Abstract
Objective
To assess the mechanistic role of group IIA phospholipase A2 (PLA2) in the process of local and distant organ injury after intestinal ischemia–reperfusion.
Summary Background Data
Intestinal ischemia–reperfusion produces lung injury by a mechanism that involves PLA2 activation, but it is unclear which isozyme is responsible for this phenomenon. Group IIA PLA2, one of the secreted forms of PLA2, is known to play a pivotal role in a variety of inflammatory reactions.
Methods
Rats underwent 45 minutes of superior mesenteric artery occlusion in the presence and absence of pretreatment with group IIA PLA2 inhibitor, S-5920/LY315920Na (20 mg/kg, subcutaneously, 30 minutes before clamping). At 2 hours of reperfusion, intestinal and lung leak was assessed by 125I-albumin tissue/blood ratio, and liver injury was estimated by serum alanine aminotransferase. PLA2 activities in tissues and sera were quantitated by phosphatidyl-glycerol/sodium cholate mixed micelle assay. PLA2 activities in tissues were also measured after in vitro preincubation with EDTA, S-5920/LY315920Na, or antirat group IIA PLA2 antibody.
Results
Intestinal ischemia–reperfusion provoked intestinal leak, liver injury, and lung leak, whereas tissue PLA2 activity was decreased in the intestine, unchanged in the liver, and increased in the lung. Serum PLA2 activities were increased in the portal and systemic circulation during ischemia. Pretreatment with S-5920/LY315920Na eliminated PLA2 activities in all tissues and sera and only abolished lung leak. The in vitro experiment revealed that most of the intestinal and lung PLA2 activities were inhibited by EDTA, S-5920/LY315920Na, and antirat group IIA PLA2 antibody, but hepatic PLA2 activity was not.
Conclusion
Intestinal ischemia–reperfusion appears to produce lung injury by a mechanism that involves group IIA PLA2 activation. Intestinal ischemia–reperfusion is likely to promote intestinal and hepatic injury independent of group IIA PLA2.
Intestinal ischemia appears to play an important role in the pathogenesis of acute respiratory distress syndrome (ARDS) and multiple organ failure. Patients with gastric intramucosal acidosis have an increased incidence of multiple organ failure and a high death rate. Prevention and treatment of gastric intramucosal acidosis by administration of fluids and vasoactive drugs improve the outcome. 1–3 In animal models, intestinal ischemia–reperfusion (I/R) produces distant organ injury by various mechanisms such as neutrophils, reactive oxygen metabolites, and cytokines. 4–6 Recently, Magnotti et al 7,8 demonstrated that hemorrhagic shock-induced lung injury was completely prevented by the division of mesenteric lymphatics, indicating that this lung injury is produced by intestinal I/R.
Phospholipase A2 (PLA2) is an ubiquitous enzyme that catalyzes the hydrolysis of the sn-2 fatty acyl bond of phosphoglycerides to liberate free fatty acids and lysophosphoglycerides. PLA2 plays a central role in diverse cellular processes, including signal transduction, host defense, membrane remodeling, and general lipid metabolism. 9,10 PLA2 also provides precursors for eicosanoid generation when the cleaved fatty acid is arachidonic acid or for platelet-activating factor formation when the sn-1 position contains an alkyl ether linkage. PLA2s are now classified into 10 subtypes with regard to function, localization, mechanism, sequence, structure, and role of divalent metal ions. 10,11 Group IIA PLA2 is one of the 14-kD, Ca2+-dependent secretory PLA2s, and its involvement in the process of inflammation has been emphasized since its discovery. 12,13 Group IIA PLA2s have been found in various body fluids from humans with sepsis, ARDS, and multiple organ failure. 14,15 Increased levels of group IIA PLA2 correlate well with the severity and prognosis of these disorders.
We have previously reported that intestinal I/R produced lung injury by a mechanism that involves PLA2 activation. 16,17 In this study we hypothesized that group IIA PLA2 is the responsible isozyme in this process.
METHODS
Adult male Sprague-Dawley rats (Sankyo Laboratory, Tokyo, Japan) were housed in an approved facility at Nippon Medical School. This animal study was approved in advance by the Institutional Animal Care and Use Committee. Rats weighing 250 to 350 g were maintained at 25°C, fed rat chow and given water ad libitum, and subjected to 12-hour light/dark cycles. All chemicals and reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted.
Intestinal I/R Model
Surgical procedures were performed under general anesthesia with 80 mg/kg ketamine (Sankyo Co., Tokyo, Japan) and 8 mg/kg xylazine given intraperitoneally. Through a midline laparotomy, the superior mesenteric artery was isolated and a bulldog arterial clamp was applied at the aortic origin. The abdomen was then covered with a sterile plastic wrap. After 45 minutes of intestinal ischemia, the arterial clamp was removed, the laparotomy incision was closed with a running 4–0 nylon suture, and the animal was allowed to awaken.
Group IIA PLA2 Inhibitor, S-5920/LY315920Na
S-5920/LY315920Na ([[3-(aminooxoacetyl)-2-ethyl-1-(phenylmethyl)-1 H-indol-4-yl]oxy] acetate; Lot 268SB4) was synthesized at Lilly Research Laboratories (Indianapolis, IN). 18 S-5920/LY315920Na acts at the active site of the human group IIA PLA2, similar to findings on structural analogs that were cocrystallized in the active site of the human group IIA PLA2. 19 Selectivity of S-5920/LY315920Na was apparent by 40-fold weaker activity against human group IB pancreatic secretory PLA2, which has the same catalytic mechanism as the human group IIA PLA2. S-5920/LY315920Na failed to inhibit the catalytic activity of human group IV cytosolic PLA2. S-5920/LY315920Na also strongly and selectively inhibited the activities of purified rat group IIA PLA2, with an IC50value of 2.4 nmol/L (unpublished data). IC50 against rat group IB PLA2 was 27.0 nmol/L.
Experimental Design
Rats were randomly allocated into three groups: sham laparotomy (n ≥ 5), intestinal I/R (45 minutes/2 hours, n ≥ 5), and intestinal I/R pretreated with S-5920/LY315920Na (20 mg/kg, subcutaneously, 30 minutes before the induction of ischemia, n ≥ 5).
Tissue Sampling
At the end of reperfusion, 2 mL blood was obtained from the inferior vena cava and spun at 1,000 g, and the serum was separated. The 3- to 5-cm-long distal ileum (10–12 cm apart from the cecum) was excised, the contents were removed, and the ileal segment was cleansed in saline at 0°C. When the lungs were harvested, a tracheostomy was performed and the animals were ventilated with room air at 60 breaths/min, 9 cm H2O peak inspiratory pressure, 2 cm positive end-expiratory pressure. A median sternotomy was then performed, and lungs were heparinized (500 U) by the right ventricle and perfused with modified Krebs-Henseleit solution (pH 7.4, 37°C, containing 4 gm% Ficoll 70 and 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) at 0.04 mL/g body weight per minute. The left lung was then rinsed externally with saline and the left lung was sampled.
Bronchoalveolar Lavage Procedure
The whole lungs underwent bronchoalveolar lavage with 3 mL normal saline. This was repeated two times, resulting in a total bronchoalveolar lavage fluid (BALF) return averaging 5.7 mL. The fluid was then centrifuged at 500 g for 10 minutes at 4°C to remove cells and was immediately stored at −80°C for subsequent analysis.
PLA2 Activity Assay
Tissue samples were homogenized for 30 seconds with threefold volume (v/w) of ice-cold 7.7 mmol/L ethylenediaminetetraacetic acid (EDTA) containing 1.5 μg/mL prostaglandin E1 using a Polytron-type homogenizer (Hitachi, Tokyo, Japan). The standard assay mixture contained 1 mmol/L 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) (Avanti Polar Lipids, Inc., Alabaster, AL), 2 mmol/L sodium cholate, 100 mmol/L Tris-HCl buffer (pH 8.0), 150 mmol/L NaCl, 10 mmol/L CaCl2, 1 mg/mL bovine serum albumin, and the enzyme sample in a final volume of 100 μL. Each sample volume was intestine 0.1 μL, liver 2 μL, lung 1 μL, BALF 10 μL, systemic serum 4 μL, and portal serum 1 μL. The substrate was used in the form of mixed micelles of sodium cholate/POPG at a molar ratio 2:1, obtained by a combination of evaporation under a stream of N2, with drying in vacuo and addition of an appropriate amount of buffer and vortex mixing until the solution became clarified. The enzyme reactions were initiated by addition of the enzyme sample to the substrate mixture. Enzyme content and reaction time were adjusted to ensure linear kinetics in all experiments. The reaction was carried out at 40°C for the defined time periods and was stopped by adding 400 μL Dole’s reagent (2-propanol:n-heptane:2 N sulfuric acid, 40:10:1, v/v/v), with 6 nmol margaric acid (Nu-Chek-Prep, Inc., Elysian, MN) added as an internal standard. Fatty acids were extracted according to Dole’s extraction system 20 followed by silicic acid treatment (Merck, Darmstadt, Germany). PLA2 activity was determined according to the method of Tojo et al, 21 measuring 9-anthryldiazomethane–labeled fatty acid with high-pressure liquid chromatography.
In the next in vitro experiments, PLA2 activities in homogenates were measured with EDTA, S-5920/LY315920Na, or antirat group IIA PLA2 antibody. In the first series, 5 mmol/L EDTA was added, in place of CaCl2, to the standard assay mixture, and each tissue PLA2 activity was determined in sham and I/R animals. In the case of S-5920/LY315920Na, S-5920/LY315920Na solution, dissolved in 5% dimethyl sulfoxide, was added to the standard assay mixture to the final concentration of 0.01, 0.1, or 1 μmol/L, and tissue PLA2 activities were quantitated. These PLA2 activities were compared with those that were measured without EDTA or S-5920/LY315920Na.
In the second experiment, the intestinal and lung homogenates, obtained from sham and I/R animals, were first coincubated with 0.22 mg/mL rabbit antirat group IIA PLA2 IgG (Shionogi Co., Osaka, Japan) or 0.22 mg/mL normal rabbit IgG in the standard assay mixture at 25 °C for 30 minutes. After coincubation, the PLA2 assay was initiated and performed by adding substrate and CaCl2 to the mixture. The PLA2 activities measured with antirat group IIA PLA2 IgG were compared with those with control IgG.
Intestinal Permeability
A second set of animals was used for the measurement of intestinal and pulmonary permeability. The animals were reanesthetized 30 minutes before the end of reperfusion, and 1.0 μCi 125I-labeled albumin (ICN Radiochemicals, Irvine, CA) in 0.5 mL phosphate-buffered saline was injected into the inferior vena cava. At the end of reperfusion, 1 mL heparinized blood was obtained from the inferior vena cava, and the 3- to 5-cm-long distal ileum was harvested (10–12 cm apart from the cecum), the contents were removed, and the ileal segment was cleansed in saline. The leakage of the radioisotope from blood into the intestinal interstitium was expressed as isotopic flux, calculated as the ratio of 125I-intestinal sample counts/g to 125I-blood counts/mL.
Hepatocellular Injury
Alanine aminotransferase (ALT) was measured by a spectrophotometric assay in which 0.25 mL of sample is added to 1.25 mL 1040 mmol/L L-alanine, 1.234 mmol/L nicotinamide adenine dinucleotide, 1560 U/L lactate dehydrogenase, 104 mmol/L phosphate buffer (pH 7.4), and 292 mmol/L 2-oxoglutarate and incubated for 1 minute at 25°C. The absorbance at 340 nm versus a water standard is read immediately and at 1-minute intervals for the next 3 minutes. The ALT level was determined by the mean absorbance change per minute.
Pulmonary Permeability
The animals were reanesthetized 30 minutes before the end of reperfusion, and 1.0 μCi 125I-labeled albumin in 0.5 mL phosphate-buffered saline was injected into the inferior vena cava. At 2 hours of reperfusion, a tracheostomy was performed and the animals were ventilated with room air at 60 breaths/minutes, 9 cm H2O peak inspiratory pressure, 2 cm positive end-expiratory pressure. A median sternotomy was performed and 1.0 mL heparinized blood was obtained from the inferior vena cava. Lungs were heparinized (500 U) by the right ventricle and perfused with modified Krebs-Henseleit solution (pH 7.4, 37°C, containing 4 gm% Ficoll 70 and 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) at 0.04 mL/g body weight per minute. The left lung was then rinsed externally with saline and the left lung and 1.0 mL blood were counted for 125I radioactivity. Lung protein flux, as reflected by 125I-albumin lung uptake, was calculated as the ratio of 125I-lung counts to 125I-blood counts/mL.
Statistics
Data are expressed as mean ± standard error of the mean. Results were evaluated using one-way analysis of variance followed by the Fisher test. Significance was accepted at P < .05.
RESULTS
Tissue PLA2 Activity
Intestinal PLA2 activity in sham animals was 340 ± 15 nmol/min/mg (Fig. 1). Intestinal PLA2 activity significantly decreased after 45 minutes of intestinal ischemia and 2 hours of reperfusion. This activity was further reduced by S-5920/LY315920Na pretreatment.

Figure 1. Intestinal phospholipase A2 activities after sham laparotomy, intestinal ischemia–reperfusion (I/R; 45 minutes/2 hours), and I/R pretreated with S-5920/LY315920Na. *P < .05 vs. the other groups.
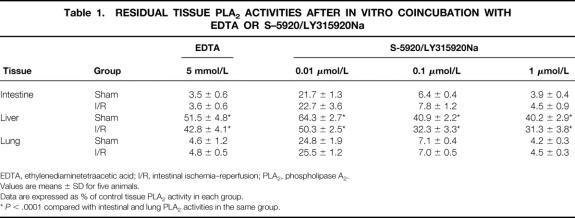
PLA2 activities in the liver were low (Fig. 2). Compared with the sham animals, intestinal I/R did not alter hepatic PLA2 activity. Intestinal I/R animals pretreated with S-5920/LY315920Na demonstrated significantly lower hepatic PLA2 activity than the sham and I/R alone animals.
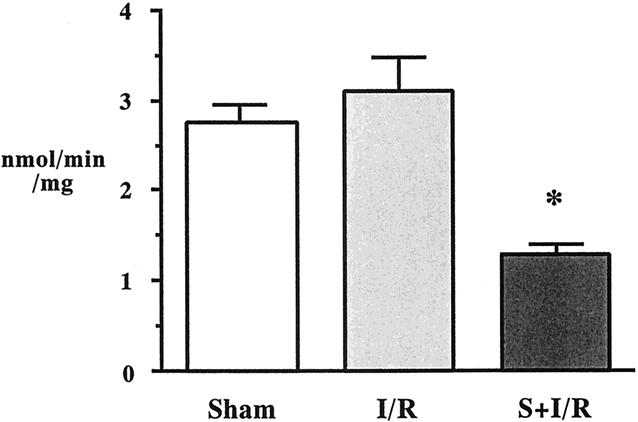
Figure 2. Liver phospholipase A2 activities after sham laparotomy, intestinal ischemia–reperfusion (I/R; 45 minutes/2 hours), and I/R pretreated with S-5920/LY315920Na. *P < .05 vs. the other groups.
Lung PLA2 activity was 20.4 ± 2.7 nmol/min/mg in sham animals, and intestinal I/R significantly increased this level (Fig. 3). S-5920/LY315920Na pretreatment abolished intestinal I/R-induced lung PLA2 activation.
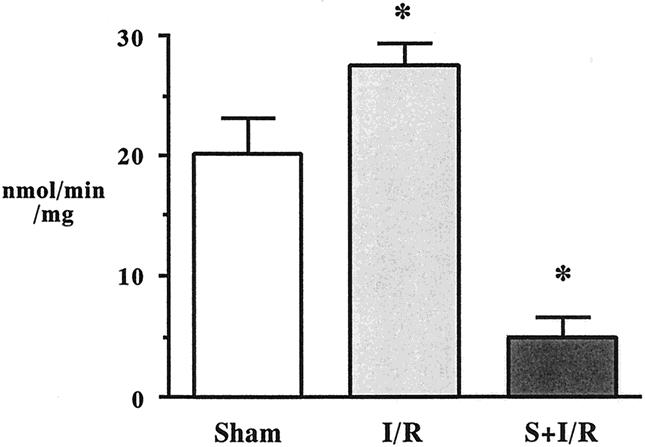
Figure 3. Lung phospholipase A2 activities after sham laparotomy, intestinal ischemia–reperfusion (I/R; 45 minutes/2 hours), and I/R pretreated with S-5920/LY315920Na. *P < .05 vs. the other groups.
Intestinal Permeability
Radioactive flux in the intestinal wall was significantly greater in the I/R group compared with the sham group (Fig. 4). S-5920/LY315920Na pretreatment did not alter the increased level of intestinal permeability after intestinal I/R.
Figure 4. Intestinal microvascular leak assessed by 125I-labeled albumin gut/blood ratio after sham laparotomy, intestinal ischemia–reperfusion (I/R; 45 minutes/2 hours), and I/R pretreated with S-5920/LY315920Na. *P < .05 vs. the other groups.
Hepatocellular Injury
Compared with sham animals, ALT levels rose significantly in the animals undergoing intestinal ischemia (Fig. 5). This hepatocellular injury was not attenuated by S-5920/LY315920Na pretreatment.

Figure 5. Serum alanine aminotransferase (ALT) levels after sham laparotomy, intestinal ischemia–reperfusion (I/R; 45 minutes/2 hours), and I/R pretreated with S-5920/LY315920Na. *P < .05 vs. the other groups.
Pulmonary Permeability
The 125I-albumin lung/blood ratio in sham animals was 0.033 ± 0.004, and intestinal I/R significantly increased this ratio (Fig. 6). The microvascular leak produced by intestinal I/R was abolished by pretreatment with S-5920/LY315920Na.

Figure 6. Lung microvascular leak assessed by 125I-labeled albumin lung/blood ratio after sham laparotomy, intestinal ischemia–reperfusion (I/R; 45 minutes/2 hours), and I/R pretreated with S-5920/LY315920Na. *P < .05 vs. the other groups.
BALF PLA2 Activity
BALF PLA2 activity after intestinal I/R (20.8 ± 2.4 nmol/min/mL, n = 7) was not different from that of sham animals (20.2 ± 2.8 nmol/min/mL, n = 5). S-5920/LY315920Na pretreatment did not cause a significant decrease in this activity (17.8 ± 2.4 nmol/min/mL, n = 5).
Serum PLA2 Activities in Portal and Systemic Veins
Serum PLA2 activities in the portal and systemic circulation are depicted in Figure 7. At the end of ischemia, PLA2 activity in the portal vein was significantly increased to 843.5 ± 379.7 nmol/min/mg (sham, 43.5 ± 4.6 nmol/min/mg). On reperfusion, serum PLA2 activities tended to decrease in the I/R animals, and the level at 2 hours of reperfusion was not different from that in the sham animals (I/R, 263.7 ± 69.7 nmol/min/mg; sham, 539.3 ± 301.5 nmol/min/mg). Pretreatment with S-5920/LY315920Na eliminated I/R-induced portal PLA2 activity throughout the study period. The kinetics of systemic serum PLA2 activities were quite similar to those in the portal vein. Of note, serum PLA2 activities in the portal vein were 10 times greater than those in the systemic circulation in both sham and I/R groups.
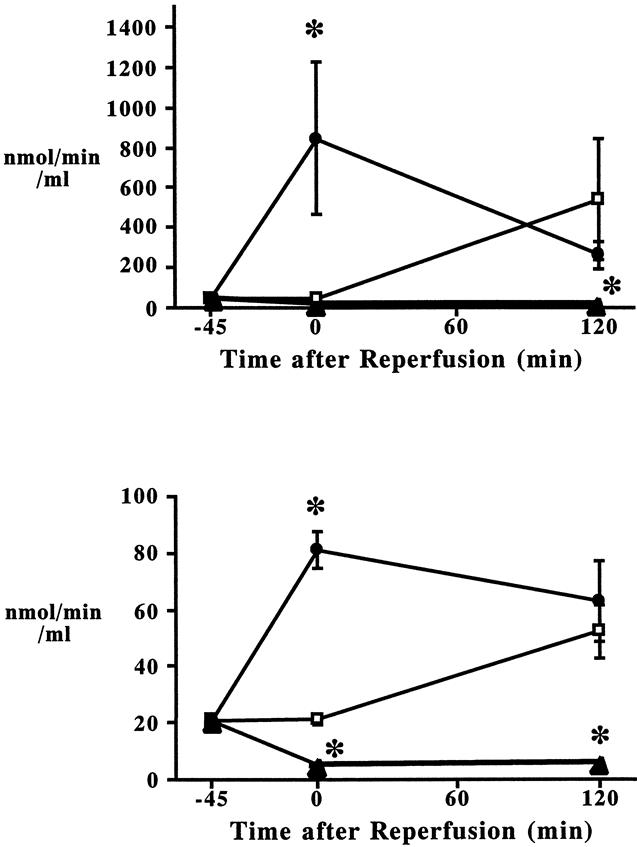
Figure 7. Kinetics of serum phospholipase A2 activities in the portal (A) and systemic circulation (B) after sham laparotomy (□), intestinal ischemia–reperfusion (I/R; 45 minutes/2 hours, •), and I/R pretreated with S-5920/LY315920Na (▴). *P < .05 vs. the other groups at the identical time point.
Characteristics of Tissue PLA2 Activities
PLA2 activity measurement after in vitro preincubation with 5 mmol/L EDTA or the indicated amounts of S-5920/LY315920Na revealed that most of the intestinal and pulmonary PLA2 activities were Ca2+-dependent and S-5920/LY315920Na-inhibitable PLA2 (Table 1). Another in vitro experiment using antirat group II PLA2 antibody confirmed that the dominant PLA2 isozyme in the ileum and lung was group IIA PLA2 (Fig. 8). The liver appeared to contain various PLA2 isozymes.
Table 1. RESIDUAL TISSUE PLA2 ACTIVITIES AFTER IN VITRO COINCUBATION WITH EDTA OR S–5920/LY315920Na
EDTA, ethylenediaminetetraacetic acid; I/R, intestinal ischemia–reperfusion; PLA2, phospholipase A2.
Values are means ± SD for five animals.
Data are expressed as % of control tissue PLA2 activity in each group.
*P < .0001 compared with intestinal and lung PLA2 activities in the same group.
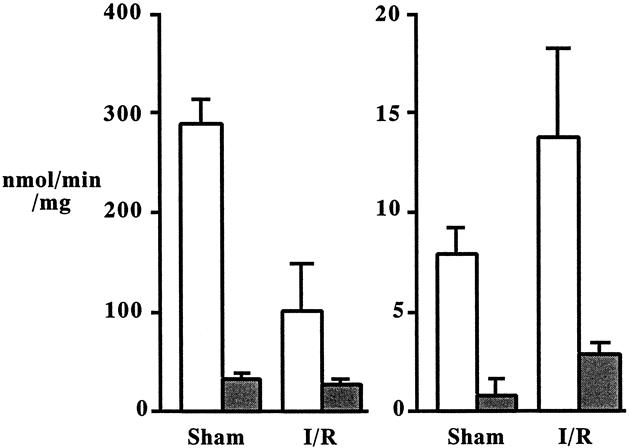
Figure 8. Intestinal (A) and lung (B) phospholipase A2 (PLA2) activities in sham laparotomy and intestinal ischemia–reperfusion (I/R; 45 minutes/2 hours) animals. PLA2 activities were determined after in vitro preincubation with antirat group IIA PLA2 antibody (shaded box). PLA2 activities were compared with the activities determined with the control immunoglobulin G (open box). Each value is the average of three experiments.
DISCUSSION
Forty-five minutes of intestinal ischemia followed by 2 hours of reperfusion increased 125I-labeled albumin transudation into the intestinal wall and lung and elevated serum ALT levels. After Intestinal I/R, PLA2 activity decreased in the intestine, did not alter in the liver, and increased in the lung. Circulating PLA2 activity was elevated during ischemia, and portal PLA2 activity was much greater than that of systemic blood. The prophylactic treatment with human group IIA PLA2 inhibitor, S-5920/LY315920Na, ameliorated PLA2 activities in all tissues and sera. Although pretreatment with S-5920/LY315920Na did not prevent intestinal and liver injury, the key finding in this study was that S-5920/LY315920Na completely blocked exacerbated pulmonary microvascular permeability. We have previously demonstrated that intestinal I/R-induced lung injury was abrogated by quinacrine, a nonspecific PLA2 inhibitor. 16,17 The current study, therefore, suggested that group IIA PLA2 plays an important role in this phenomenon.
When PLA2 activities were compared between tissues, intestinal PLA2 activity was 10-fold greater than that of lung and 100-fold greater than that of liver. PLA2 activities in the intestine and lung were largely inhibited by coincubation with 5 mmol/L EDTA, S-5920/LY315920Na, or antigroup IIA PLA2 antibody, indicating that the dominant PLA2 in these tissues is group IIA PLA2 (see Table 1 and Fig. 8). However, PLA2 activity in the liver was only blocked by half in the presence of EDTA or S-5920/LY315920Na (see Table 1). Intestine is a rich source of group IIA PLA2 in both rats and humans. 21,22 Group IIA PLA2 is increased in the serum and the colonic mucosa in patients with Crohn disease and ulcerative colitis. 23,24 Group IIA PLA2 is synthesized and stored by Paneth cells, whereas other cell types in the intestine seem incapable of synthesizing this enzyme. The rich sources of group IIA PLA2 in normal rats are reported as, in descending order, platelets, stomach, ileum, spleen, colon, lung, pancreas, liver, kidney, thymus, heart, epididymis, and brain. 21
We and others have previously shown that intestinal I/R increases intestinal PLA2 activity. 17,25 In the present study, however, intestinal PLA2 activity was decreased after intestinal I/R. It appears that these contradictory data mainly resulted from the different substrates we used for the analysis of PLA2 activity. The new PLA2 assay introduced in this study specifically extracts group IIA PLA2 activity. The substrate we previously used was 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidyl-choline (2-oleoyl PC), whereas the substrate in the present study was 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidyl-glycerol (2-oleoyl PG). Group IIA PLA2 is known to show much higher activity when 2-oleoyl PG is the substrate. 26 As shown in Table 1, most of the intestinal PLA2 activity was eliminated by S-5920/LY315920Na when the substrate was 2-oleoyl PG. However, when 2-oleoyl PC was used in the same experiment, intestinal PLA2 activity was attenuated only by half (unpublished data). These findings indicate that PLA2 activities measured with 2-oleoyl PC were derived from various kinds of PLA2 isozymes. The difference in detergents used in the present and previous studies also may have contributed to the different PLA2 activities.
High PLA2 activities were observed in both portal and systemic sera at the end of ischemia. PLA2 activity in the portal blood was 10-fold greater than that of systemic blood, suggesting that serum PLA2 activities were released from the ischemic gut. Using an intestinal graft ischemia model, Sonnino et al 27 measured secretory PLA2 activity in the preservation media and found that PLA2 activity was accumulated rapidly during the first 6 hours of ischemia. They found that this is a secretory event rather than leakage from dying cells, because PLA2 levels increased earlier than the rise in lactate dehydrogenase. 28 The current study, however, indicates that group IIA PLA2 does not play a major role in the pathogenesis of intestinal I/R-induced local injury.
Several reports have supported the hypothesis that PLA2 is a potent mediator of lung inflammation. In isolated perfused lungs, addition of PLA2 in the perfusate progressively increased pulmonary microvascular permeability. 29,30 Direct intratracheal instillation of PLA2 extracted from Naja naja venom showed a high cumulative death rate and histologic evidence of acute lung injury characterized by interstitial and alveolar edema. 29,31 Endotoxin is known to increase PLA2 activity in the lung. Ljungman et al 32 identified that the major PLA2 isozyme that increased after systemic or intratracheal endotoxin injection was group II PLA2. Using blood-free, salt-perfused lungs, they also found elevated group II PLA2 activity in the lung when endotoxin was added to the perfusate.
PLA2 activation appears to be important in the pathophysiology of ARDS. Increased PLA2 activity has been detected in the BALF from patients with ARDS, and the dominant PLA2 isozyme was identified as group II PLA2. 33 PLA2 levels in BALF correlated positively with lung injury score. In the present study, intestinal I/R increased PLA2 activity in the pulmonary tissue but not in the BALF. The mechanism of PLA2 activation in the lung is unclear. Circulating group IIA PLA2 is known to adhere easily to microvascular endothelial cells. 34 Pulmonary PLA2 activation, therefore, may have been provoked by intrinsic PLA2 activation in the lung tissue, or by attachment of circulating group IIA PLA2 to the pulmonary microvascular endothelium, or both. Forty-five minutes of brief intestinal ischemia does not produce measurable alveolar albumin leak after reperfusion, but albumin leakage in the BALF becomes apparent when the ischemic time exceeds 60 minutes. 35 This may explain why BALF PLA2 activity did not increase in this model.
Although S-5920/LY315920Na successfully decreased hepatic PLA2 activity, intestinal I/R-induced liver damage was not prevented by this treatment. The findings that intestinal I/R did not influence hepatic PLA2 activity, group IIA PLA2 is not the dominant PLA2 isozyme in the liver, and hepatic PLA2 activity is much lower than in other organs may explain this phenomenon. PLA2 appears to play a central role in the pathogenesis of reperfusion injury of the kidney, brain, heart, and pancreas; however, hepatic I/R has been shown to produce hepatic injury independent of PLA2. Terao et al 36 clamped the portal and hepatic artery, supplying the left and middle lobes, for 1 hour and detected no enhancement of hepatic PLA2 activity after predetermined periods of reperfusion up to 24 hours. In a different set of experiments, they confirmed renal PLA2 activation after renal I/R.
CONCLUSION
Group IIA PLA2 inhibitor, S-5920/LY315920Na, completely prevented intestinal I/R-induced lung leak, suggesting that group IIA PLA2 plays a major role in this process. Group IIA PLA2 has been postulated to have an antibacterial role against gram-positive and gram-negative organisms. 37,38 The antibacterial action of group IIA PLA2 against gram-negative bacteria appears to require the presence of other antibacterial agents, such as bactericidal/permeability-increasing protein, whereas this requirement is not necessary against gram-positive bacteria. With careful precautions against infectious complications, group IIA PLA2 inhibitor may become a useful tool to treat acute lung injury and ARDS in critically ill patients.
Footnotes
Correspondence: Kaoru Koike, MD, PhD, Dept. of Emergency and Critical Care Medicine, Nippon Medical School, 1–1-5, Sendagi, Bunkyo, Tokyo, 113-8603, Japan.
E-mail: koike@nms.ac.jp
Accepted for publication November 10, 1999.
References
- 1.Gutierrez G, Palizas F, Doglio G, et al. Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet 1992; 339:195–199. [DOI] [PubMed] [Google Scholar]
- 2.Maynard N, Bihari D, Beale R, et al. Assessment of splanchnic oxygenation by gastric tonometry in patients with acute circulatory failure. JAMA 1993; 270:1203–1210. [PubMed] [Google Scholar]
- 3.Mythen MG, Webb AR. Intra-operative gut mucosal hypoperfusion is associated with increased post-operative complications and cost. Intens Care Med 1994; 20:99–104. [DOI] [PubMed] [Google Scholar]
- 4.Koike K, Moore FA, Moore EE, et al. Gut ischemia mediates lung injury by a xanthine oxidase-dependent neutrophil mechanism. J Surg Res 1993; 54:469–473. [DOI] [PubMed] [Google Scholar]
- 5.Koike K, Moore EE, Moore FA, et al. CD11b blockade prevents lung injury despite neutrophil priming after gut ischemia/reperfusion. J Trauma 1995; 39:23–28. [DOI] [PubMed] [Google Scholar]
- 6.Caty MG, Guice KS, Oldham KT, et al. Evidence for tumor necrosis factor-induced pulmonary microvascular injury after intestinal ischemia–reperfusion injury. Ann Surg 1990; 212:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnotti LJ, Upperman JS, Xu D, et al. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg 1998; 228:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upperman JS, Deitch EA, Guo W, et al. Post-hemorrhagic shock mesenteric lymph is cytotoxic to endothelial cells and activates neutrophils. Shock 1998; 10:407–414. [DOI] [PubMed] [Google Scholar]
- 9.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem 1994; 269:13057–13060. [PubMed] [Google Scholar]
- 10.Dennis EA. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci 1997; 22:1–2. [DOI] [PubMed] [Google Scholar]
- 11.Cupillard L, Koumanov K, Mattei MG, et al. Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J Biol Chem 1997; 272:15745–15752. [DOI] [PubMed] [Google Scholar]
- 12.Vadas P, Pruzanski W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab Invest 1986; 55:391–404. [PubMed] [Google Scholar]
- 13.Nevalainen TJ. Serum phospholipases A2 in inflammatory diseases. Clin Chem 1993; 39:2453–2459. [PubMed] [Google Scholar]
- 14.Vadas P, Pruzanski W. Induction of group II phospholipase A2 expression and pathogenesis of the sepsis syndrome. Circ Shock 1993; 39:160–167. [PubMed] [Google Scholar]
- 15.Nyman KM, Uhl W, Forsstrom J, et al. Serum phospholipase A2 in patients with multiple organ failure. J Surg Res 1996; 60:7–14. [DOI] [PubMed] [Google Scholar]
- 16.Koike K, Moore EE, Moore FA, et al. Phospholipase A2 inhibition decouples lung injury from gut ischemia–reperfusion. Surgery 1992; 112:173–180. [PubMed] [Google Scholar]
- 17.Koike K, Moore EE, Moore FA, et al. Gut phospholipase A2 mediates neutrophil priming and lung injury after mesenteric ischemia–reperfusion. Am J Physiol 1995; 268:G397–G403. [DOI] [PubMed] [Google Scholar]
- 18.Draheim SE, Bach NJ, Dillard RD, et al. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 3. Indole-3-glyyoxamides. J Med Chem 1996; 39:5159–5175. [DOI] [PubMed] [Google Scholar]
- 19.Snyder DW, Bach NJ, Dillard RD, et al. Pharmacology of LY315920Na/S-5920, [[3-(aminooxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]acetate, a potent and selective secretory phospholipase A2 inhibitor: a new class of anti-inflammatory drugs, SPI. J Pharmacol Exp Ther 1999; 288:1117–1124. [PubMed] [Google Scholar]
- 20.Dole VP, Meinertz HJ. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem 1960; 235:2595–2599. [PubMed] [Google Scholar]
- 21.Tojo H, Ono T, Okamoto M. Reverse-phase high-performance liquid chromatographic assay of phospholipases: application of spectrometric detection to rat phospholipase A2 isozymes. J Lip Res 1993; 34:837–844. [PubMed] [Google Scholar]
- 22.Lilja I, Smedh K, Olaison G, et al. Phospholipase A2 gene expression and activity in histologically normal ileal mucosa and in Crohn’s ileitis. Gut 1995; 37:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haapamaki MM, Gronroos JM, Nurmi H, et al. Gene expression of group II phospholipase A2 in intestine in ulcerative colitis. Gut 1997; 40:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haapamaki MM, Gronroos JM, Nurmi H, et al. Elevated group II phospholipase A2 mass concentration in serum and colonic mucosa in Crohn’s disease. Clin Chem Lab Med 1998; 36:751–755. [DOI] [PubMed] [Google Scholar]
- 25.Otamiri T, Tagesson C. Role of phospholipase A2 and oxygenated free radicals in mucosal damage after small intestinal ischemia and reperfusion. Am J Surg 1989; 157:562–565. [DOI] [PubMed] [Google Scholar]
- 26.Ono T, Tojo H, Kuramitsu S, et al. Purification and characterization of a membrane-associated phospholipase A2 from rat spleen. J Biol Chem 1988; 263:5732–5738. [PubMed] [Google Scholar]
- 27.Sonnino RE, Pigatt L, Schrama A, et al. Phospholipase A2 secretion during intestinal graft ischemia. Dig Dis Sci 1997; 42:972–981. [DOI] [PubMed] [Google Scholar]
- 28.Arcuni J, Wang L, Yousef K, et al. Secretory event in intestinal grafts during preservation ischemia. J Surg Res 1999; 84:233–239. [DOI] [PubMed] [Google Scholar]
- 29.Niewoehner DE, Rice K, Duane P, et al. Induction of alveolar epithelial injury by phospholipase A2. J Appl Physiol 1989; 66:261–267. [DOI] [PubMed] [Google Scholar]
- 30.Selig WM, Durham SK, Welton AF. Pulmonary responses to phospholipase A2 in the perfused guinea pig lung. J Appl Physiol 1989; 67:2495–2503. [DOI] [PubMed] [Google Scholar]
- 31.Edelson JD, Vadas P, Villar J, et al. Acute lung injury induced by phospholipase A2. Structural and functional changes. Am Rev Respir Dis 1991; 143:1102–1109. [DOI] [PubMed] [Google Scholar]
- 32.Ljungman AG, Tagesson C, Lindahl M. Endotoxin stimulates the expression of group II PLA2 in rat lung in vivo and in isolated perfused lungs. Am J Physiol 1996; 270:L752–L760. [DOI] [PubMed] [Google Scholar]
- 33.Kim DK, Fukuda T, Thompson BT, et al. Bronchoalveolar lavage fluid phospholipase A2 activities are increased in human adult respiratory distress syndrome. Am J Physiol 1995; 269:109–118. [DOI] [PubMed] [Google Scholar]
- 34.Murakami M, Kudo I, Inoue K. In vivo release and clearance of rat platelet phospholipase A2. Biochim Biophys Acta 1989; 1005:270–276. [DOI] [PubMed] [Google Scholar]
- 35.Simpson R, Alon R, Kobzik L, et al. Neutrophil and nonneutrophil-mediated injury in intestinal ischemia–reperfusion. Ann Surg 1993; 218:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terao Y, Shibata O, Goto S, et al. Phospholipase A2 is activated in the kidney but not in the liver during ischemia–reperfusion. Res Comm Molec Pathol Pharmacol 1997; 96:277–289. [PubMed] [Google Scholar]
- 37.Weinrauch Y, Elsbach P, Madsen LM, et al. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest 1996; 97:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madsen LM, Inada M, Weiss J. Determinants of activation by complement of group II phospholipase A2 acting against Escherichia coli. Infect Immun 1996; 64:2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]