Abstract
Objective
To determine whether interleukin-1 (IL-1) affects the cellular homeostasis of small bowel mucosa, the authors studied apoptosis and proliferation in small bowel epithelium in two groups of C57 mice: an IL-1 receptor knockout group, and a control wild-type group.
Summary Background Data
Gut mucosal integrity is maintained by a balance of cell proliferation and cell death. Recent reports suggest that IL-1, a proinflammatory cytokine, increases cell death by apoptosis in some epithelial cells.
Methods
Twenty-four male C57BL6 IL-1 receptor (type I) knockout mice were killed, and small bowel was removed for study. Twenty-four wild-type mice (C57-BL6) served as controls. Body weights, bowel length, and mucosal morphology were examined for phenotypic differences. Apoptosis was quantified by terminal deoxyuridine nick-end labeling (TUNEL) immunohistochemical staining and cellular proliferation by proliferation cell nuclear antigen staining. Whole mucosal protein was analyzed for nuclear factor-κB expression. Groups were analyzed by t test.
Results
The absence of IL-1 type I receptor in knockout mice was verified by reverse transcriptase–polymerase chain reaction. IL-1 receptor null mice were larger than wild-type controls, with a longer small bowel; however, the index of small bowel length to total body weight did not differ between groups. The percentage of apoptotic cells was higher in IL-1 receptor null mice than in wild-type mice; the proliferation index also increased. Mucosal height and other measures of mucosal morphology were not different. Genotypic absence of IL-1 receptors was associated with decreased expression of nuclear factor-κB in whole mucosal protein extracts.
Conclusions
Both apoptosis and proliferation increased in gut epithelial cells of mice without IL-1 receptors, suggesting increased cell turnover with no change in net balance. This model represents an opportunity to examine potential mechanisms of gut epithelial turnover in vivo, under both normal conditions and in conditions of mucosal proliferation and atrophy.
Mucosal epithelium is maintained by a balance in turnover between cell proliferation and cell death by apoptosis. Dysregulation of gut cellular turnover has been implicated in several diseases, among them the modulation of sepsis and multiorgan failure after injury, inflammatory bowel disease, and cancer. Severe cutaneous burn has been shown to cause atrophy of small bowel mucosa, 1–3 which is associated with accelerated turnover of gut epithelial cells. 4 The causes of the increased rates of cellular apoptosis and proliferation are not known.
Burn injury increases expression of several cytokines, one of which is interleukin-1β (IL-1β). 5 IL-1β plays a role in both cell death and survival under appropriate conditions:
1. IL-1β pretreatment rendered glomerular mesangial cells more susceptible to apoptotic death from oxidant stress. 6
2. IL-1β induced thyrocyte apoptosis, which was blocked by the addition of Fas antibodies. 7
3. Treatment of pancreatic islet cells with a combination of IL-1β, tumor necrosis factor (TNF)-α, and interferon-γ increased cell death by apoptosis. 8
4. IL-1β type I receptor antagonist inhibited apoptosis in neurons and fibroblasts. 9
5. Pretreatment with mature IL-1β provided an antiapoptotic effect for induced apoptosis, which was attributed to downregulation of the receptor and thus the signal. 9
Others have shown that IL-1 is a growth factor for a variety of cells. 10,11 For example, IL-1 protects fibroblasts against TNF-mediated cell death by arresting cell cycling. 12
With these findings, we wondered whether IL-1β plays a role in gut mucosal cell turnover by influencing apoptotic cell death. Our hypothesis was that loss of the IL-1 signal would decrease gut epithelial cell apoptosis and improve cell survival. We chose to test this hypothesis in vivo in IL-1 receptor type I knockout mice.
METHODS
Twenty-four IL-1 type I receptor knockout C57-BL6 mice were killed by decapitation after acclimation to standard laboratory animal environment. Total body weight was measured before they were killed, and the small bowel was taken for analysis. Absence of IL-1 type I receptors was verified by reverse transcriptase–polymerase chain reaction analysis with the nonsense allele. 13 Twenty-four age-matched wild-type mice (C57-BL6) raised in the same laboratory were killed under the same conditions as controls. Mice were fed ad libitum before being killed. This study was approved by the Animal Care and Use Committee of the University of South Florida, Tampa.
In pilot studies of small bowel epithelial homeostasis, we found the proximal gut to be more responsive to experimental manipulation than the distal bowel. Therefore, we focused on the proximal small bowel. After measuring the length of the small bowel (ligament of Treitz to the ileocecal valve) from each animal, the proximal 10-cm segment was isolated. A 1-cm segment was excised from the proximal end and was immersed immediately in 10% buffered formalin for fixation and histologic analysis. The remaining 9 cm was immediately flushed with saline, opened longitudinally, and blotted dry. The mucosa was then carefully scraped from the underlying seromuscular layer with a glass slide and was frozen immediately in liquid nitrogen for storage at −70°C.
Immunohistochemistry
We used the terminal deoxyuridine nick-end labeling (TUNEL) immunohistochemical method (Apoptag; Oncor, San Francisco, CA) to identify apoptotic cells. 4 Briefly, formalin-fixed tissue was processed and embedded in paraffin. Sections of 3 μm were deparaffinized, rehydrated in graded alcohol (100%, 95%, and 70%), and washed with deionized water. Protein was digested using proteinase K (20 μL/mL in phosphate-buffered saline [PBS]), and endogenous peroxidase activity was quenched with 2% H2O2 in PBS. Seventy-five μL equilibration buffer was placed on each section, and then diluted terminal proxythymidine (1:1) enzyme solution was applied and incubated at 37°C for 1 hour. After incubation, slides were placed in stop/watch buffer and 55 μL antidigoxigenin peroxidase was added, followed by incubation at room temperature for 30 minutes. The sections were washed, and diaminobenzidine-hydrogen peroxide was applied for color development. Sections were then counterstained with either 1% methyl green or hematoxylin, and mounted for examination.
Six sections of each block of tissue obtained at 40- to 50-μm intervals were examined. In each section, 10 full-length villi were selected for counting TUNEL-positive cells. Apoptotic cells were identified by either brown staining in the nucleus or by apoptotic bodies engulfed in adjoining cells. Intraepithelial lymphocytes were excluded by morphology. All epithelial cells in the villi were counted, and apoptosis was expressed as a percentage of apoptotic cells for each section. Values for all six histologic sections were averaged to reach a percentage apoptosis for each mouse.
Proliferation was measured in a similar fashion, by immunohistochemical staining for proliferating cell nuclear antigen (PCNA). Deparaffinized and rehydrated sections were incubated with PCNA-horseradish peroxidase conjugate (Santa Cruz Biotechnology, SC-56, Santa Cruz, CA) at a 1:50 dilution overnight at 4°C and washed with PBS, and diaminobenzidine-hydrogen peroxide was applied for color development. The tissue studied was from identical bowel segments used in the apoptosis study. The sections studied were from the same tissue blocks used for apoptosis quantitation. Hematoxylin was used for counterstaining. PCNA-positive cells were counted on six sections from each mouse. Proliferation was expressed as a percentage of PCNA-positive cells among the total cells counted from the base of the crypt to the villus tip. Ten such determinations were made from each section and averaged to reach a percentage of proliferating cells for each mouse. In both the TUNEL and PCNA studies, counting was done by a person unaware of the receptor status of the tissue.
Mucosal Height and Morphology
As an estimate of mucosal height, 10 complete villi from each section were measured for crypt depth and villus height. Measurements were made with a calibrated micrometer attached to the microscope eyepiece, villus width at midvillus was measured in the same 10 villi, and cell size was graded on a scale from 1 to 4 by a masked observer. The sections were randomly distributed between groups.
Western Blotting for Nuclear Factor-κB Protein Expression
Pooled whole mucosal extracts (40 μg) from each group were dissolved in SDS-PAGE (10% acrylamide) and electrotransferred to an Immobilon membrane (Millipore, Bedford, MA). Membranes were incubated in PBS containing 5% nonfat milk for 60 minutes at room temperature to block nonspecific binding and then incubated with nuclear factor (NF)-κB p65 (C-20) rabbit polyclonal antibody (Santa Cruz Biotechnology, SC-372, Santa Cruz, CA). After incubation with the primary antibody, the blots were washed in PBS containing 0.1% Tween and incubated with the secondary antibody. The filters are washed four times with PBS containing 0.1% Tween. To detect the immunocomplexes, the ECL-Western blotting detection system (Amersham, Piscataway, NJ) was used.
Statistical Analysis
Statistical analysis was performed by the Student t test and the Kruskal-Wallis method for between-group comparisons.
RESULTS
Age-matched mice without IL-1β receptors were larger than wild-type mice (24.9 ± 8 g vs. 20.7 ± 0.3 g;P < .05). IL-1 receptor knockout mice also had longer jejunoileal (small bowel) lengths (36.4 ± 0.9 cm vs. 31.6 ± 0.2 cm;P < .05). The ratios of small bowel length to total body weight were not different between groups, indicating a proportional increase in small bowel length for increased total body weight (Fig. 1). Measures of mucosal morphology (villus height, crypt depth, cell size, villus width) were not different between groups (Table 1). Apoptosis was increased significantly in IL-1 receptor knockout mice compared with wild-type mice (Fig. 2). PCNA analysis showed significant increases in proliferating cells in the IL-1 receptor knockout mice compared with wild-type animals (Fig. 3).
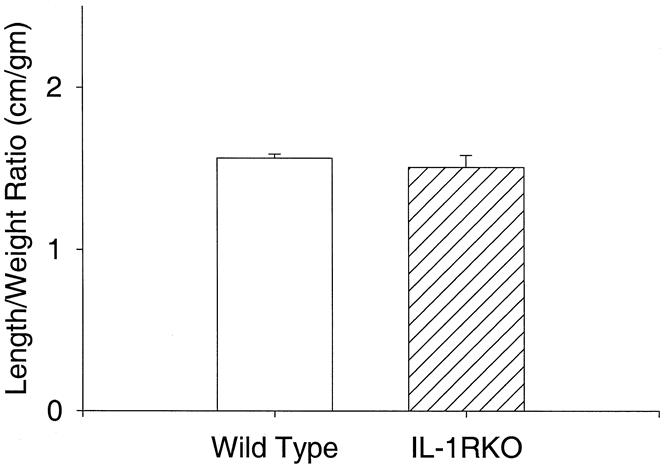
Figure 1. Small bowel length to total body weight ratios. No differences were found between groups, indicating proportional increases in gut mass for total body mass.
Table 1. MUCOSAL MORPHOLOGY MEASUREMENTS
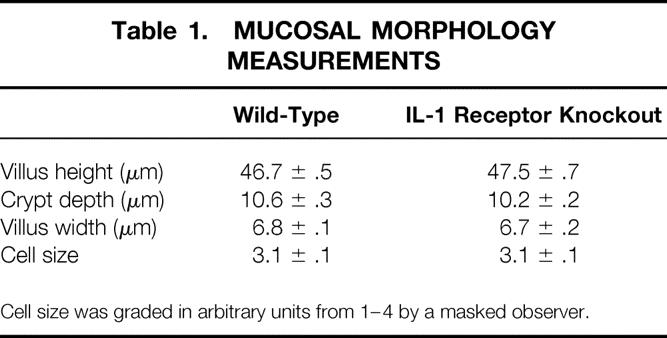
Cell size was graded in arbitrary units from 1–4 by a masked observer.
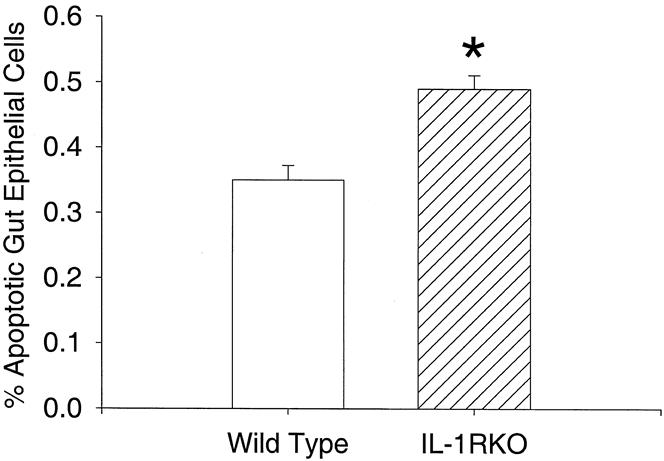
Figure 2. Percentage of apoptotic cells in proximal small bowel epithelium. *P < .001.
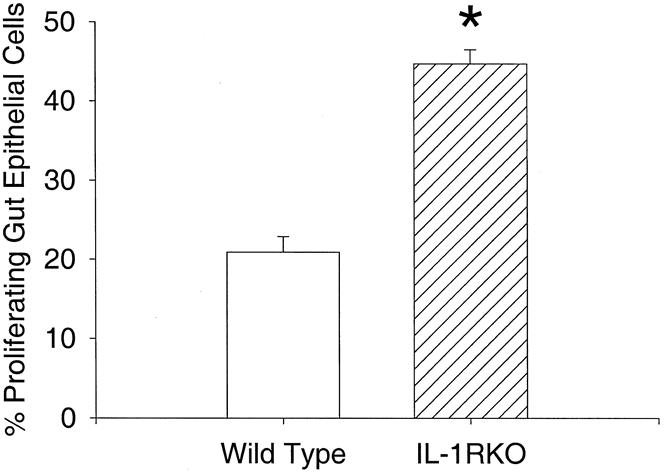
Figure 3. Percentage of proliferating cells in the proximal gut epithelium. *P < .001.
Recent evidence suggests that IL-1 receptor signals are transduced through NF-κB. 14,15 We examined the expression of NF-κB in whole mucosal extracts from the proximal bowel and found decreased NF-κB expression in IL-1 receptor knockout animals compared with controls (Fig. 4).
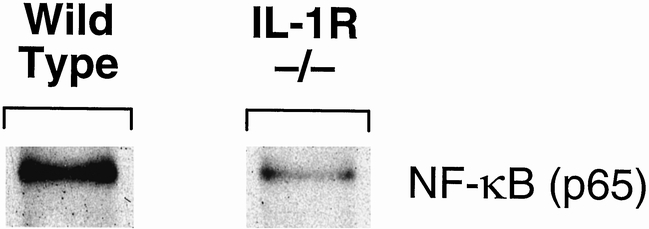
Figure 4. Nuclear factor-κB expression by Western blot.
DISCUSSION
Interleukin-1β is linked to both increased apoptosis and increased cell survival under differing conditions in vitro. 6–12 In most of these studies, IL-1β stimulated programmed cell death, which was inhibited by IL-1β antagonists. This led to our hypothesis that apoptosis decreases in mice without IL-1 receptors in the gut epithelium. We found that cell death did not decrease but actually increased in mice without IL-1 type I receptors. This increase was matched by an increase in proliferation, presumably to maintain cellular balance. These findings indicate increased gut epithelial cell turnover in mice without IL-1 receptors.
We found that IL-1β receptor knockout mice were phenotypically larger with a longer small bowel than wild-type mice. Mucosal height was not grossly different, indicating proportional increases in mucosal mass for body weight, and no hypertrophy or atrophy relative to the wild-type animals. The rate of cell death must be roughly equal to the rate of cell proliferation to maintain gut homeostasis. Then, either cell death or cell proliferation was stimulated by the absence of the IL-1 receptor. The opposite factor then increased in compensation. An alternate hypothesis would be that both apoptosis and proliferation were stimulated. In this model, we could not assess which of the three possibilities incited these findings.
IL-1 signals are transduced through the type I receptor, which has two ligands, IL-1α and IL-1β. IL-1α is normally produced and released in mice and has been proposed to regulate cellular differentiation. 16–18 IL-1β is not normally expressed except under inflammatory stimuli. The animals in this experiment were not stressed; therefore, an explanation for these findings may lie in the absence of the IL-1α effects on cellular homeostasis.
As a corroborative measure of altered cellular regulation in IL-1 receptor knockout mice compared with wild-type mice, we chose to examine NF-κB expression in whole mucosal extracts. IL-1 type I receptors activate NF-κB 19; NF-κB activation was required for cytoprotective responses to TNF-induced apoptosis, although it was insufficient alone to inhibit apoptosis. 20 We found that NF-κB expression was decreased in the gut mucosa of IL-1 receptor knockout mice, and this was associated with increased gut epithelial apoptosis in vivo. These findings bolster the conclusion that NF-κB has cytoprotective effects, although the mechanisms by which this occurs are unknown. We are examining the genetic mechanisms responsible for these findings.
In summary, we found that IL-1 receptor knockout mice have relatively similar gut mucosal mass and morphology to wild-type mice. However, the knockout mice have increased rates of apoptosis and proliferation, characterizing increased cellular turnover. This increased turnover was associated with decreased expression of NF-κB, which corroborates the finding of increased apoptosis. This model represents an opportunity to examine potential mechanisms of gut mucosal turnover.
Footnotes
Correspondence: Steven E. Wolf, MD, Shriners Burns Hospital-Galveston, 815 Market St., Galveston TX 77550.
Supported by NIH Grant DDK-34567806 and Shriners Hospitals for Children Grant 8570.
Presented at the Association for Academic Surgery, Seattle, WA, November 20–24, 1998.
E-mail: swolf@sbi.utmb.edu
Accepted for publication December 10, 1999.
References
- 1.Chung DH, Evers BM, Townsend CM Jr, et al. Burn-induced transcriptional regulation of small intestinal ornithine decarboxylase. Am J Surg 1992; 163:157–163. [DOI] [PubMed] [Google Scholar]
- 2.Huang KF, Chung DH, Herndon DN. Insulin-like growth factor reduces gut atrophy and bacterial translocation after severe burn injury. Arch Surg 1993; 128:47–54. [DOI] [PubMed] [Google Scholar]
- 3.Chung DH, Evers BM, Townsend CM Jr, et al. Role of polyamine synthesis during gut mucosal adaptation after burn injury. Am J Surg 1993; 165:144–149. [DOI] [PubMed] [Google Scholar]
- 4.Wolf SE, Ikeda H, Matin S, et al. Cutaneous burn increases apoptosis in the gut epithelium of mice. J Am Coll Surg 1999; 188:10–16. [DOI] [PubMed] [Google Scholar]
- 5.Vindenes HA, Ulvestad E, Bjerknes R. Concentrations of cytokines in plasma of patients with large burns: their relation to time after injury, burn size, inflammatory variables, infection, and outcome. Eur J Surg 1998; 164:647–656. [DOI] [PubMed] [Google Scholar]
- 6.Yokoo T, Kitamura M. IL-1β depresses expression of the 70 kD heat shock protein and sensitizes glomerular cells to oxidant-initiated apoptosis. J Immunol 1997; 159:2886–2892. [PubMed] [Google Scholar]
- 7.Giordano C, Stassi G, DeMaria R, et al. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science 1997; 275:960–963. [DOI] [PubMed] [Google Scholar]
- 8.Delaney CA, Pavlovic D, Hoorens A, et al. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology 1997; 138:2610–2614. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander RM, Gagliardini V, Rotello RJ, Yuan J. Functional role of IL-1β in IL-1B converting enzyme mediated apoptosis. J Exp Med 1996; 184:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonnemann G, Shapiro L, Engler-Blum G, et al. Cytokines in human renal interstitial fibrosis. I. Interleukin-1 is a paracrine growth factor for cultured fibrosis derived kidney fibroblasts. Kidney Int 1995; 57:837–834. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Warner SJ, Friedman GB. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth inhibitory prostanoids. J Clin Invest 1988; 81:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belizario JE, Dinarello CA. Interleukin 1, interleukin 6, tumor necrosis factor, and transforming growth factor β increase cell resistance to tumor necrosis factor cytotoxicity by growth arrest in the G1 phase of the cell cycle. Cancer Res 1991; 51:2379–2385. [PubMed] [Google Scholar]
- 13.Denham W, Yang J, Fink G, et al. Gene targeting demonstrates additive detrimental effects of interleukin 1 and tumor necrosis factor during pancreatitis. Gastroenterology 1997; 113:1741–1746. [DOI] [PubMed] [Google Scholar]
- 14.Zhang FX, Kirschning CJ, Mancinelli R, et al. Bacterial lipopolysaccharide activates nuclear factor kappa B through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem 1999; 274:7611–76114. [DOI] [PubMed] [Google Scholar]
- 15.Awane M, Andres PG, Li DJ, Reinecker HC. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J Immunol 1999; 162:5337–5344. [PubMed] [Google Scholar]
- 16.Dinarello CA. Biologic basis for IL-1 in disease. Blood 1996; 87:2095–2147. [PubMed] [Google Scholar]
- 17.Maier JA, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science 1990; 249:1570–1574. [DOI] [PubMed] [Google Scholar]
- 18.Maier JA, Statuto M, Ragnotti G. Endogenous interleukin 1 alpha must be transported to the nucleus to exert its activity in human endothelial cells. Mol Cell Biol 1994; 14:1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung K, Betts JC, Xu L, Nabel GJ. The cytoplasmic domain of the interleukin-1 receptor is required for nuclear factor-kappa B signal transduction. J Biol Chem 1994; 269:1579–1582. [PubMed] [Google Scholar]
- 20.Natoli G, Costanzo A, Guido F, et al. Nuclear factor κB-independent cytoprotective pathways originating at tumor necrosis factor receptor-associated factor 2. J Biol Chem 1998; 273:31262–31272. [DOI] [PubMed] [Google Scholar]


