in individuals to enhance the development of young surgeons. Mentoring can be one of the most powerful aspects of the academic surgeon’s professional life. Young people often identify with their mentors, and this may be a major reason why individuals choose a career in academic surgery.
Many departments track their graduates, in particular those who have spent time in a research effort during their residency and subsequently develop an independent research agenda. When a new investigator is awarded an extramural grant from a competitive agency, such as the NIH, it is a seal of approval and a cause for celebration. When young investigators build a productive research program and are successful in their career development and professional growth, it is a positive reflection on the department that was responsible for their training.
Customer Satisfaction
The performance of the department’s research enterprise can also be evaluated relative to the preferences and desires of stakeholders that provide resources to the organization. Who are surgical research’s customers? They include funding agencies, philanthropists, industry, and patients. The needs and wants of these customers must be met because customer loyalty (and retention) from all of these groups is vital. The department of surgery is becoming more dependent on longstanding relationships with industry, continued support from benefactors, and successful renewal of extramural grants. These stakeholders enjoy being courted and frequently require feedback concerning their investment.
Different customers have varying expectations and distinct feedback requirements. Large industrial grants are usually accompanied by stringent oversight requirements. Representatives from industry are responsible to shareholders (or venture capital dollars), and they expect that the laboratory will produce results by performing certain experiments. Milestone payments are frequently included on completion of specified tasks, and such remuneration is often part of the contractual agreement.
NIH (or other government) grants are often difficult to obtain, but once in hand these awards are rarely closely monitored. Government grants focus on timely investigative topics and pressure the investigator to practice “safe science,” thus reducing the potential for innovation. One learns to play the “NIH grant game” (have the work completed before the grant is submitted); the only worry is the next competitive renewal grant that will be submitted 3 to 5 years later. Unfortunately, the government is rarely as sensitive as is industry to its research investment in terms of outcomes. Hence, the emphasis is not structured to evaluate research results critically.
Monies (gifts, endowments) from philanthropists require another type of response to satisfy this type of customer. Frequently, the individual or family has been affected by a disease or has close ties to a principal investigator. Name recognition is often important: using the benefactor’s name for a laboratory or building is often necessary. Press releases, which discuss new discoveries, should acknowledge the benefactor and may provide ongoing satisfaction to this customer. Introducing the benefactor to patients who have in some way benefited from the contributions greatly personalizes the process and helps the contributor justify his or her support.
Patient groups or their advocates may also support a laboratory. Firefighters’ associations often contribute to burn research, and patients and their families with similar illnesses participate in fund-raising ventures to support research. The Shriners organization is notable for its research and care of children. Attention to these groups and often participation by the investigator or members of the laboratory are important to ensure communication and encourage continued funding.
Market Share
Although market share is commonly used as an indicator of clinical vitality, it is less frequently applied to the research enterprise. Market share is frequently used by the university hospital as an index of its market clout: a research laboratory might judge its rank and status in a particular area of research on the basis of the number of papers (or grants) it generates in that specific field. Data are available through various sources (e.g., Index Medicus, NIH) that record the number of papers published on a particular topic in a given year, or the number of grants awarded in that field. The relative contribution of an individual research unit to the total output can be determined. Of course, this performance metric does not take into account factors such as research quality or innovation.
Financial Indices
Cost
Cost issues relate to the expenses involved in managing the laboratory and producing results. In addition to fixed and overhead costs, efficiency and resource utilization must be addressed. Traditional cost accounting measures determine what it costs to accomplish a task—for example, to perform a certain experiment. Expenses would include the use of equipment, personnel costs, and supplies. Activity-based costing also records the costs of nonperformance, 5 such as the cost of not using the scintillation counter and having it sit idle, personnel downtime, and the cost of dealing with imperfect approaches and techniques that require repeating the experiment. Activity-based costing, therefore, provides a more accurate assessment of cost and also encourages efficiency (e.g., sharing of core facilities). 12
Traditional cost accounting assumes that a certain experiment has to be done (or a specific measurement has to be made) and that it has to be performed in a specific laboratory. Activity-based costing asks: Does it have to be done? (Do we need the answer, or is there a less expensive means of getting the answer?) And, if so, where is it best done? (Could it be outsourced? For example, is it more efficient to pay someone to make this monoclonal antibody rather than make it myself?). Using this approach, activity-based costing can substantially lower costs. Such decisions must be balanced by the cost of training a research fellow. In the latter case, the question must also be asked, “Does the fellow need to learn how to make a monoclonal antibody?”
Although the costs associated with running the laboratory can be measured in terms of monetary outlays, they can also be measured by opportunity losses. Resources are never free (even when no money exchanges hands), and two types of opportunity costs are relevant to the surgical investigator. The first relates to the decision to invest in a particular project: any decision to commit resources to one study diverts the resources from another. The second relates to the opportunity cost of human investment. When the surgeon is in the laboratory, he or she cannot be in the operating room; clinical revenues are therefore lost as a result of the decision to perform surgical research. Thus, the trade-off becomes one of estimating the value of each exercise (value is defined as quality divided by cost). Unfortunately, in many departments the value of having the investigator generate clinical revenues is judged to be more important than the value associated with developing a research agenda.
Profitability
Although for-profit organizations use financial performance (profits, return on investment) as the primary means of assessing corporate performance, it can be, by itself, a misleading marker. It is difficult to measure a rate of return on surgical research because many of the dividends are difficult to measure and value. However, with this drawback in mind, the department can ask: How much additional funding, new patents, and innovative technology have our research programs generated?
The value added by a particular research program is highest where its relative strength is greatest and the strategic importance of the program is crucial to the department’s business objectives. For example, research programs that are unrelated to the clinical enterprise (e.g., nondisease-oriented research) or merely supportive (e.g., a tissue-typing laboratory) do not create a competitive edge for the department. These efforts offer no real advantage, because rivals can easily mimic them. Strategically, unique research programs that enhance the clinical agenda (e.g., a high-powered clinical trials program) can create a competitive advantage for the department by offering innovative therapies to patients, particularly if the department’s research strength in the field is superior to that of its competitors.
FUTURE STRATEGIES: ALIGNING AND LINKING THE RESEARCH ENTERPRISE WITH BUSINESS STRATEGY
In deciding how to improve and position its research enterprise to achieve a competitive edge, the department of surgery must address several additional questions: What are the implications for research in my business? How do I leverage research to enhance our clinical initiatives? Is the core competence of the research inside or outside my department? Should our clinical strategy be business-driven (and research-supported) or research-driven? Failure to take advantage of the premium value from research investments is often due to the lack of alignment between business and research strategies and aggressive management of the research enterprise.
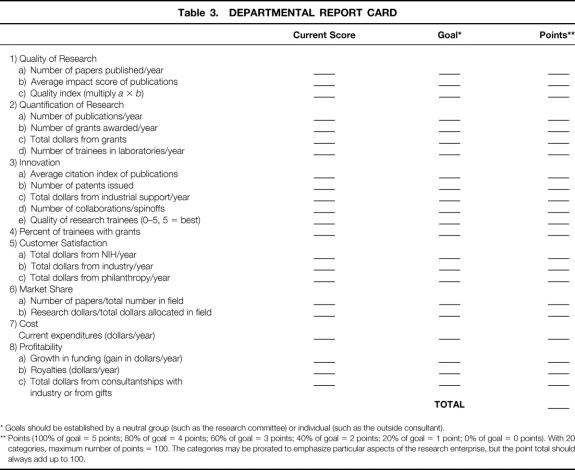
It may be useful for the department to generate a “report card” that evaluates its current research activities and links this performance to future goals (Table 3). This exercise, in conjunction with a departmental checklist (see Table 1) will enable the chair (or designee) to identify and correct absent or weak linkages between the research programs and the clinical enterprise. The grading categories in Table 3 are listed as being equal but may be ranked (or prorated) to reflect the importance of each category to the department’s (or division’s) overall goals. For example, a department of surgery that has a high-powered research program may wish to ascribe more points (say 8 to 10, rather than the average of 5) to categories such as innovation, quality, and profitability and give fewer points to factors such as cost and market share. In contrast, a department that is more clinically oriented, with research activities designed principally to provide the residents with research opportunities, might rank quantity and market share above innovation. In all cases, the final possible points should add up to 100. A major strength of this grading system is that it allows each department or division to set its own goals and compete with itself, so to speak, rather than competing against some arbitrarily created national system.
Table 3. DEPARTMENTAL REPORT CARD
* Goals should be established by a neutral group (such as the research committee) or individual (such as the outside consultant).
** Points (100% of goal = 5 points; 80% of goal = 4 points; 60% of goal = 3 points; 40% of goal = 2 points; 20% of goal = 1 point; 0% of goal = 0 points). With 20 categories, maximum number of points = 100. The categories may be prorated to emphasize particular aspects of the research enterprise, but the point total should always add up to 100.
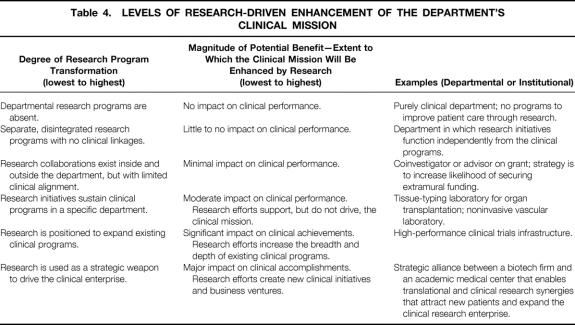
Alignment between the research and clinical missions involves a strategic fit and functional integration. 13 Fit demands that business strategies and research strategies be linked and complementary. The research strategy must be articulated in terms of how the department is positioned in the marketplace (external) and how the research infrastructure should be managed within the department (internally). The position of the department in the biomedical research marketplace centers around the scope of the research enterprise (breadth and depth of research programs), its core competencies (specific research expertise that is distinctive), and its governance (collaborations, joint ventures). The internal research domain centers on research architecture (facilities), processes (efficiency, monitoring, control), and research skills (training, knowledge development). No single alignment model between business strategy and research strategy fits all departments of surgery, and there are several levels at which the research enterprise can enhance the department’s clinical mission. In general, the greater the degree of transformation of the research program, the greater the potential benefit (Table 4).
Table 4. LEVELS OF RESEARCH-DRIVEN ENHANCEMENT OF THE DEPARTMENT’S CLINICAL MISSION
In its business strategy, the department of surgery achieves its goals and objectives by selling clinical services. Ultimately, these services (whether they are dependent on a superior outcome from a surgical procedure or access to a novel clinical trial) must attract and retain customers. In its research strategy, the department configures and positions its academic programs so they serve one or more of three strategic purposes: to support or expand existing businesses, to drive new business ventures, or to increase the breadth and depth of existing research and technological capabilities.
Support and Expand Existing Businesses
The department can use its research capabilities and expertise to support and expand existing businesses. This strategy was applied when Partners Healthcare (the alliance between the Brigham and Women’s Hospital and the Massachusetts General Hospital) entered into a joint venture with the Dana-Farber Cancer Institute in 1995. The new entity, Dana-Farber/Partners Cancer Care (DF/PCC), leveraged its collective cancer patient base to create a clinical research enterprise that would support and expand its business strategy of increasing its market share of the cancer care business in eastern Massachusetts. An integral part of the strategic plan was the creation of a unified clinical trials infrastructure that enabled DF/PCC to differentiate patient care through the advantages of clinical research. The single clinical trials core offered several benefits: the ability to offer cancer patients access to a wide variety of clinical trials, an enhanced ability to perform clinical research in a timely fashion, an increase in negotiating power for clinical trials funding from industry, and integration of disease programs and centers around a common process, which would enhance efficiency and productivity.
The University of Pennsylvania Medical Center used a similar strategy when substantial resources were committed to the establishment of cutting-edge programs in molecular biology and gene therapy. 14 Betting on the concept that gene therapy will revolutionize the way many complex diseases are treated during the next decade, Penn’s strategy was to invest in these initiatives, train physicians and scientists who were actively involved in hands-on human research, and create a translational research enterprise that offered cutting-edge therapies to patients with genetic disorders.
Drive New Business Ventures
Several academic medical centers are using their research capabilities and expertise to create new business opportunities. This requires a stronger linkage between biomedical research, whose purpose is the creation of new knowledge, and development, the application of this knowledge to achieve practical results. The research goal is the production of knowledge that will enable the department to participate in the forefront of new technology (devices, treatments) or to lay the scientific foundation for the development of new products or processes.
Stanford University has been a leader in technology transfer, fostering the growth of biotechnology in northern California and providing a model for academic medical centers across the country. 15 It is exploiting research creativity to achieve a competitive advantage and has taken a more aggressive posture regarding equity. These current approaches to marketing university intellectual capital have created new challenges. Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts, which has also been aggressive in leveraging its research enterprise, has established a set of policies and rules that govern academic–industrial relationships. 15 These rules prohibit startup companies in which MIT owns equity from hiring students doing related university research, require that data generated by university laboratories be published when available and not delayed until a product is ready for market, and forbids the use of funds from the startup company for research on campus.
Increase the Breadth and Depth of Existing Research and Technological Capabilities
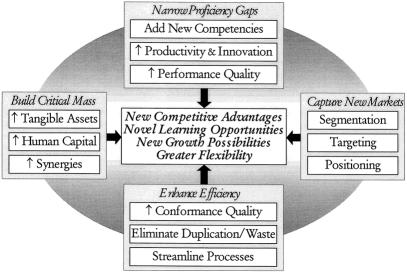
In addition to supporting current business opportunities and creating new ones, managing the research enterprise strategically can also enable the department to broaden and deepen existing research and development capabilities. When Massachusetts General Hospital and Brigham and Women’s Hospital formed Partners, the alliance brought together the two largest hospital-based research enterprises in the country. A primary impetus for this cooperation was that it would enhance the research enterprise. On a smaller scale, interdepartmental collaborations serve as an effective means of importing new techniques and skills into the laboratory. Many surgical residents choose to spend their research time in a basic scientist’s laboratory, a partnership that can plug competency gaps, provide access to new research tools, and pave the way for new research interactions. Research collaborations and alliances can add value by narrowing proficiency gaps, building critical mass, and increasing efficiency (Fig. 2). 16
Figure 2. How research collaborations and alliances add value.
Strategic alliances with commercial firms are creating new clinical research opportunities for academic medical centers. Duke University Medical Center has actively solicited pharmaceutical firms to support research in its clinical research center and in so doing has become a leader in evidence-based medicine. 17 According to Ralph Snyderman, MD, Chancellor of Duke University Medical Center, the academic–industrial research relationships that have been developed have been successful: “I think it’s been a success in terms of the academic role it plays. It’s been a success financially in that it pays for itself and generates the ability to fund a large number of clinical faculty, which is important. The number of excellent papers that have been published is well over 100. I think they publish 50 or more papers per year out of the Clinical Research Institute—peer-reviewed papers—and I think that’s important.”18
Need for a New Organizational Structure
The specific research structure used by a particular department will depend in part on its goals, culture, and values. No one would advocate the elimination of funded research activities that had no clear-cut clinical applicability. Seemingly unimportant new discoveries could serve as a stimulus for future investigations that lead to new, clinically relevant knowledge. Moreover, such research activities do serve an important role in training young people in terms of the scientific method.
The particular organizational structure used will also depend on the department’s strategy. If the strategy were solely to obtain grants, the department should hire people who are successful in this capacity. The current organizational department structure would change very little. If the strategy were based on the premise that a sustainable clinical enterprise depends on innovation and research, then the structure of the department requires some change. At the very least, such change would require greater intra- and interdepartmental collaboration. At the other extreme, one could envision a series of focused factories (disease centers), with elimination of the existing departmental structure. Given the existing cultural and political obstacles in academic medical centers and the need to train residents in an acute care hospital setting, some type of hybrid organization seems to be most appropriate.
No structure is right for all surgery departments, but the shift to a matrix organization seems to enhance the research enterprise in many circumstances. If for no other reason, such a change is necessary because physicians must recognize that consumerism in healthcare is a force to be addressed, and they must adopt some of the practices of other successful businesses, including strategic market planning. For many of the high-end services provided by academic medical centers to appeal to existing or targeted consumer market segments, they must be innovative enough to deliver extra quality, particularly in a rapidly changing environment where many services are now viewed as commodities. The development of centers of excellence (cancer center, heart center, brain center, transplant center) in many institutions can deliver such added value. These programs add value to the care of patients with complex problems by shifting the focus of delivery of care from the provider to the patient and the disease. Experts from multiple disciplines are brought together simultaneously to see patients and design treatment plans collectively (“one-stop shopping”). Simultaneously, such a model enhances the development of a more integrated research effort across the basic and clinical sciences to apply scientific discoveries to patient care more rapidly.
SUMMARY AND CONCLUSIONS
In the future, a tighter alignment between business and research strategies will create the need for changes in the department of surgery that challenge the existing culture, traditional hierarchical structures, and reward systems. But as advances in biomedical research change what research can do, we must change what we do with research. Research should be viewed as more than a technical support system for one of the costs of doing business. Its strategic role must be articulated throughout the department.
The department’s business strategy can no longer be divorced from its research strategy. Major challenges for the department will involve appreciating the potential scope and power of a contemporary research enterprise, selecting an alignment perspective between research and clinical initiatives that best fits the department’s goals, improving communication between clinical surgeons and basic scientists, identifying the appropriate criteria to assess research performance, and recognizing that the strategic alignment process is dynamic and continuous, not static, and requires management.
The research and development imperatives for the academic department of surgery have never been more compelling. Leaders of these departments must recognize the critical importance of their research programs as drivers of success. Successful surgery departments will accurately forecast what the future of the healthcare industry will look like. They will then leverage their competencies and capabilities so they can take advantage of that future. This recognition of research and development as a powerful entity, driving the course of a department of surgery, will ensure a rich future for surgical research.
Acknowledgments
The authors thank Nancy Erlich, C. McCollister Evarts, MD, Lazar J. Greenfield, MD, Alden H. Harken, MD, Ori D. Rotstein, MD, and Andrew L. Warshaw, MD, for their helpful suggestions.
Footnotes
Correspondence: Wiley W. Souba, Dept. of Surgery, H051, Penn State College of Medicine, 500 University Dr., Hershey, PA 17033.
Accepted for publication November 15, 2000.
References
- 1.Souba WW. Reinventing the academic medical center. J Surg Res 1999; 81:113–122. [DOI] [PubMed] [Google Scholar]
- 2.Weissman JS, Saglam D, Campbell EG, et al. Market forces and unsponsored research in academic medical centers. JAMA 1999; 281:1093–1098. [DOI] [PubMed] [Google Scholar]
- 3.Begg C, Cramer L, Hoskins W, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998; 280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 4.Souba WW. How competitive forces mold strategy in academic surgery. Surgery 1999; 125:616–629. [PubMed] [Google Scholar]
- 5.Drucker P. The information executives truly need. In: Harvard Business Review on Measuring Corporate Performance. Boston: Harvard Business School Press; 1998: 1–24.
- 6.Eccles R. The performance measurement manifesto. In: Harvard Business Review on Measuring Corporate Performance. Boston: Harvard Business School Press; 1998: 25–45.
- 7.Souba WW. No margin, no mission. J Surg Res 1996; 64:109–111. [DOI] [PubMed] [Google Scholar]
- 8.Roussel P, Saad K, Erickson T. Third-Generation R&D: Managing the Link to Corporate Strategy. Boston: Harvard Business School Press; 1991.
- 9.Bedram L, Punch-Jensen P, Jensen P, et al. Recovery after laparoscopic colonic surgery with epidural anesthesia and early oral nutrition and mobilization. Lancet 1995; 345:763–764. [DOI] [PubMed] [Google Scholar]
- 10.Souba WW. Mentoring young academic surgeons, our most precious asset. J Surg Res 1999; 82:113–120. [DOI] [PubMed] [Google Scholar]
- 11.Souba WW, Gamelli RL, Lorber MI, et al. Strategies for success in academic surgery. Surgery 1995; 117:90–95. [DOI] [PubMed] [Google Scholar]
- 12.Cooper R, Kaplan RS. Measure costs right: make the right decisions. Harvard Business Rev Sept-Oct 1988:96–105.
- 13.Venkatraman N. IT-enabled business transformation: from automation to business scope transformation. Sloan Management Rev 1994; 35:73–87. [Google Scholar]
- 14.The JIM Interview: William Kelley, MD. J Invest Med 1994; 42:606–612. [Google Scholar]
- 15.Fisher LM. Technology transfer at Stanford University. Strategy & Business 1998; 4th quarter, Issue 13:76–85.
- 16.Doz YL, Hamel G. Alliance Advantage. Boston: Harvard Business School Press; 1998.
- 17.Thompson D. More science and much more money. Time Magazine, Oct. 12, 1998:68–69.
- 18.The JIM Interview: Ralph Snyderman, MD. J Invest Med 1996; 44:398–405. [Google Scholar]