Abstract
Objective
To determine the extent of pre- and intraoperative hematogenic tumor cell dissemination in patients undergoing liver resection for metastatic colorectal cancer.
Summary Background Data
For patients with hepatic metastases of colorectal cancer, liver resection is the only potentially curative therapy. However, 38% to 53% of patients develop extrahepatic tumor recurrence, probably caused by tumor cells disseminated before or during surgery not detected by current staging systems.
Methods
Blood samples harvested before, during, and after surgery from 41 patients and bone marrow samples from 30 patients undergoing resection of liver metastases of colorectal cancer were analyzed for disseminated tumor cells using cytokeratin 20 reverse transcriptase–polymerase chain reaction.
Results
Tumor cells were detected in the blood samples of 26 of the 41 patients (63.4%) and in the bone marrow samples of 8 of the 30 patients (26.7%). Tumor cells were detected significantly more often during surgery than before or after surgery. Intraoperative tumor cell dissemination was detected in 41.7% of patients undergoing resection of two or more liver segments but only 14.3% of patients undergoing resection of one liver segment. Compared with resection of primary colorectal cancer, major liver resection carries an increased risk for intraoperative tumor cell dissemination.
Conclusions
Detection of disseminated tumor cells in patients undergoing liver resection for metastases of colorectal cancer using cytokeratin 20 reverse transcriptase–polymerase chain reaction might help to identify patients at high risk for tumor recurrence who may benefit from adjuvant therapy. Major liver resection of metastases leads to frequent intraoperative tumor cell shedding, possibly preventable by alternative surgical strategies.
The liver is the most common site for metastatic spread of colorectal cancer as a result of the portal venous drainage of the gastrointestinal tract. The frequency of synchronous liver metastases ranges from 15% to 30%, and a similar percentage of patients develop metachronous liver metastases. 1–4 For these patients, hepatic resection is the only treatment option offering a reasonable chance of long-term survival. 5,6 Patients with potentially resectable but untreated liver metastases have a 3-year survival rate of 20%, and fewer than 3% of patients survive 5 years. 7 Liver resection of metastases of colorectal cancer results in a 3-year survival rate of 30% to 61% and a 5-year survival rate of 16% to 51%, depending on patient selection. 8–12 However, after resection of liver metastases, 38% to 53% of resected patients develop extrahepatic tumor recurrence, probably caused by the release of neoplastic cells into the systemic circulation either before or during hepatic resection. 8,9,13–15 It has always been suspected, but never proven, that mechanical alteration of the liver during resection of liver metastases leads to massive intraoperative tumor cell shedding into the circulation. Detection of this intraoperative tumor cell dissemination could help to modify the technique of liver resection to avoid or reduce the assumed spread of tumor cells. In addition, detection of disseminated tumor cells of colorectal cancer in patients with liver metastases could help to identify patients at risk for tumor relapse, who might benefit from adjuvant therapy regimens. Because of the lack of appropriate detection systems, the extent of pre- and intraoperative hematogenic tumor cell dissemination in patients undergoing resection of liver metastases of colorectal cancer has not been determined.
Reverse transcriptase–polymerase chain reaction (RT-PCR)-based protocols are sensitive and specific assays for the detection of disseminated cancer cells, allowing the identification of approximately one neoplastic cell in 107 normal peripheral mononuclear blood cells. 16 Cytokeratin 20 transcripts appear to be good targets for the detection of disseminated colorectal cancer cells because they are expressed in gastrointestinal epithelium, urothelium, or Merkel cells and their respective tumors but not in other nontransformed tissues. 17–23 Recently, we demonstrated a significant intraoperative tumor cell dissemination during resection of primary colorectal cancer using cytokeratin 20 RT-PCR for detection of circulating tumor cells of colorectal cancer. 22
The purpose of this study was to determine the extent of pre- and intraoperative hematogenic tumor cell dissemination in patients undergoing liver resection of colorectal metastases using cytokeratin 20 RT-PCR.
METHODS
Blood and Bone Marrow Samples
Blood samples (10 mL) were obtained before, during, and after surgery through a central venous catheter in the superior vena cava and diluted with 10 mL phosphate-buffered saline. Bone marrow samples (10 mL) were obtained after induction of general anesthesia by aspiration from both iliac crests. After density centrifugation through Ficoll-Paque (Pharmacia, Peapack, NJ) (30 min, 400 g), mononuclear cells were harvested from the interphase and washed twice in phosphate-buffered saline. The cell pellet was then shock-frozen in liquid nitrogen and stored at −70°C.
RNA Extraction
RNA extraction from peripheral mononuclear blood cells, bone marrow samples, and frozen tissue sections of metastases was performed as previously described. 22
RT-PCR
Cytokeratin 20 RT-PCR was performed as previously described. 22 In brief, cDNA was synthesized with a reverse transcription kit as recommended by the manufacturer (Life Technologies Gibco BRL, Karlsruhe, Germany) using 1 μg RNA and the primer cytokeratin 20 558.rev in a total reaction volume of 20 μL.
PCR was performed using the following reaction conditions: primers, 25 pmoles each; dNTP, 0.2 mmol/L each; MgCl2, 1.5 mmol/L;Taq DNA polymerase, 2.5 U; PCR buffer, 20 mmol/L Tris-HCl and 50 mmol/L KCl. For the first PCR, 5 μL of the RT-reaction mixture was used to amplify cytokeratin 20 cDNAs in a total reaction volume of 100 μL using the primers 1.for (ATG GAT TTC AGT CGC AGA) and 558.rev (ATG TAG GGT TAG GTC ATC AAA G). Thirty-five rounds of amplification were performed at 30-second intervals at temperatures of 93°C, 60°C, and 72°C, with a final extension step of 10 minutes. Twenty microliters of this reaction mixture was then subjected to the nested PCR. The nested PCR reaction was performed in a total volume of 100 μL with the primers 139.for (TCC AAC TCC AGA CAC ACG GTG AAC TAT G) and 429.rev (CAG GAC ACA CCG AGC ATT TTG CAG) (35 cycles, 30 seconds 93°C, 30 seconds 72°C, and 30 seconds 72°C, final extension step 10 minutes).
PCR products were analyzed by electrophoresis on 2% agarose gels. Cytokeratin 20 PCR products were blotted onto nylon membranes (Hybond N+, Amersham Life Science, Buckinghamshire, UK) and hybridized with a chemoluminiscence-labeled oligonucleotide probe as previously described. 23
RNA quality and performance of reverse transcription of all analyzed samples was confirmed by RT-PCR amplification of glyceraldehyde phosphate dehydrogenase (GAPDH) transcripts as previously described. 24
Sensitivity of Cytokeratin 20 RT-PCR
The sensitivity of the cytokeratin 20 RT-PCR assay was determined in cell spiking experiments as previously described, allowing the detection of 10 HT29 cells in 10 mL blood. 22
Patients
Informed consent was obtained from all patients, and the study protocol was approved by the ethics committee of the University of Heidelberg.
Forty-one patients undergoing liver resection for metastatic colorectal carcinoma treated at the Department of Surgery at the University of Heidelberg (28 men, 13 women, ages 39–77, mean 62.7) from September 1995 to March 1999 were included. For the purpose of this analysis, major liver resection was defined as resection of two or more liver segments, minor liver resection as resection of one liver segment. 25 Our recent work has demonstrated a decreased probability of intraoperative tumor cell detection in blood samples of patients with high intraoperative blood loss, which is caused by a dilution effect from intraoperative blood loss and subsequent transfusion. 22 Thus, in this study only patients with a blood loss of less than 2 L were included. From each patient, three blood samples were obtained through a central venous catheter: the first after induction of anesthesia, the second after resection of metastases, and the third 24 hours after surgery.
Informed consent for bone marrow aspiration was given by 30 of the 41 patients. In all patients, the postoperative histopathologic examination of the resected specimen confirmed the presence of metastases of colorectal carcinoma.
Control Group
Blood samples from three different groups served as negative controls. The first group contained 31 healthy volunteers, the second 9 patients with benign gastrointestinal diseases, and the third 18 patients undergoing colorectal resection and 10 patients undergoing liver resection for benign disease. In addition, bone marrow samples of 30 patients without malignant disease were analyzed.
Statistical Analysis
Statistical computations were done using the software packages S-PLUS, Version 3.4 (MathSoft, Inc., Cambridge, MA) and StatXact4 for Windows (Cytel Software Corp., Cambridge, MA). P ≤ .05 was considered statistically significant.
To correlate PCR results with the timing of blood collection, the Cochran Q test was performed. 26 This test was used to find systematic changes in the distribution of the PCR results over the three time points of blood collection. For evaluation of intraoperative tumor cell dissemination, we performed a partitioned Q:Qdiff, intraoperative versus pre- or postoperative. 26
To compare the incidence of intraoperative tumor cell dissemination during resection of liver metastases with resection of primary colorectal cancer, the odds ratio (with 95% confidence interval) of intraoperative tumor cell dissemination was calculated. 27 In addition, the P value for testing the null hypothesis for an odds ratio of 1 was computed.
Tumor cell detection in blood versus bone marrow was compared using the McNemar test. 28
Tumor cell detection in blood and bone marrow was correlated to standard prognostic criteria after resection of liver metastases (number of liver metastases, size of liver metastases, patient age, disease-free interval after the primary tumor, stage of the primary tumor, grading of liver metastases) using the Fisher exact test and the Mann-Whitney test.
RESULTS
Specificity of Cytokeratin 20 RT-PCR
Blood samples from 31 healthy volunteers and 9 patients with benign gastrointestinal diseases and bone marrow samples from 30 patients without malignant disease consistently tested negative for cytokeratin 20 expression. To exclude the possibility of exfoliation of cytokeratin 20-expressing normal colon mucosa cells or liver cells during surgical manipulation, blood samples from 18 patients undergoing colorectal resection and 10 patients undergoing liver resection for benign disease were obtained before, during, and after surgery and screened for expression of cytokeratin 20. None of these revealed a PCR product. All the resected metastases tested positive for cytokeratin 20 mRNA expression.
Patient Study
In 3 of the 41 patients, only an exploratory laparotomy was performed as a result of peritoneal tumor spread. In all three patients, cytokeratin 20 transcripts were detected in preoperative blood and bone marrow samples. In 38 patients, a potentially curative liver resection was performed.
Tumor Cell Detection in Blood Samples
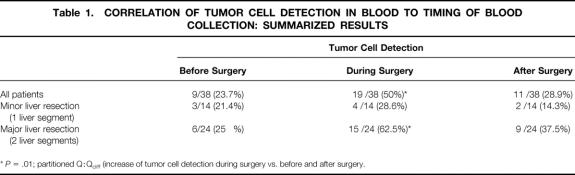
Cytokeratin 20 transcripts were detected in at least one of the three blood samples taken before, during, and after surgery in 23 of the 38 patients (60.5%) undergoing hepatic resection of colorectal metastases (Fig. 1 shows a representative PCR result). No statistical correlation to other standard prognostic criteria after resection of liver metastases could be demonstrated. The results of tumor cell detection in blood were stratified according to the timing of blood collection and the type of liver resection (major or minor) (Table 1). The Cochran Q test revealed a significant association between PCR results and the timing of blood collection for all patients (P = .025) and for patients undergoing major liver resection (P = .017), but not for patients undergoing minor liver resection (P = .819). The partitioned Q:Qdiff demonstrated a significant increase in tumor cell detection during surgery for all patients (P = .010) and for patients undergoing major liver resection (P = .008), but not for patients undergoing minor liver resection (P = .527).
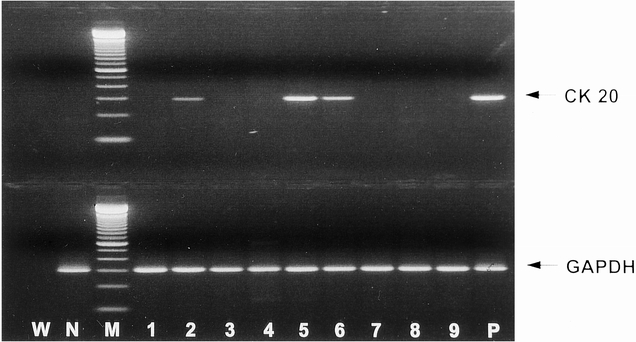
Figure 1. Cytokeratin 20 and glyceraldehyde phosphate dehydrogenase (GAPDH) reverse transcriptase–polymerase chain reaction amplification products from blood samples obtained before, during, and after surgery from three patients undergoing hemihepatectomy for liver metastasis of colorectal carcinoma. W, water negative control; N, negative control (blood of healthy person); M, molecular weight marker; P, positive control (HT29 cells). Lanes 1–3, patient 1; lanes 4–6, patient 2; lanes 7–9, patient 3. Lanes 1, 4, and 7, before surgery; lanes 2, 5, and 8, during surgery; lanes 3, 6, and 9, after surgery.
Table 1. CORRELATION OF TUMOR CELL DETECTION IN BLOOD TO TIMING OF BLOOD COLLECTION: SUMMARIZED RESULTS
*P = .01; partitioned Q:Qdiff (increase of tumor cell detection during surgery vs. before and after surgery.
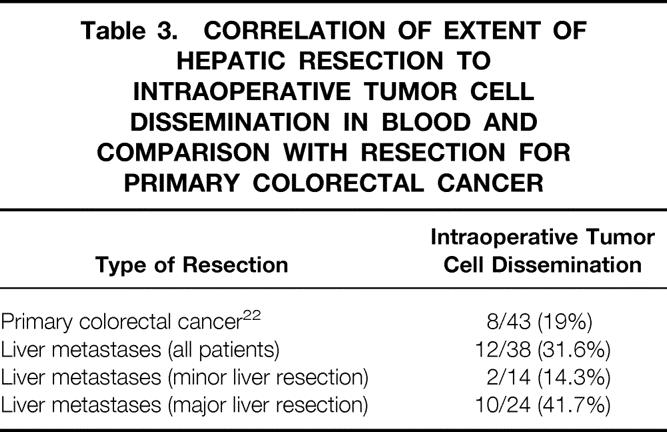
In 12 of 38 patients (31.6%), tumor cells were detected only during or during and after surgery (Table 2), possibly indicating intraoperative tumor cell dissemination. In patients undergoing major liver resection, tumor cells were detected only during or during and after surgery in 10 of 24 patients (41.7%) compared with 2 of 14 patients (14.3%) undergoing minor liver resection (Table 3). Our previous work demonstrated intraoperative tumor cell dissemination in 8 of 43 patients (19%) undergoing resection of primary colorectal cancer. 22 Statistical analysis revealed an increased risk for intraoperative tumor cell dissemination during major liver resection for metastases compared with resection of primary colorectal cancer (odds ratio = 3.07, confidence interval 0.88–11.1;P = .05).
Table 2. CORRELATION OF TUMOR CELL DETECTION IN BLOOD TO TIMING OF BLOOD COLLECTION: INDIVIDUAL RESULTS
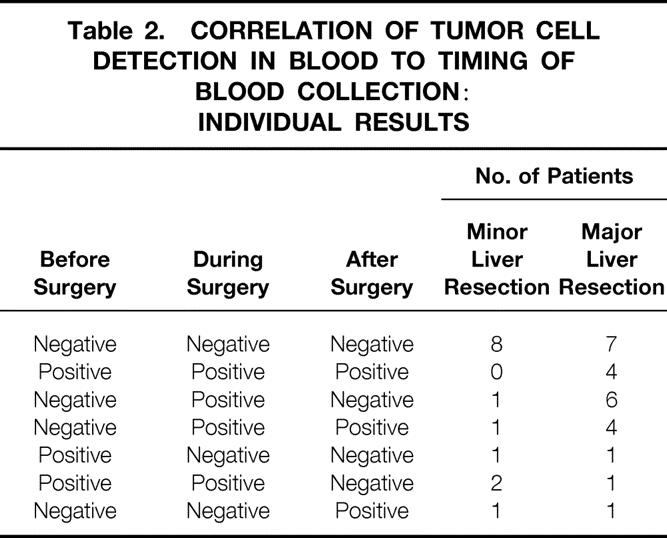
Table 3. CORRELATION OF EXTENT OF HEPATIC RESECTION TO INTRAOPERATIVE TUMOR CELL DISSEMINATION IN BLOOD AND COMPARISON WITH RESECTION FOR PRIMARY COLORECTAL CANCER
Tumor Cell Detection in Bone Marrow Samples
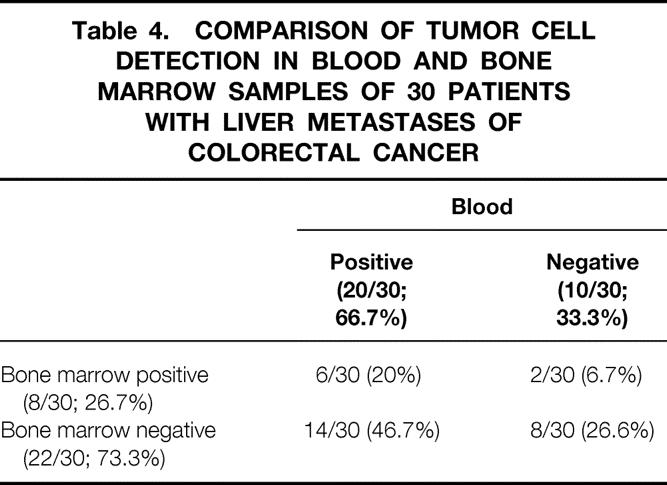
Using cytokeratin 20 RT-PCR, tumor cells were detected in 8 of 30 patients (26.7%). Tumor cell detection in bone marrow did not statistically correlate to other standard prognostic criteria. Tumor cells were detected more frequently in blood than in bone marrow samples of the respective patients (McNemar test, P = .013) (Table 4).
Table 4. COMPARISON OF TUMOR CELL DETECTION IN BLOOD AND BONE MARROW SAMPLES OF 30 PATIENTS WITH LIVER METASTASES OF COLORECTAL CANCER
DISCUSSION
Detection of Hematogenic Tumor Cell Dissemination
Patients undergoing resection of hepatic metastases of colorectal cancer have a high risk for extrahepatic tumor recurrence, probably caused by disseminated tumor cells. The objective of adjuvant therapy is to eradicate those cells, thereby decreasing the incidence of relapse and improving patient survival. In patients with liver metastases of colorectal cancer, however, no benefit of systemic, intraperitoneal, hepatic intraarterial, or portal adjuvant chemotherapy has been demonstrated in most studies. 29–31 These disappointing results may be improved by restricting adjuvant therapy to patients at high risk for relapse. To identify this patient group, adequate prognostic markers must be developed. Multiple prognostic parameters after resection of liver metastases have been suggested—for example, number of liver metastases, size of liver metastases, presence of hepatic vein invasion, width of resection margin, disease-free interval after the primary tumor, stage of the primary tumor, and grading of liver metastases. 5,12,32–36 Most of those parameters, however, could not be confirmed in other studies or merely predict intrahepatic tumor recurrence. No marker is currently available that can predict extrahepatic tumor recurrence after potentially curative resection. 37 Because extrahepatic tumor relapse is presumably caused by hematogenic disseminated tumor cells, detection of those cells may identify patients at risk for extrahepatic tumor recurrence.
In the present study, disseminated tumor cells were detected by cytokeratin 20 RT-PCR in blood samples of 26 of 41 patients (63.4%) and in bone marrow samples of 8 of 30 patients (26.7%) with liver metastases of colorectal cancer. These rates are considerably lower than the detection rates in primary colorectal cancer stage IV 20–22 (blood 83%, bone marrow 52–71%). This may be due to tumor cell reduction through preoperative chemotherapy, which 30 of the 41 patients had received. Tumor cell detection in blood and bone marrow showed no correlation to other standard prognostic criteria after liver resection. This might be explained by the above limitations in predicting extrahepatic tumor recurrence using those criteria. This, however, must be reevaluated in a larger patient group. We detected tumor cells significantly more often in blood than in bone marrow samples. This might reflect the fact that implantation of circulating tumor cells is very inefficient, and the vast majority of tumor cells circulating in the bloodstream are rapidly destroyed. 38–41 The presence of circulating tumor cells therefore does not necessarily predict subsequent metastatic disease. The long-term follow-up of our patient group will provide data on the prognostic relevance of disseminated tumor cells in blood and bone marrow in patients with liver metastases of colorectal cancer.
Intraoperative Tumor Cell Dissemination
Manipulation of tumors during surgical resection carries the risk of intraoperative tumor cell dissemination, which was demonstrated in animal and clinical studies for different tumor entities. 22,42–49 To prevent intraoperative tumor cell dissemination, Barnes 50 and Turnbull et al 51 described the “no-touch isolation” technique with initial lymphovascular ligation for resection of primary colorectal cancer.
In patients with liver resection for primary or metastatic cancer, intraoperative tumor cell dissemination is also a major concern, but no conclusive data are available. Especially compared with resection of primary colorectal cancer, liver resection for metastases of colorectal cancer should result in a higher incidence of mechanically induced intraoperative hematogenic tumor cell dissemination, because extensive mobilization of the liver with massive mechanical manipulation of the metastases is usually performed before occlusion of venous drainage. 52 Only one report describes the detection of tumor cells in the right atrium during resection for hepatocellular carcinoma. 53 However, cytologic blood smear analysis was used for tumor cell detection in this study, a method with low sensitivity and specificity. Detection of intraoperative tumor cell dissemination in patients undergoing resection of hepatocellular carcinoma was also attempted using an alpha-fetoprotein RT-PCR, but this study was not conclusive because of the low specificity of this assay for detection of malignant liver cells in blood samples during liver resection. 54
In this study, blood samples were taken before, during, and after surgery in patients undergoing resection of liver metastases of colorectal cancer to detect hematogenic tumor cell dissemination. Comparison of detection of tumor cells in blood at different times always has the problem of statistical sampling errors, because only 10 mL of blood was examined and tumor cell release into the bloodstream may not be a continuous process. 38,55,56 To differentiate the intraoperative increase in tumor cell detection from the effects caused by sampling errors, all detection rates were statistically analyzed. This analysis demonstrated a significant increase in tumor cell detection during surgery in patients undergoing major liver resection, but not in patients undergoing minor liver resection. This observation is in accordance with the concept of intraoperative tumor cell dissemination, because a higher degree of intraoperative manipulation of the liver and tumor in more extensive resections should result in a higher incidence of tumor cell shedding. Intraoperative tumor cell dissemination was detected more frequently in patients undergoing liver resection compared with our previous results in patients undergoing resection of primary colorectal cancer. 22 Statistical analysis revealed an odds ratio of 3.07 for intraoperative tumor cell dissemination during major liver resection for metastases versus tumor cell dissemination during resection of primary colorectal cancer. We conclude that patients undergoing major liver resection for metastases of colorectal cancer are at higher risk for intraoperative tumor cell dissemination compared with patients undergoing resection of primary colorectal cancer. These results support our above hypotheses and stress the potential importance of occlusion of venous drainage before surgical manipulation of tumors.
The high frequency of circulating tumor cells released during hepatic resection suggests that prevention of intraoperative tumor cell dissemination could be effective in preventing metastatic relapse in patients undergoing curative surgery for liver metastases of colorectal cancer. Intraoperative tumor cell dissemination might be prevented by alternative surgical strategies with prevention of liver manipulation before venous occlusion—for example, using the previously described anterior approach for liver resection. 57,58 In addition, perioperative antibody or cytotoxic therapy may prevent tumor cell implantation. These hypotheses need to be evaluated in further studies.
We conclude that cytokeratin 20 RT-PCR is a sensitive and specific tool for the detection of disseminated tumor cells in the blood and bone marrow of patients undergoing curative resection of liver metastases of colorectal cancer. Detection of tumor cells using cytokeratin 20 RT-PCR might identify patients at high risk for tumor relapse who could possibly benefit from adjuvant therapy. In addition, major liver resection of colorectal cancer metastases frequently leads to intraoperative tumor cell shedding, which might be prevented by alternative surgical strategies or perioperative adjuvant therapy.
Footnotes
Correspondence: Magnus von Knebel Doeberitz, MD, Division for Molecular Diagnostics and Therapy, Dept. of Surgery, University of Heidelberg, INF 110, D-69120 Heidelberg, Germany.
Accepted for publication October 8, 1999.
References
- 1.Bengmark S, Hafström L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer 1969; 23:198–202. [DOI] [PubMed] [Google Scholar]
- 2.Bengtsson G, Carlsson G, Hafström L, Jönsson PE. Natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg 1981; 141:586–589. [DOI] [PubMed] [Google Scholar]
- 3.Tong D, Russel AH, Dawson LE, Wisbeck W. Second laparotomy for proximal colon cancer: sites of recurrence and implications for adjuvant therapy. Am J Surg 1983; 145:382–385. [DOI] [PubMed] [Google Scholar]
- 4.Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg 1986; 73:732–735. [DOI] [PubMed] [Google Scholar]
- 5.Lehnert T, Otto G, Herfarth C. Therapeutic modalities and prognostic factors for primary and secondary liver tumors. World J Surg 1995; 19:252–263. [DOI] [PubMed] [Google Scholar]
- 6.Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg 1999; 86:158–169. [DOI] [PubMed] [Google Scholar]
- 7.Wagner JS, Adson MA, van Heerden JA, et al. The natural history of hepatic metastases from colorectal cancer. Ann Surg 1984; 199:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekberg H, Tranberg K-G, Andersson R, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg 1986; 73:727–231. [DOI] [PubMed] [Google Scholar]
- 9.Iwatsuki S, Esquivel CO, Gordon RD, Starzl T. Liver resection for metastatic colorectal cancer. Surgery 1986; 100:804–810. [PMC free article] [PubMed] [Google Scholar]
- 10.Little JM, Hollands M. Hepatic resection for colorectal metastases-Selection of cases and determinants of success. Austral NZ J Surg 1987; 57:355–359. [DOI] [PubMed] [Google Scholar]
- 11.Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer: competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol 1990; 16:360–365. [PubMed] [Google Scholar]
- 12.Yamamoto J, Shimada K, Kosuge T, et al. Factors influencing survival of patients undergoing hepatectomy for colorectal metastases. Br J Surg 1999; 86:332–337. [DOI] [PubMed] [Google Scholar]
- 13.Hughes KS, for the Registry of Hepatic Metastases. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery 1986; 100:278–284. [PubMed] [Google Scholar]
- 14.Butler J, Attiyeh FF, Daly JM. Hepatic resection for metastases of the colon and rectum. Surg Gynecol Obstet 1986; 162:109–113. [PubMed] [Google Scholar]
- 15.Nordlinger B, Parc R, Delva E, et al. Hepatic resection for colorectal liver metastases: influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg 1987; 205:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson PWM, Burchill SA, Selby PJ. The molecular detection of circulating tumor cells. Br J Cancer 1995; 72:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moll R, Löwe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Path 1992; 140:427–447. [PMC free article] [PubMed] [Google Scholar]
- 18.Moll R, Zimbelmann R, Goldschmidt MD, et al. The human gene encoding cytokeratin 20 and its expression during fetal development and in gastrointestinal carcinomas. Differentiation 1993; 53:75–93. [DOI] [PubMed] [Google Scholar]
- 19.Burchill SA, Bradbury MF, Pittman K, et al. Detection of epithelial cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. Br J Cancer 1995; 71:278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soeth E, Röder C, Juhl H, et al. The detection of disseminated tumor cells in bone marrow from colorectal-cancer patients by a cytokeratin 20-specific nested reverse transcriptase–polymerase chain reaction is related to the stage of disease. Int J Cancer 1996; 69:278–282. [DOI] [PubMed] [Google Scholar]
- 21.Soeth E, Vogel I, Röder C, et al. Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res 1997; 57:3106–3110. [PubMed] [Google Scholar]
- 22.Weitz J, Kienle P, Lacroix J, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res 1998; 4:343–348. [PubMed] [Google Scholar]
- 23.Weitz J, Kienle P, Magener A, et al. Detection of disseminated colorectal cancer cells in lymph nodes, blood and bone marrow. Clin Cancer Res 1999; 5:1830–1836. [PubMed] [Google Scholar]
- 24.Hsu EM, Mc Nicol PJ, Guijon FB, Paraskevas M. Quantification of HPV-16 E6–E7 transcription in cervical intraepithelial neoplasia by reverse transcriptase polymerase chain reaction. Int J Cancer 1993; 55:397–401. [DOI] [PubMed] [Google Scholar]
- 25.Bismuth H, Houssin DH, Castaing D. Major and minor segmentectomies in liver surgery. World J Surg 1982; 6:10–24. [DOI] [PubMed] [Google Scholar]
- 26.Fleiss JL. Statistical Methods for Rates and Proportions, 2d ed. New York: John Wiley & Sons; 1973.
- 27.Breslow NE, Day NE. Statistical methods in cancer research, Vol. 1. The analysis of case-control studies. IARC Scientific Publication No. 32. Lyon: IARC; 1980. [PubMed]
- 28.Agresti A. Categorical Data Analysis. New York: John Wiley & Sons; 1990.
- 29.O’Connell JM, Adson MA, Schutt AJ, et al. Clinical trial of adjuvant chemotherapy after surgical resection of colorectal cancer metastatic to the liver. Mayo Clin Proc 1985; 60:517–520. [DOI] [PubMed] [Google Scholar]
- 30.Elias D, Lasser P, Rougier P, et al. Early adjuvant intraportal chemotherapy after curative hepatectomy for colorectal liver metastases: a pilot study. Eur J Surg Oncol 1987; 13:247–250. [PubMed] [Google Scholar]
- 31.Lorenz M, Müller H-H, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. Ann Surg 1998; 228:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 1991; 110:13–29. [PubMed] [Google Scholar]
- 33.Pedersen IK, Burcharth F, Roikjaer O, Baden H. Resection of liver metastases from colorectal cancer. Dis Colon Rectum 1994; 37:1078–1082. [DOI] [PubMed] [Google Scholar]
- 34.Busch E, Kemeny MM. Colorectal cancer: hepatic-directed therapy—the role of surgery, regional chemotherapy and novel modalities. Semin Oncol 1995; 22:494–508. [PubMed] [Google Scholar]
- 35.Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Br J Surg 1997; 84:977–980. [DOI] [PubMed] [Google Scholar]
- 36.Elias D, Cavalcanti A, Sabourin JC, et al. Resection of liver metastases from colorectal cancer: the real impact of the surgical margin. Eur J Surg Oncol 1998; 24:174–179. [DOI] [PubMed] [Google Scholar]
- 37.Ekberg H, Tranberg KG, Andersson R, et al. Pattern of recurrence in liver resection for colorectal secondaries. World J Surg 1987; 11:541–547. [DOI] [PubMed] [Google Scholar]
- 38.Fidler IJ. Metastasis: quantificative analysis of distribution and fate of tumor cell emboli labeled with 125I-iodo-2′-deoxyuridine. J Natl Cancer Inst 1970; 45:773–782. [PubMed] [Google Scholar]
- 39.Weiss L. Metastatic inefficiency. Adv Cancer Res 1990; 54:159–211. [DOI] [PubMed] [Google Scholar]
- 40.Fidler IJ. The biology of human cancer metastasis. Acta Oncol 1991; 30:669–675. [DOI] [PubMed] [Google Scholar]
- 41.Fidler IJ. Macrophages and metastasis: a biological approach to cancer therapy. Cancer Res 1985; 45:4714–4726. [PubMed] [Google Scholar]
- 42.Romsdahl MM, McGrath RG, Hoppe E, McGrew EA. Experimental model for the study of tumor cells in the blood. Acta Cytol 1965; 9:141–145. [PubMed] [Google Scholar]
- 43.Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessel and pulmonary metastasis following tumor implantation. Cancer Res 1974; 34:997–1004. [PubMed] [Google Scholar]
- 44.Tyzzer EE. Factors in the production and growth of tumor metastasis. J Med Res 1913; 28:309–331. [PMC free article] [PubMed] [Google Scholar]
- 45.Nishizaki T, Matsumata T, Kanematsu T. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res 1990; 49:92–97. [DOI] [PubMed] [Google Scholar]
- 46.Brown DC, Purushotham AD, Birnie GD, George WD. Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription–polymerase chain reaction. Surgery 1995; 117:96–101. [DOI] [PubMed] [Google Scholar]
- 47.Glaves D, Huben RP, Weis L. Haematogenous dissemination of cells from human renal adenocarcinomas. Br J Cancer 1988; 57:32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willeke F, Ridder R, Mechtersheimer G, et al. Analysis of FUS-CHOP fusion transcripts in different types of soft tissue liposarcoma and their diagnostic implications. Clin Cancer Res 1998; 4:1779–1884. [PubMed] [Google Scholar]
- 49.Eschwege P, Dumas F, Blanchet P, et al. Hematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 1995; 346:1528–1530. [DOI] [PubMed] [Google Scholar]
- 50.Barnes JP. Physiologic resection of the right colon. Surg Gynecol Obstet 1952; 94:722–726. [PubMed] [Google Scholar]
- 51.Turnbull RB, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg 1967; 166:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg 1982; 6:3–9. [DOI] [PubMed] [Google Scholar]
- 53.Koo J, Fung K, Siu KF, et al. Recovery of malignant tumor cells from the right atrium during hepatic resection for hepatocellular carcinoma. Cancer 1983; 52:1952–1956. [DOI] [PubMed] [Google Scholar]
- 54.Lemoine A, Le Bricon T, Salvussi M, et al. Prospective evaluation of circulating hepatocytes by alpha-fetoprotein mRNA in humans during liver surgery. Ann Surg 1997; 226:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer 1983; 48:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayhew E, Glaves D. Quantitation of the tumorigenic disseminating and arrested cancer cells. Br J Cancer 1984; 50:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai ECS, Fan ST, Lo CM, et al. Anterior approach for difficult major right hemihepatectomy. World J Surg 1996; 20:314–318. [DOI] [PubMed] [Google Scholar]
- 58.Hohenberger P. Right hemihepatectomy: frontal approach. Alternative to conventional procedures. Chirurgie 1996; 7:944–948. [DOI] [PubMed] [Google Scholar]