Abstract
Objective
To analyze the clinical value of reverse transcriptase–polymerase chain reaction (RT-PCR) recognition of mRNA coding for carcinoembryonic antigen (CEA) and cytokeratin 20 in blood obtained from patients with colorectal carcinoma.
Summary Background Data
RT-PCR has been applied to identify very small numbers of tumor cells. Molecular detection is thought to provide useful information for the clinical management of perioperative prophylaxis of tumor cell implantation or postoperative adjuvant therapy regimens.
Methods
From 52 patients with colorectal cancer, peripheral blood specimens were obtained before and after surgical manipulation; also, a specimen of mesenteric venous blood draining the colorectal tumor was obtained just before tumor resection. Using cDNA primers specific for CEA and cytokeratin 20, RT-PCR was performed to detect tumor cells. Subsequently, the 52 patients were divided into two groups, a group positive for both CEA and cytokeratin 20 and a group negative for CEA, cytokeratin 20, or both.
Results
On the basis of 450 days of follow-up data, the PCR-positive group had a significantly shorter overall survival than the PCR-negative group only with the mesenteric venous blood specimens. Multivariate analysis indicated that detection of the simultaneous presence of CEA and cytokeratin 20 mRNA in mesenteric venous blood is a potent prognostic factor independent of the traditional pathologic parameters. Of the eight peripheral blood specimens found to be PCR-positive, five showed a change of PCR from negative to positive during surgery, and liver metastases developed 11 months later in one of these five patients.
Conclusions
Molecular detection of both CEA and cytokeratin 20 mRNA in mesenteric venous blood may be of prognostic value for patients with colorectal carcinoma. Molecular detection in the peripheral blood at surgery suggests that hematogenic tumor cell dissemination is a common and early event and that surgical manipulation enhances this release of tumor cells into the circulation.
Approximately 20% to 45% of colorectal cancer patients who undergo a histologic curative surgical procedure nevertheless subsequently develop metastatic disease, and the sites of metastases are lymph nodes, peritoneum, liver, lung, and bone marrow. 1,2 In an attempt to increase the sensitivity of detection of these early metastases, several approaches have been used. Using conventional cytologic and immunohistochemical methods, colorectal cancer cells have been detected in peripheral and mesenteric venous blood draining tumors. 3–5 However, the prognostic and clinical value of this phenomenon is not clear. 4 Further technical advances have made it possible to detect micrometastases at the molecular level in bone marrow and even in circulating blood. 6–8 More recent studies have reported and discussed the clinical significance of such detection. 9–13
In this study, we detected the presence of carcinoembryonic antigen (CEA) mRNA and cytokeratin 20 mRNA in the circulating blood specimens of patients with colorectal carcinoma. Because CEA mRNA is expressed in the vast majority of epithelial cells but not in nonepithelial cells, and this expression can be detected at the molecular level despite serum CEA (protein) volumes, we used it as a promising marker of epithelial cells. 7,14 Despite using well-designed CEA-specific primers, 14 false-positive results were observed in our previous study 15 as well as those of other investigators. 16,17 To increase the specificity, we used in addition cytokeratin 20, a member of the epithelial subgroup of the multigene family of intermediate filament proteins and an important constituent of the mammalian cytoskeleton. 18 The expression of cytokeratin 20 is almost entirely confined to the gastrointestinal epithelium, urothelium, and Merkel cells; it has not been found in normal hematopoietic cells. 19 On the basis of our results, we discuss the clinical value of the detection of CEA and cytokeratin 20 mRNA in the circulating blood.
PATIENTS AND METHODS
Patients
Fifty-two colorectal cancer patients aged 24 to 86 years were evaluated (32 men, 20 women). UICC staging showed 5 stage I patients, 17 stage II, 21 stage III, and 9 stage IV. Of the stage IV group, eight patients had liver metastases and one had lung metastases at surgery. The surgical resections (20 right hemicolectomy, 3 transverse colectomy, 15 sigmoidectomy, 10 lower anterior resection, 4 abdominoperineal resection) were performed at more than 10 cm from the edge of the primary tumor, with lymphadenectomy of pericolic, perirectal, and named vascular truncal regions, in our department from July 1997 through November 1997. Patients whose tumor could not be resected were excluded. Peripheral blood was collected both before and after surgical manipulation, and mesenteric venous blood draining the tumor was collected just before the tumor resection.
As a control group, we also obtained peripheral blood from 10 patients with benign disease (6 cholelithiasis patients who had undergone laparoscopic cholecystectomy, 1 patient with a benign mammary tumor, 2 inguinal hernias, and 1 umbilical hernia) at surgery and 10 healthy volunteers. Extraction of RNA from blood specimens was carried out within 4 hours after collection.
Cell Lines
Colorectal cancer cell lines (colo 320) were also investigated as positive controls for the detection sensitivity in these experiments.
Isolation of Total RNA
Isolation was performed on 10-mL peripheral blood specimens (before and after surgical manipulation) and a 10-mL mesenteric venous blood specimen on Ficoll-Isopaque (Pharmacia, Freiburg, Germany), and total RNA was extracted with ISOGEN (Nippon Gene, Osaka, Japan) according to the manufacturer’s instructions. The purity of recovered RNA was determined using Ultraspec 2000 (Pharmacia, Cambridge, UK).
Reverse Transcriptase–Polymerase Chain Reaction
Total RNA (isolated from 2.0 μg) diluted in 10 μL DEPC-treated distilled water was denatured at 70° for 10 minutes and quickly chilled on ice. The cDNA was synthesized in a 20-μL reaction mixture containing 4 μL 5 × first-strand buffer, 500 μmol/L dNTP, 100 μmol/L solution of random primers, and 400 units of Moloney Leukemia Virus Reverse Transcriptase (Gibco BRL, Gaithersburg, MD). The reaction mixture was incubated at 42° for 60 minutes and then heated to 90° for 2 minutes to inactivate the reverse transcriptase, and the mixture was stored at −20°.
To check the integrity of the synthesized cDNA, 2 μL of the mixture was subjected to reverse transcriptase–polymerase chain reaction (RT-PCR) for glyceraldehyde-3-phosphate dehydrogenase. 20
We adopted a two-step PCR for the amplification of CEA cDNA and cytokeratin 20 cDNA to enhance the specificity. Nested PCR with CEA was performed with CEA-specific primers. 14 Nested PCR with cytokeratin 20 was based on our previous study 15 using the following cytokeratin 20-specific primers: A: 5′-CGT CTA ACA GTG GAA GCT GAT CTC-3′ for the outer sense, B: 5′-TCG GGC GTT CCA TGT TAC TC-3′ for the outer antisense, C: 5′-AAG CAT CTG GGC AAC ACT GTC A-3′ for the inner sense, D: 5′-AAC GGG CCT TGG TCT CCT CTA-3′ for the inner antisense.
The first cytokeratin 20 PCR was performed using primers A and B, followed by the second PCR using primers C and D. The final PCR products of CEA and cytokeratin 20 mRNA were a 132-bp and a 313-bp DNA fragment, respectively.
The two-step PCR was carried out as follows: for the first PCR, 2 μL cDNA was blended into 23 μL reaction mixture including 2.5 μL 10 × buffer (10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, and 1.5 mmol/L MgCl2), 200 μmol/L dNTP, 0.5 μmol/L of each primer, 0.625 units Taq DNA polymerase (Takara Biomedicals, Otsu, Japan), and 12.5 μL DEPC-treated distilled water. A PCR thermal cycler MP (Takara Biomedicals, Otsu, Japan) was used for the reactions. The first reaction was carried out for 20 cycles for CEA (95°, 1 minute; 72°, 2 minutes) and for 30 cycles for cytokeratin 20 (95°, 1 minute; 67°, 1 minute; 72°, 1 minute) with a final step of 10 minutes. Two microliters of the first PCR product was transferred into a second tube and amplified as follows: 25 cycles for CEA (95°, 1 minute; 69°, 1 minute; 72°, 1 minute) and 30 cycles for cytokeratin 20 (95°, 1 minute; 67°, 1 minute; 72°, 1 minute) with a final step of 10 minutes. We reexamined each specimen at least once to confirm the accuracy. The PCR products were subjected to electrophoresis on 2% agarose gels and visualized using ethidium bromide. The amplified products were sequenced using a fmol DNA Cycle Sequencing System (Promega, Madison, WI) and determined to be identical to those expected.
Cell Spiking
Cell spiking experiments were used to test the potential sensitivity of this technique for detection of colon cancer cells in blood. To confirm the sensitivity, known numbers of colo 320 cells were added to whole blood specimens, total RNA was extracted, RT-PCR was performed for both CEA and cytokeratin 20 mRNA, and subsequently the nested PCRs were performed with each primer.
Statistical Analysis
The Fisher exact probability test was used for the statistical analyses relating to the detection of PCR and the traditional clinical pathologic parameters (divided into two groups, early groups vs. advance groups). The Mann-Whitney test was used to compare means. Data are presented as mean values ± standard error of the mean. Kaplan-Meier survival curves were constructed and analyzed by the log-rank test. The influence of each variable on survival was assessed by a Cox regression analysis. All tests were performed using SPSS for Windows Release 8.01J (SPSS Japan Inc., Tokyo, Japan). P < .05 was considered to indicate statistical significance.
RESULTS
Overall, 52 paired mesenteric venous blood and peripheral blood specimens, each pair from the same patient with colorectal cancer, and 20 peripheral blood specimens from 10 benign disease patients and 10 healthy volunteers were examined with the CEA- and cytokeratin 20-specific nested PCR.
Cell Lines
The sensitivity of the CEA and cytokeratin 20 nested RT-PCR was investigated by cell spiking experiments, which confirmed that 101 colo 320 cells diluted in 2 mL human whole blood could be identified (Fig. 1).
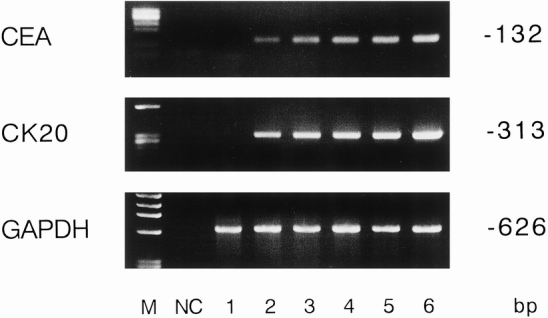
Figure 1. Different numbers of cancer cells were added to 2 mL whole blood. The number of tumor cells in the lanes is 0, 101, 102, 103, 104, and 105 from left to right (lanes 1–6). (M, molecular weight marker; NC, water negative control.)
Control Blood and Patient Blood Analysis
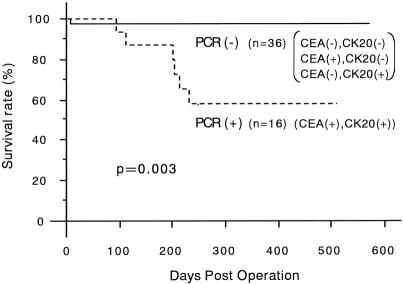
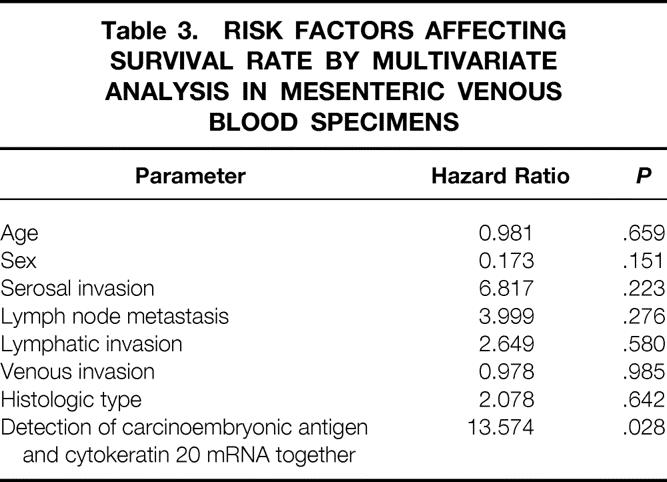
Of the 52 mesenteric venous blood specimens, 23 displayed a distinct and clearly visible CEA and/or cytokeratin 20 PCR product (Fig. 2). A CEA PCR product was detected in 20 of 52 (38.4%) and a cytokeratin 20 PCR product in 19 of 52 (36.5%). The RT-PCR results in each stage are shown in Table 1. The association between the detection of CEA and cytokeratin 20 mRNA together and clinicopathologic parameters is shown in Table 2. A significant association was found between PCR detection and stage (P = .006), depth of invasion (P = .018), and nodal metastases (P = .034). The prognosis of the PCR-positive group was significantly shorter than that of the PCR-negative group (P = .003;Fig. 3). Multivariate analysis indicated that detection of both CEA and cytokeratin 20 mRNA together (P = .028) was an independent prognostic factor (Table 3).
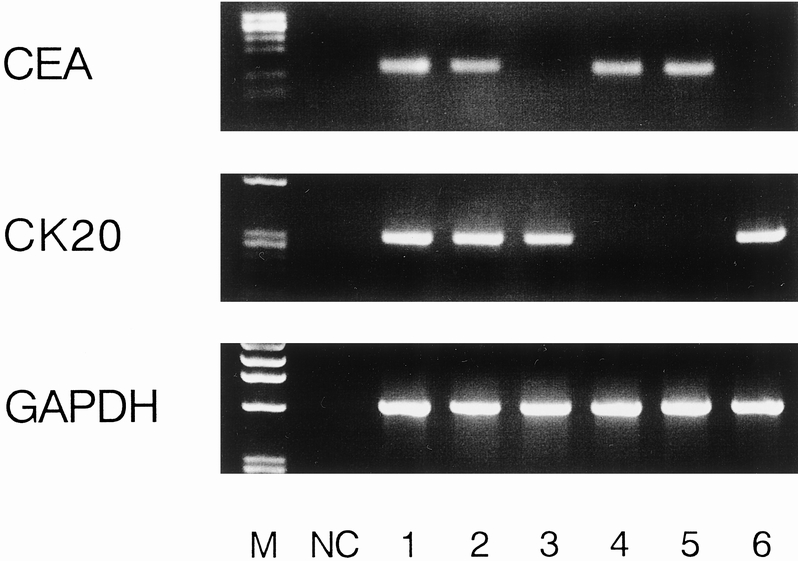
Figure 2. Representative results of reverse transcriptase–polymerase chain reaction assay using mesenteric venous blood specimens. Lanes 1 and 2 were obtained from the two stage IV patients; lanes 3, 4, 5, and 6 were obtained from the four stage III patients.
Table 1. RESULTS OF NESTED RT-PCR IN MESENTERIC VENOUS BLOOD WITH CEA AND CK20 BY TUMOR STAGE
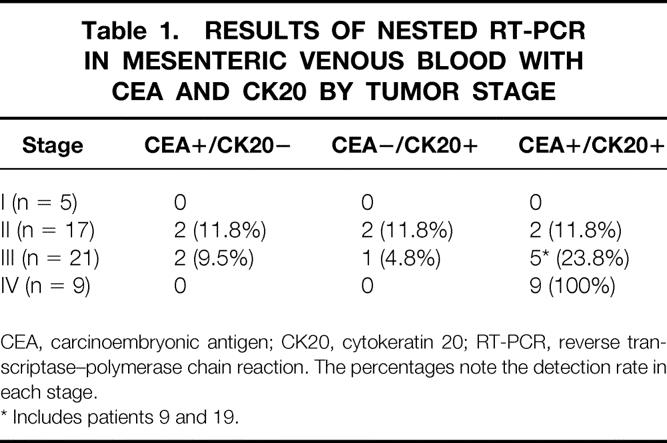
CEA, carcinoembryonic antigen; CK20, cytokeratin 20; RT-PCR, reverse transcriptase–polymerase chain reaction. The percentages note the detection rate in each stage.
* Includes patients 9 and 19.
Table 2. RELATION BETWEEN DETECTION OF CEA AND CK20 mRNA TOGETHER IN MESENTERIC VENOUD BLOOD AND THE CLINICOPATHOLOGIC CHARACTERISTICS
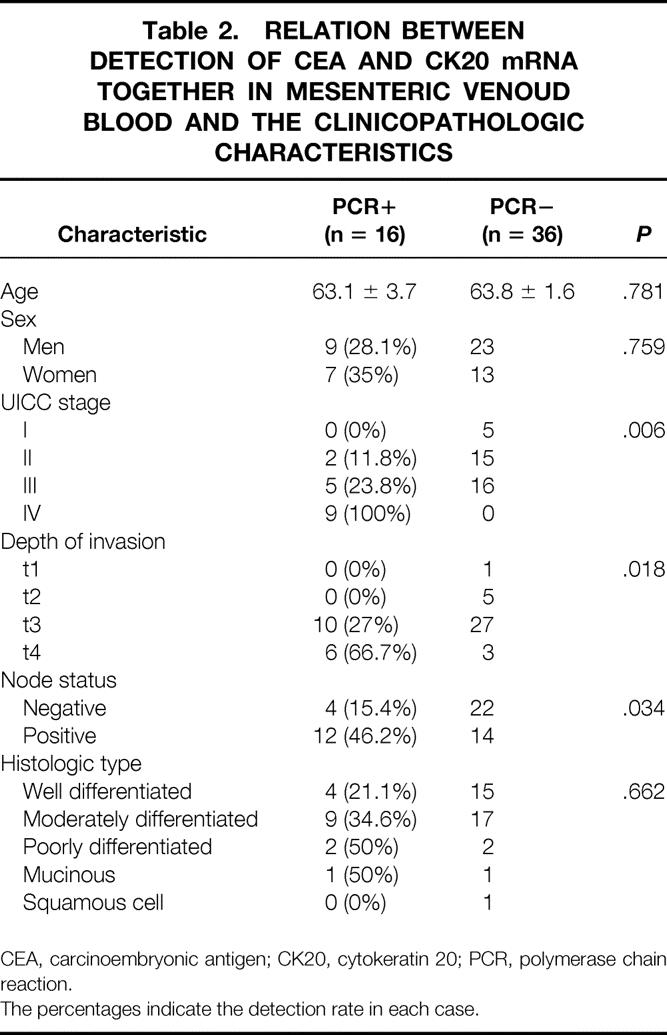
CEA, carcinoembryonic antigen; CK20, cytokeratin 20; PCR, polymerase chain reaction.
The percentages indicate the detection rate in each case.
Figure 3. Survival curves of the 52 patients with colorectal cancer according to the expression of both carcinoembryonic antigen and cytokeratin 20 mRNA in the mesenteric venous blood specimens. The survival curves were determined by the Kaplan-Meier method. The negative group includes one patient, patient 40, who died of pulmonary vein thrombosis on postoperative day 5 (P value determined using log-rank test).
Table 3. RISK FACTORS AFFECTING SURVIVAL RATE BY MULTIVARIATE ANALYSIS IN MESENTERIC VENOUS BLOOD SPECIMENS
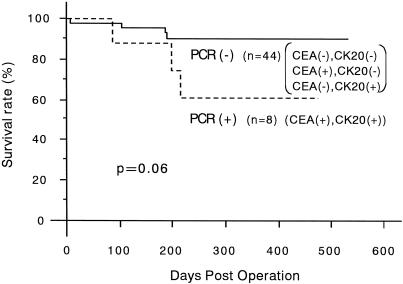
With regard to peripheral blood specimens, 37 specimens from patients with colorectal cancer displayed a distinct and clearly visible PCR product, whereas none of the 20 specimens from 10 patients with benign disease and 10 healthy volunteers did (Fig. 4). The PCR results for each stage for specimens collected before and after surgical manipulation are summarized in Table 4. The relation between the detection of both CEA and cytokeratin 20 mRNA together and clinicopathologic parameters is shown in Table 5. A significant association was found between PCR detection and age (P = .012) and histologic type (P < .001). The difference in prognosis between the PCR-positive and PCR-negative groups was not significant (P = .06;Fig. 5), and multivariate analysis indicated that detection of both CEA and cytokeratin 20 mRNA together, like other factors, was not an independent prognostic factor (Table 6).
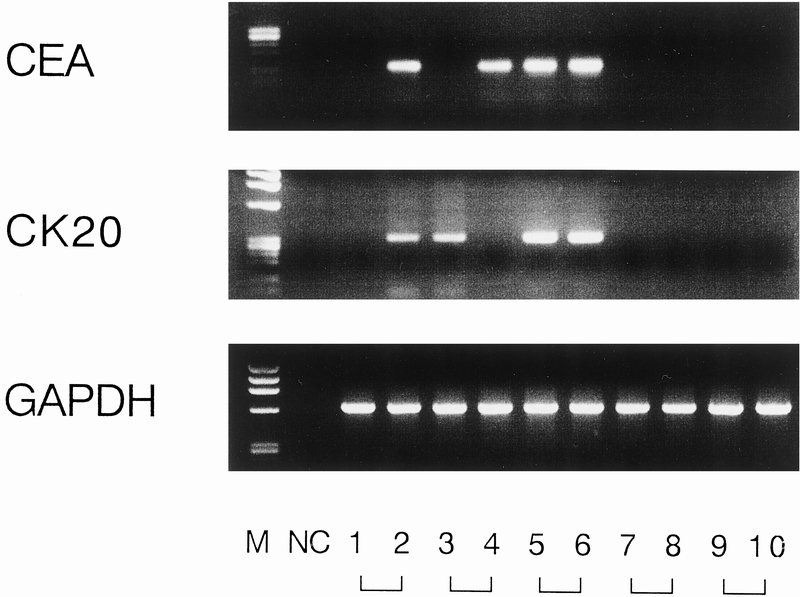
Figure 4. Representative results of reverse transcriptase–polymerase chain reaction assay using peripheral blood specimens. Lanes 1 and 2 were obtained from patient 19 (stage III) before and after surgery, respectively; lanes 3 and 4 were from patient 9 (stage III); lanes 5 and 6 from patient 35 (stage IV); lanes 7 and 8 from patient 15 (stage I); and lanes 9 and 10 from a patient with benign disease (laparoscopic cholecystectomy).
Table 4. RESULTS OF NESTED RT-PCR IN PERIPHERAL BLOOD SPECIMENS*
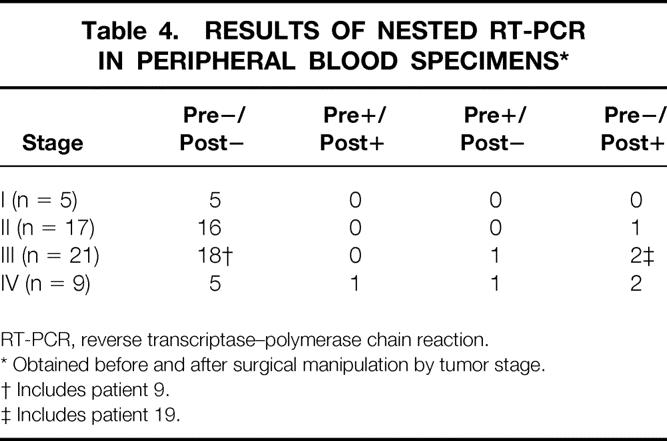
RT-PCR, reverse transcriptase–polymerase chain reaction.
* Obtained before and after surgical manipulation by tumor stage.
† Includes patient 9.
‡ Includes patient 19.
Table 5. RELATION BETWEEN DETECTION OF CEA AND CK20 mRNA TOGETHER IN PERIPHERAL BLOOD AND CLINICOPATHOLOGIC CHARACTERISTICS
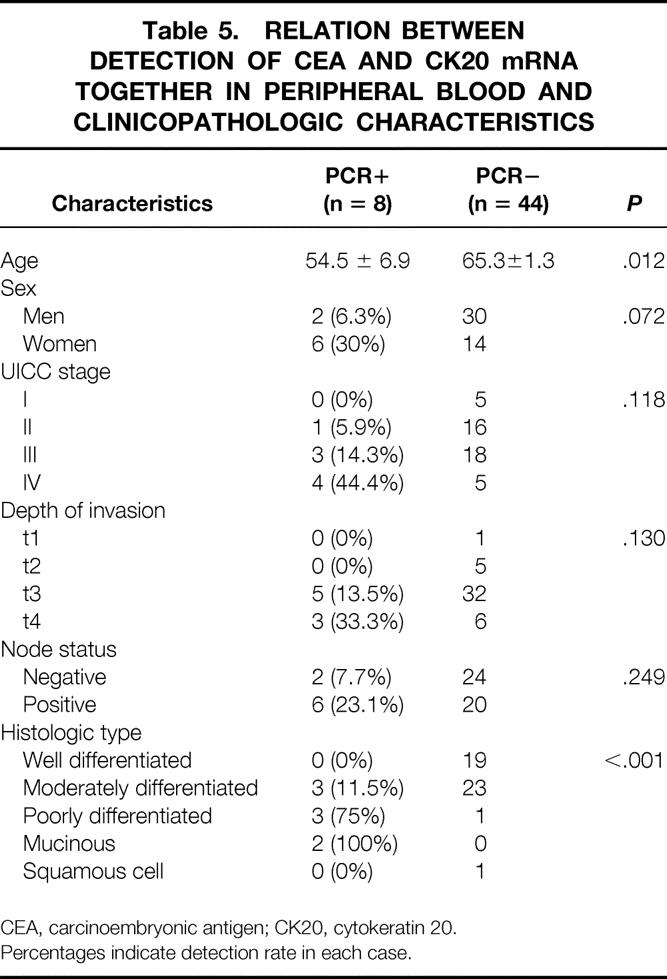
CEA, carcinoembryonic antigen; CK20, cytokeratin 20.
Percentages indicate detection rate in each case.
Figure 5. Survival curves of the 52 patients with colorectal cancer according to the expression of both carcinoembryonic antigen and cytokeratin 20 mRNA in peripheral blood specimens. The negative group includes patient 40 (P value determined using log-rank test.)
Table 6. RISK FACTORS AFFECTING SURVIVAL RATE BY MULTIVARIATE ANALYSIS IN PERIPHERAL BLOOD SPECIMENS
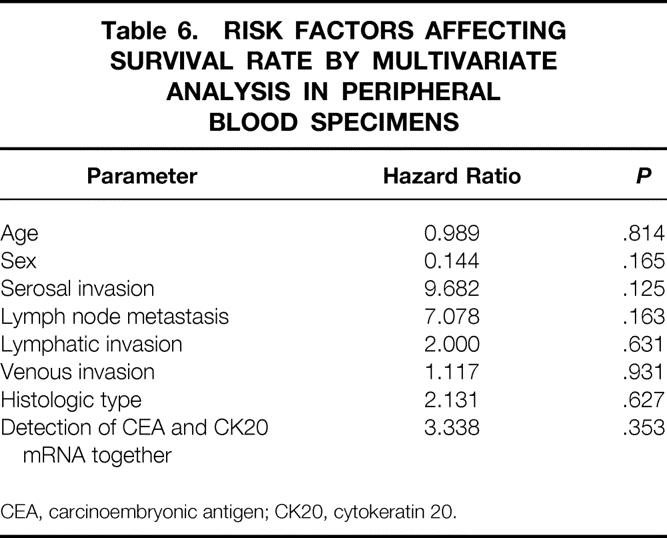
CEA, carcinoembryonic antigen; CK20, cytokeratin 20.
DISCUSSION
There have been several reports on RT-PCR detection of very small numbers of cancer cells in circulating blood, 6–8 including mesenteric venous blood. 21 Recently, the prognostic and clinical value of this molecular detection method has been the focus of discussion. 9–13 We have demonstrated previously that RT-PCR using CEA mRNA and cytokeratin 20 mRNA 15 is a highly specific and sensitive detection method for the presence of colorectal cancer cells in lymph nodes. Here we have used an improved version of this method to analyze the peripheral blood and mesenteric venous blood, and as a result we were able to show the clinical value of this molecular detection. In previous studies, many target genes have been used to detect micrometastases, such as CEA, 7,13,15 mucin 1 (MUC1), 22 gastrointestinal tumor-associated antigen 733.2 (GA733.2), 23 cytokeratin 8, 6 cytokeratin 18, 24 cytokeratin 19, 6,22,25 and cytokeratin 20. 6 Although it has been reported that unlike the other target genes, cytokeratin 20 mRNA was not detected in any normal blood, 2,8,25 recently false-positive results have been detected with cytokeratin 20 in normal control blood. 9,10 However, the lower frequency (3–8%) of false-positive results compared with CEA (0–33%) 12,16,17 and the absence of a cytokeratin 20 pseudogene suggest that cytokeratin 20 may be a more useful target for RT-PCR detection of epithelial-derived cancers than previously described target genes. In this study, to overcome the false-positive problem with CEA and also cytokeratin 20, we defined PCR-positive as the situation where both CEA and cytokeratin 20 were positive.
In the mesenteric venous blood specimens, the detection rate of both CEA and cytokeratin 20 was 16 of 52 (31%). There has been only one previous study using mesenteric venous blood, with a detection rate of 4%. 21 As the report suggested, the low detection rate might be due to their K-ras point mutation system. However, another study analyzing intraoperative portal blood from patients with pancreatic cancer reported 100% detection using RT-PCR with CEA mRNA. 26 Our detection rate in mesenteric venous blood was similar to that in previous testing for bone marrow. 8,9 The detection rate with combined CEA and cytokeratin 20 increased with the stage of the tumor, and there were significant differences in the expression of CEA and cytokeratin 20 mRNA between the early stages I/II and the advanced stages III/IV (P = .035, Fisher exact test; see Table 1). Especially in stage IV, all of the nine cases were detected in combined CEA and cytokeratin 20 testing. However, in terms of depth of invasion, the ratios of the PCR-negative results in t3 and t4 cases were 73% and 33.3%, respectively. One possible explanation is that tumor cells may be intermittently flowing into the bloodstream of the bowel wall; another is that sampling errors may have occurred (see Table 2). 7,27 A third possible explanation is heterogeneity of the tumor cells. It cannot be ruled out that the expression of CEA differs between circulating tumor cells and the primary tumor. As with the detection rate by stage, it is significant that the detection rate increased with advancing depth of invasion (Fisher exact test).
Because our study was prospective, the determination of CEA and cytokeratin 20 mRNA expression preceded the prognostic analysis. All analyses were performed without knowledge of the corresponding clinical data and the postoperative therapeutic schedules. The actual results of analysis of the association between the expression of CEA and cytokeratin 20 mRNA together and the survival time after surgery demonstrated the prognostic value of the detection (see Fig. 3 and Table 3). Thirty-seven patients with normal serum CEA levels also showed the same association between the expression of both types of mRNA and the survival time (P = .0143, data not shown). Of the 43 patients without liver metastases at surgery, two patients (patients 9 and 19 in Table 1) whose mesenteric venous blood specimen expressed both CEA and cytokeratin 20 mRNA developed liver metastases 7 and 11 months later, respectively.
However, in peripheral blood specimens, the detection rate of CEA and cytokeratin 20 mRNA together was 8 of 52 (15.4%), which was consistent with that in a previous study. 9 The rate of detection was significantly lower than in mesenteric venous blood specimens (P = .033, McNemar test). Of the seven patients with liver metastases at surgery, three (42.9%) expressed both CEA and cytokeratin 20 mRNA in the peripheral blood specimens, whereas in the mesenteric venous blood specimen, both CEA and cytokeratin 20 mRNA were detected in all patients. It is obvious that one reason for the lower detection rate in peripheral blood is that the tumor cells are diluted in the systemic circulation blood. The detection rate in peripheral blood specimens from patients with liver metastases is similar to the results obtained in a previous study, 10 and our study also showed no association between the molecular detection of CEA and cytokeratin 20 mRNA together in peripheral blood specimens and the patient’s prognosis (see Fig. 5).
From the viewpoint of surgical manipulation, it is significant that a direct association was observed between the time when the specimen was obtained and molecular detection of tumor cells. Of the eight patients who were PCR-positive in peripheral blood, more than half (5/8) were PCR-positive only after surgery (see Table 4). Further, patient 19, mentioned above, whose peripheral blood specimen showed a change from negative to positive during surgery, developed liver metastases 11 months after surgery. This might hint that surgical manipulation induced hematogenic tumor cell dissemination through the bloodstream. This is supported by animal studies, where it was reported that surgical manipulation caused tumor cell dissemination into the bloodstream, 28 and recent studies with PCR technique where a change from a negative to a positive result during surgery was reported. 7,26,29,30 These molecular-level findings show tumor cell dissemination and the significance of “no-touch” isolation techniques. However, because of the small number of patients with changes in CEA and cytokeratin 20 positivity in the peripheral blood in this study, more patients will be necessary to draw conclusions. However, two patients showed a positive result only before surgery. Also, the detection rate in peripheral blood showed an increase with the stage of disease as well as in mesenteric venous blood (see Tables 1 and 4). One possible explanation is tumor dissemination as a result of systemic disease, and probably as a result of intermittent shedding and sampling errors a positive result was obtained only before surgery. Even in the early stage of solid cancer, tumor cell dissemination is considered a systemic disease. 31 Therefore, we also must keep in mind the possibility of detection not only from surgical manipulation but also from advanced disseminated systemic disease.
Our results demonstrate at the molecular level the prognostic value of testing the mesenteric venous blood, and the probability that cancer cell dissemination is enhanced by surgical manipulation. This is the first comparative analysis of paired mesenteric venous blood and peripheral blood (before and after surgery) from the same patient. However, a study with long-term follow-up in a larger patient population is required to confirm the clinical usefulness of our results.
Footnotes
Correspondence: Kazuya Yamaguchi, MD, Second Department of Surgery, Gifu University School of Medicine, 40 Tsukasa-machi, Gifu 500-8705, Japan.
E-mail: ykazuya@poplar.ocn.ne.jp
Accepted for publication December 28, 1999.
References
- 1.Goldberg RM, Fleming TR, Tangen CM, et al. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection. Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med 1998; 129:27–35. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Research Society for Cancer of the Colon and Rectum. Multi-Institutional Registry of Large Bowel Cancer in Japan. Vol 11. Osaka; 1995.
- 3.Fisher ER, Turnbull RB Jr. The cytologic demonstration and significance of tumor cells in the mesenteric venous blood in patients with colorectal carcinoma. Surg Gynecol Obstet 1955; 100:102–108. [PubMed] [Google Scholar]
- 4.Griffiths JD, McKinna JA, Rowbotham HD, et al. Carcinoma of the colon and rectum: circulating malignant cells and five-year survival. Cancer 1973; 31:226–236. [DOI] [PubMed] [Google Scholar]
- 5.Kashitsuka T. Clinical and experimental studies on metachronous liver metastasis of colorectal cancer II: Tumor releasing effects by intraoperative manipulation of human colorectal cancer [in Japanese]. Acta Schol Med Univ Gifu 1994; 42:463–467. [Google Scholar]
- 6.Burchill SA, Bradbury MF, Pittman K, et al. Detection of epithelial cancer cells in peripheral blood by reverse transcriptase–polymerase chain reaction. Br J Cancer 1995; 71:278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori M, Mimori K, Ueo H, et al. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer 1996; 68:739–743. [DOI] [PubMed] [Google Scholar]
- 8.Soeth E, Roder C, Juhl H, et al. The detection of disseminated tumor cells in bone marrow from colorectal cancer patients by a cytokeratin-20-specific nested reverse transcriptase–polymerase chain reaction is related to the stage of disease. Int J Cancer 1996; 69:278–282. [DOI] [PubMed] [Google Scholar]
- 9.Soeth E, Vogel I, Roder C, et al. Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res 1997; 57:3106–3110. [PubMed] [Google Scholar]
- 10.Wyld DK, Selby P, Perren TJ, et al. Detection of colorectal cancer cells in peripheral blood by reverse transcriptase–polymerase chain reaction for cytokeratin 20. Int J Cancer 1998; 79:288–293. [DOI] [PubMed] [Google Scholar]
- 11.Funaki NO, Tanaka J, Ohshio G, et al. Cytokeratin 20 mRNA in peripheral venous blood of colorectal carcinoma patients. Br J Cancer 1998; 77:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castells A, Boix L, Bessa X, et al. Detection of colonic cells in peripheral blood of colorectal cancer patients by means of reverse transcriptase and polymerase chain reaction. Br J Cancer 1998; 78:1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori M, Mimori K, Ueo H, et al. Clinical significance of molecular detection of carcinoma cells in lymph nodes and peripheral blood by reverse transcription–polymerase chain reaction in patients with gastrointestinal or breast carcinomas. J Clin Oncol 1998; 16:128–132. [DOI] [PubMed] [Google Scholar]
- 14.Gerhard M, Juhl H, Kalthoff H, et al. Specific detection of carcinoembryonic antigen-expressing tumor cells in bone marrow aspirates by polymerase chain reaction. J Clin Oncol 1994; 12:725–729. [DOI] [PubMed] [Google Scholar]
- 15.Futamura M, Takagi Y, Koumura H, et al. Spread of colorectal cancer micrometastases in regional lymph nodes by reverse transcriptase–polymerase chain reaction for carcinoembryonic antigen and cytokeratin 20. J Surg Oncol 1998; 68:34–40. [DOI] [PubMed] [Google Scholar]
- 16.Jonas S, Windeatt S, O-Boateng A, et al. Identification of carcinoembryonic antigen-producing cells circulating in the blood of patients with colorectal carcinoma by reverse transcriptase–polymerase chain reaction. Gut 1996; 39:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko Y, Klinz M, Totzke G, et al. Limitations of the reverse transcription–polymerase chain reaction method for the detection of carcinoembryonic antigen-positive tumor cells in peripheral blood. Clin Cancer Res 1998; 4:2141–2146. [PubMed] [Google Scholar]
- 18.Moll R, Franke WW, Schiller DL, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982; 31:11–24. [DOI] [PubMed] [Google Scholar]
- 19.Moll R, Lowe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. Am J Pathol 1992; 140:427–447. [PMC free article] [PubMed] [Google Scholar]
- 20.Tokunaga K, Nakamura Y, Sakata K, et al. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res 1987; 47:5616–5619. [PubMed] [Google Scholar]
- 21.Fujita S, Sugano K, Fukayama N, et al. Detection of K-ras point mutations in mesenteric venous blood from colorectal cancer patients by enriched polymerase chain reaction and single-strand conformation polymorphism analysis. Jpn J Clin Oncol 1996; 26:417–421. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi S, Aihara T, Motomura K, et al. Detection of breast cancer micrometastases in axillary lymph nodes by means of reverse transcriptase–polymerase chain reaction. Comparison between MUC1 mRNA and keratin 19 mRNA amplification. Am J Pathol 1996; 148:649–656. [PMC free article] [PubMed] [Google Scholar]
- 23.Szala S, Froehlich M, Scollon M, et al. Molecular cloning of cDNA for the carcinoma-associated antigen GA733–2. Proc Natl Acad Sci USA 1990; 87:3542–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumaier M, Gerhard M, Wagener C. Diagnosis of micrometastases by the amplification of tissue-specific genes. Gene 1995; 159:43–47. [DOI] [PubMed] [Google Scholar]
- 25.Bostick PJ, Chatterjee S, Chi DD, et al. Limitations of specific reverse transcriptase–polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol 1998; 16:2632–2640. [DOI] [PubMed] [Google Scholar]
- 26.Funaki NO, Tanaka J, Hosotani R, et al. Quantitative analysis of carcinoembryonic antigen messenger RNA in peripheral venous blood and portal blood of patients with pancreatic ductal adenocarcinoma. Clin Cancer Res 1998; 4:855–860. [PubMed] [Google Scholar]
- 27.Glaves D, Huben R, Weiss L. Haematogenous dissemination of cells from human renal adenocarcinoma. Br J Cancer 1988; 54:32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishizaki T, Matsumata T, Kanematsu T, et al. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastases. J Surg Res 1990; 49:92–97. [DOI] [PubMed] [Google Scholar]
- 29.Brown DC, Purushotham AD, Birnie GD, et al. Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription–polymerase chain reaction. Surgery 1995; 117:96–101. [DOI] [PubMed] [Google Scholar]
- 30.Weitz J, Kienle P, Lacroix J, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res 1998; 4:343–348. [PubMed] [Google Scholar]
- 31.Heiss MM, Allgayer H, Gruetzner KU, et al. Individual development and uPA-receptor expression of disseminated tumor cells in bone marrow: a reference to early systemic disease in solid cancer. Nature Med 1995; 1:1035–1089. [DOI] [PubMed] [Google Scholar]