Abstract
Objective
To evaluate the impact of technical modifications on living-donor liver transplants in children since their introduction in 1989.
Summary Background Data
Although more than 4,000 liver transplants are performed every year in the United States, only approximately 500 are performed in children. Living-donor liver transplantation has helped to alleviate the organ shortage for small children in need of liver transplantation. Few centers have amassed a sufficient number of cases to evaluate the impact of the different techniques used in pediatric living-donor liver transplantation.
Methods
From 1989 through 1997, 104 primary living-donor liver transplants were performed at the University of Chicago. Three phases of the living-donor liver transplant program can be defined based on the techniques of vascular reconstruction: phase 1, November 1989 to November 1994 (n = 78); phase 2, November 1994 to January 1996 (n = 6); and January 1996 to present (n = 20). The patients’ charts were reviewed retrospectively. The incidence and type of vascular complications and patient and graft survival rates were analyzed.
Results
Although the demographics of the patients have not changed during the three phases of the living-donor liver transplant program, the outcomes have improved. Without the use of conduits, the incidence of portal vein complications has significantly decreased from 44% to 8%. The incidence of hepatic artery thrombosis has decreased from 22% to 0% with the use of microvascular techniques. The combined use of both techniques has led to a significant increase in graft survival, from 74% to 94%.
Conclusions
The living-donor liver transplant recipient operation has undergone significant technical changes since its introduction in 1989. These changes have decreased the vascular complications associated with this type of graft. Avoiding the use of vascular conduits and performing microvascular hepatic artery anastomoses are the critical steps in improving graft survival.
Clinical liver transplantation was originally described and performed on children. 1 However, because of the low prevalence of disease that progresses to end-stage liver disease, a shortage of organs appropriate for pediatric transplantation, and the lack of surgical expertise, the accumulated experience in pediatric liver transplantation is significantly less than in adult liver transplantation. Living-donor liver transplantation was first reported by Raia et al in 1989 2 and by Strong et al in 1990. 3 To expand the donor pool and thus allow timely transplantation of children with end-stage liver disease, the University of Chicago instituted the first prospective systematic application of living-donor liver transplantation in 1989. 4 Since then, we have performed more than 100 such transplants. The experience at the University of Chicago accounts for almost 50% of the living-donor liver transplants performed in the United States during this period.
After the successful reports from Australia and Chicago in 1992, the use of living-donor liver transplantation has gained widespread acceptance for the treatment of children with liver disease, especially in Japan, where it has supplied most of the grafts for liver transplantation. 5–14 The advent of this procedure was accompanied by several new technical challenges in an already technically demanding field. As with most innovative advances, subsequent modifications have been made in an attempt to resolve unforeseen complications. 15 At the University of Chicago, we have defined various phases of the living-donor liver transplant program by the technical modifications of the recipient operation. These modifications affect the performance of living-donor transplants and the growing number of split-liver transplants being performed.
We analyzed the first 104 living-donor liver transplants performed at the University of Chicago from November 1989 to January 1998, with particular focus on the technical conduct of the operation, to identify factors important to a favorable outcome.
METHODS
Preoperative Evaluation
The evolution of the donor evaluation was recently described. 16 Briefly, all potential donors undergo an extensive medical and psychosocial evaluation, followed by an abdominal computed tomography scan and a visceral angiogram. If the results of these tests are suitable, the transplant can be electively performed at a future date. Recipients are evaluated similar to any other potential liver transplant recipient. No additional tests need to be performed for recipients of living-donor liver transplantation. For patients with chronic liver disease, the transplant is usually delayed until the recipient develops a manifestation of decompensation, such as growth failure or portal hypertensive bleeding. Patients with acute liver disease undergo transplantation as soon as the donor evaluation is completed.
Donor Operation
The donor operation is performed similar to the original description by Broelsch et al. 4 Grewal et al 16 have reported recent modifications, techniques, and complications. Significant aspects that affect the recipient operation include dividing the portal vein and hepatic artery immediately distal to the bifurcation to obtain as much length as possible and performing intraoperative cholangiography to minimize the incidence of separate segment 2 and 3 hepatic ducts. There is no need to impinge on the outflow of the middle hepatic vein, because a short left hepatic vein minimizes the possibility of graft rotation in the recipient. Ninety-three of the grafts obtained were left lateral segments (segments 2 and 3), whereas 11 were full left lobes (segments 2, 3, and 4).
Surgical Techniques
Three phases of the living-donor liver transplant program can be defined on a technical basis.
The first phase is from November 1989 to November 1994. This period is defined by anastomosing the graft’s hepatic vein to the recipient’s right hepatic vein. In addition, most of these patients received an extension of the right hepatic vein orifice down onto the anterior surface of the inferior vena cava to decrease venous outflow obstruction. 17 This position of the graft necessitated placement of vascular conduits on both the portal vein and the hepatic artery. 4
The discovery of late portal vein thrombosis and stenosis involving the cryopreserved vein grafts led to the desire to eliminate the portal vein conduit and produced phase 2 of the program. 15 This phase, initiated in November 1994, is characterized by anastomosing the donor’s hepatic vein to the orifice of the combined left and middle hepatic vein orifices. This maneuver allowed elimination of the portal vein conduit. One patient in this group received a portal vein conduit; this patient received a transplant for hepatoblastoma, and we wanted to remove most of the recipient portal vein because of the possibility of tumor involvement.
During these two technical phases of the program, the arterial reconstruction was performed with a saphenous vein (from the living donor) extension graft anastomosed to the graft’s hepatic artery and then anastomosed to either the aorta (infrarenal or supraceliac) or to branches of the celiac axis.
The third phase of progression, initiated in January 1996, has been to eliminate both the hepatic artery and the portal vein conduits and to perform a microvascular hepatic arterial anastomosis, with 8–0 monofilament polypropylene suture, of the donor’s left hepatic artery to the recipient’s hepatic artery. This is performed using a Leica operating microscope (Model OH2, Leica AG, Heerbrugg, Switzerland). The portal vein anastomosis is performed as a branch patch of the recipient’s left and right portal branch to the donor’s left portal branch with 6–0 monofilament polypropylene suture. The hepatic vein anastomosis is performed at the confluence of the left and middle hepatic vein of the recipient with 4–0 monofilament polyglycolate suture.
Postoperative Management
The postoperative management of living-donor liver transplant recipients does not significantly differ from that of reduced-size liver transplant recipients. It has been previously described and has not significantly changed during the three periods. 18 The only significant modification that would affect vascular thrombosis is the addition of an anticoagulation protocol instituted for patients receiving transplants after November 1994. This current protocol requires anticoagulation for all recipients less than 20 kg and recipients of allografts from donors less than 20 kg. This policy initiates heparin when the prothrombin time is 18 seconds or less and there is no evidence of bleeding. Heparin is initiated with a therapeutic goal of an activated partial thromboplastin time of 50 to 60 seconds. Heparin is continued for 5 postoperative days, except if hypotension caused by hypovolemia related to hemorrhage occurs or if a liver biopsy is needed. Reassessment of the risks and benefits of heparin is made when bleeding necessitates reexploration, patients require more than 20 mg/kg packed red blood cells during a 24-hour period, and there is a platelet count of less than 50,000/high-power field. Ultrasonography to evaluate vascular patency and flow is performed on the first postoperative day and when clinically indicated thereafter. Immunosuppression is initiated with cyclosporine, prednisone, and azathioprine. Initial cyclosporine trough levels are maintained at 250 to 300 ng/mL. Initial rejection episodes are treated with a steroid pulse and taper. Additional rejection episodes are treated with conversion to tacrolimus. Cytomegalovirus prophylaxis is achieved with ganciclovir for the first 14 days and then acyclovir for 3 months. Epstein-Barr virus surveillance is performed with polymerase chain reaction. Pneumocystis carinii pneumonia prophylaxis is given for the first 6 months after the transplant.
All patients have had at least 1 year of follow-up (mean follow-up 4.4 years).
All procedures, including informed consent, were conducted in accord with the ethical standards of the Committee on Human Experimentation of the University of Chicago.
Statistical Analysis
Parametric data were analyzed with the Student t test, nonparametric data were analyzed with the Mann-Whitney test, the Fisher exact test for incidence of occurrences between groups, and the Mantel-Cox log-rank test for survival data. Statistical analysis was performed using Statview v4.1 by Abacus Concepts (San Diego, CA). Significance was established at P < .05.
RESULTS
Demographics
The demographic characteristics of the patients during this period (Table 1) have been stable: none of the values were statistically different from each other.
Table 1. RECIPIENT DEMOGRAPHICS
Technical Modifications
Hepatic Vein
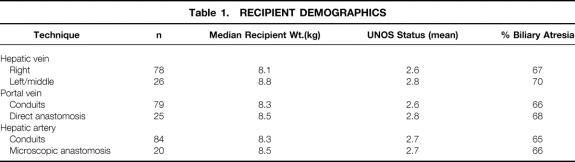
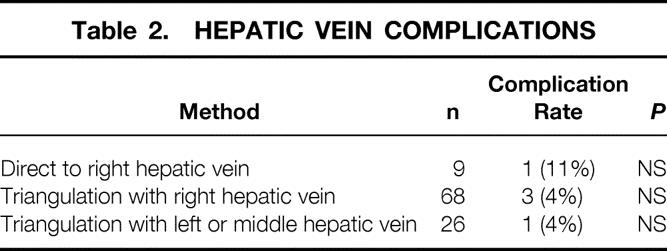
The early experience of hepatic vein reconstruction involved the recipient’s right hepatic vein orifice. This orifice was chosen in an attempt to decrease movement of the graft, which could produce twisting of the vena cava and venous outflow obstruction. The direct anastomosis to the right hepatic vein was modified to a triangulation technique in which an extension of the orifice was made by incising the anterior surface of the vena cava. A similar incision was made along the posterior surface of the graft’s left hepatic vein. This technique produced a low incidence of hepatic outflow obstruction. The desire to eliminate the portal vein conduit necessitated a change from the right hepatic vein to the confluence of the left and middle hepatic vein. A similar triangulation technique is also performed (Fig. 1). Table 2 summarizes the low incidence of complications from these techniques of hepatic vein reconstruction.
Figure 1. Current method of hepatic vein reconstruction. The right hepatic vein is oversewn and the left and middle hepatic veins are joined by incising the septum. An incision is made along the anterior surface of the cava to decrease the possibility of obstruction.
Table 2. HEPATIC VEIN COMPLICATIONS
Portal Vein
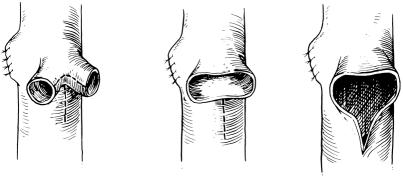
The use of the right hepatic vein caused a rotation of the donor graft’s neohilum to the right and necessitated the use of vascular conduits to provide tension-free anastomoses. The use of cryopreserved iliac vein provided acceptable early patency rates but produced an unacceptably high late stenosis and thrombosis rate. 15 This observation led to the elimination of the routine use of venous conduits. We have been able to avoid the use of portal vein conduits in all but two patients. Although the mean follow-up for this group (2.5 years) is shorter than for the group with extension grafts (4.5 years), the complication rate is significantly less in the patients without a portal venous conduit (15% vs. 42%, P < .01) (Table 3). Further, the mean follow-up of the patients without a portal venous conduit is now longer than the mean interval for diagnosis of late portal vein stenosis and thrombosis seen in patients with venous conduits (2.0 ± 0.9 years). 15 The current method of portal vein reconstruction is shown in Figure 2.
Table 3. PORTAL VEIN COMPLICATIONS
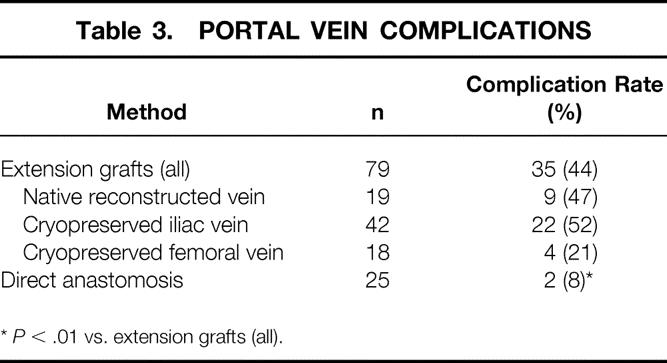
*P < .01 vs. extension grafts (all).
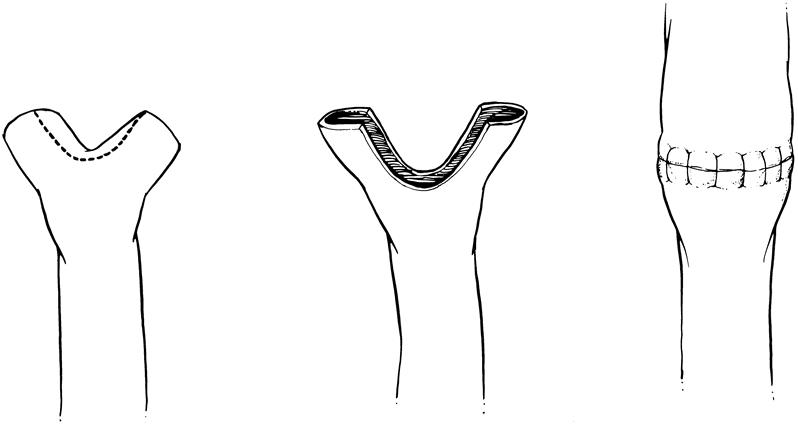
Figure 2. Current method of portal vein reconstruction. The recipient’s right and left portal veins are individually tied and then used as a branch patch to the donor graft’s portal vein.
Hepatic Artery
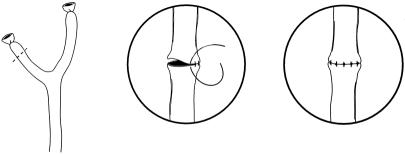
During the period in which the right hepatic vein was used, hepatic arterial reconstruction required a saphenous vein extension graft that was anastomosed to the left hepatic artery on the back table. The saphenous vein was subsequently anastomosed to the recipient. The most common site of anastomosis was the infrarenal aorta. Branches of the celiac truck were also used. This produced a hepatic arterial thrombosis rate slightly higher than in cadaveric pediatric liver transplantation for similar-sized recipients. In January 1996, we adopted a microvascular technique for arterial reconstruction without the use of extension grafts (Fig. 3). From January 1996 to January 1998, we used this technique 20 times and did not experience an arterial thrombosis. The data concerning hepatic arterial reconstruction are shown in Table 4.
Figure 3. Hepatic arterial anastomosis performed with the operating microscope.
Table 4. HEPATIC ARTERIAL COMPLICATIONS
* Microvascular anastomosis to hepatic artery.
Graft and Patient Survival
There were no intraoperative deaths during any of the 104 transplant operations. The impact of the different phases is shown in Figure 4. Clearly, the introduction of microvascular hepatic arterial reconstruction has contributed the most to improving early graft survival. The 1-year graft survival rate for grafts placed without vascular conduits is 94%, compared with 74% with at least one vascular conduit. The difference in patient survival rate (Fig. 5) is statistically significant, but the current technique has contributed to an increase in the 1-year patient survival rate from 87% to 94%. The only patient and graft loss since the introduction of the current technique was in a child with fulminant hepatic failure who developed venous outflow obstruction in the early postoperative period, became hemodynamically unstable, and died.
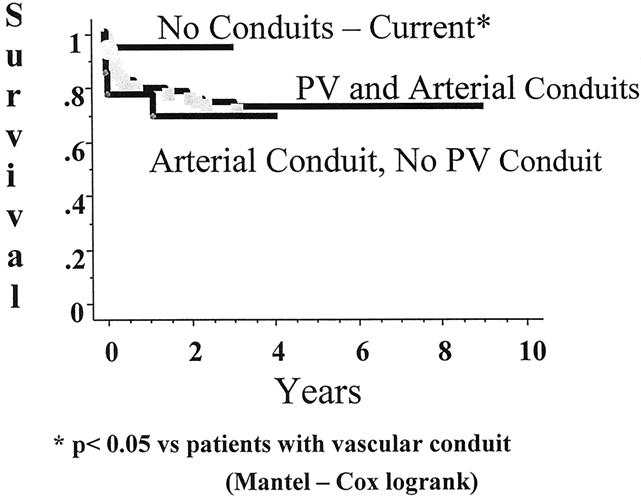
Figure 4. Graft survival after living-donor liver transplantation. *P < .05 vs. patients with vascular conduit (Mantel-Cox log-rank).
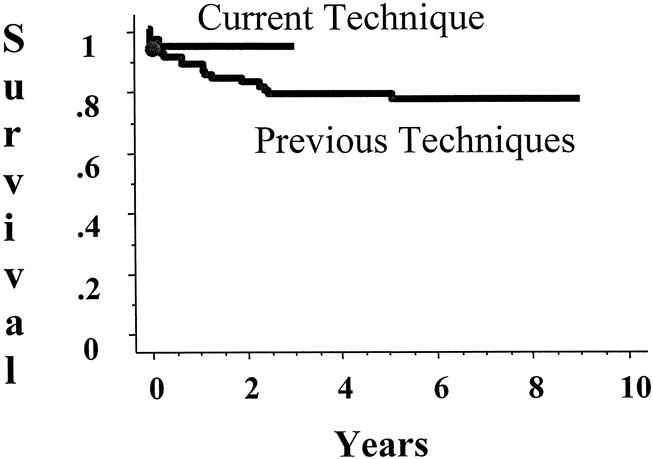
Figure 5. Patient survival after living-donor liver transplantation.
DISCUSSION
The first liver transplant in humans was reported more than 35 years ago, 1 but there is still controversy over the optimal method for perform liver transplantation. Much of this debate is centered around topics associated with adult liver transplantation, such as biliary reconstruction and stenting, indications for venovenous bypass, and routine use of piggyback techniques with or without portal caval shunting. 19–31 Because pediatric liver transplantation is approximately one-tenth as common as adult liver transplantation, technical modifications have not developed as rapidly. Routine use of reduced-size liver transplants became common approximately 10 years after the widespread application of liver transplantation in the early 1980s. 32 Living-donor liver transplantation followed reduced-size liver transplantation by several years, and there continue to be differing opinions concerning the techniques used for graft implantation. 4,9,15,33–37 The initiation of the living-donor liver transplant program at the University of Chicago introduced a set of technical challenges to the evolving field of pediatric liver transplantation. 4
Since the introduction of living-donor liver transplantation, the University of Chicago has maintained an active living-donor program. There have been several modifications to the donor and recipient operations. This report concentrates on the modifications of the recipient operation. We have identified three distinct phases of the evolution to this point, and all the changes have been designed to improve graft survival by eliminating early and late vascular thrombosis. The initial description of living-donor liver transplantation involved the right hepatic vein with triangulation of the vein into the inferior vena cava to decrease the potential for graft rotation and kinking of the hepatic vein. 4 Soon after the original series in Chicago, Tanaka began a series of living-donor liver transplants in Kyoto, and with this development the process of modification was underway. The Kyoto group reported its initial experience in 1992 and described hepatic vein reconstruction using the middle and left hepatic vein orifice. They reported a hepatic vein complication rate of 10% in their initial series. 38 The same group has subsequently reported its experience with its first 152 living-donor transplants and found a decrease in the incidence of hepatic vein complications, from 8% to 4%, with the addition of the triangulation technique. 9 The technique described by Emond et al, 17 incorporating a triangulation technique for hepatic vein reconstruction in reduced-size liver transplants, has the advantages of a large surface area and anchor sites in three different orientations. However, perhaps the most important aspect of the triangulation technique is that it mandates a short length of hepatic vein along the inferior aspect of the anastomosis, which limits the ability of the graft to rotate and occlude the hepatic venous outflow.
In this series, we report a very low incidence of hepatic outflow obstruction (4%), regardless of the hepatic vein orifice used (4%), as long as the hepatic vein cuffs are kept short by the use of triangulation or other techniques. In our series, the incidence of outflow complications did not increase with the use of the left and middle hepatic veins.
The original description of portal vein reconstruction in living-donor liver transplants involved venous extension conduits. Conduits for portal vein extension were necessary secondary to the graft rotation imposed by the use of the right hepatic vein orifice. This rotation of the graft’s neohilum to the right upper quadrant increased the distance between the graft and recipient portal vein and hepatic artery. This additional distance mandated the use of conduits. A variety of conduits were used in this series. Their advantages and disadvantages have been previously reported. 15 The results of the most recent group of patients in this series emphasize the advantages of not using a venous conduit for the portal vein. The 9% complication rate for these patients is similar to that in other reports for this size recipient in either cadaveric or living-donor transplantation. 39–41 This lower incidence of portal vein complications is a significant improvement compared with the 44% complication rate when venous conduits were used earlier in the experience. However, continued portal vein thrombosis and low flow continues to be a postoperative problem in a subset of patients. Recipients with small, sclerotic portal vein with a long segment that is not distensible while the portal vein branches are ligated represents this high-risk group. It is only in these patients that we believe portal vein manipulations are warranted. Tanaka’s group has described such manipulations. 39 We would advocate a direct anastomosis of the graft portal vein to the splenic vein–superior meseuteric vein confluence if the anastomosis can be accomplished without tension. Additional measures may involve enlarging the diameter of the portal vein with a segment of donor saphenous vein or recipient internal jugular vein. Certainly, our previous experience with cryopreserved iliac vein would limit our enthusiasm for this technique. 15 Combining these techniques could further reduce the incidence of portal vein thrombosis.
The incidence of hepatic artery thrombosis without microvascular anastomosis in pediatric liver transplantation is approximately 10% in most studies. 35–37,42–45 Tanaka’s group initiated the use of microsurgical techniques for hepatic arterial anastomosis in living-donor liver transplants. This group reported its initial experience in 1994 and has extended its series in subsequent publications. 33,46 The acceptance of this technique was delayed in Western countries because cadaveric retransplantation could salvage patients who had hepatic artery thrombosis, and because of the increased technical difficulty of the procedure. We began our experience with microvascular arterial anastomosis of the hepatic artery soon after modifying the graft position to eliminate the portal vein conduits. This rotational change lined up both the portal vein and the hepatic artery, facilitating primary anastomosis without conduits. The microvascular technique has significantly decreased the incidence of hepatic artery thrombosis and has significantly increased actuarial graft survival. As the demand for organs becomes even more critical, it is important to use every means possible to maximize graft survival. The data from this series support the routine use of microvascular arterial anastomosis in living-donor liver transplantation and, by extension, any transplant, such as split-liver transplantation, in which the arterial pedicle is not benefiting from a branch or Carrel patch.
In conclusion, there have been numerous technical modifications to the living-donor liver transplant since its original description and widespread application. The combination of elective timing of the operation and technical improvements has increased the patient and graft survival rates to levels rarely seen for these small children. The introduction of the microvascular hepatic artery reconstruction has provided the most benefit in terms of improved early graft survival, whereas the elimination of cryopreserved portal vein conduits has aided in decreasing late portal vein complications. Further modifications will certainly continue to improve patient and graft survival rates.
Footnotes
Correspondence: J. Michael Millis, MD, Section of Transplant Surgery, Dept. of Surgery, MC 5027, University of Chicago, Chicago, IL 60637.
Dr. Broelsch is currently with the Department of Surgery, University of Essen, Essen, Germany.
E-mail: mmillis@surgery.bsd.uchicago.edu
Accepted for publication November 10, 1999.
References
- 1.Starzl TE, Marchiaro TL, Von Kaulla K, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963; 117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 2.Raia S, Nery JR, Mies S. Liver transplantation from live donors [letter]. Lancet 1989; 2 (8661):497. [DOI] [PubMed] [Google Scholar]
- 3.Strong RW, Lynch SV, Ong TH, et al. Successful liver transplantation from a living donor to her son. N Engl J Med 1990; 322:1505–1507. [DOI] [PubMed] [Google Scholar]
- 4.Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg 1991; 214:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaoka Y, Ozawa K, Tanaka A, et al. New devices for harvesting a hepatic graft from a living donor. Transplantation 1991; 52:157–160. [DOI] [PubMed] [Google Scholar]
- 6.Makuuchi M, Kawarazaki H, Iwanaka T, et al. Living related liver transplantation. Surg Today 1992; 22:297–300. [DOI] [PubMed] [Google Scholar]
- 7.Kasai H, Makuuchi M, Kawasaki S, et al. Intraoperative color Doppler ultrasonography for partial-liver transplantation from the living donor in pediatric patients. Transplantation 1992; 54:173–175. [PubMed] [Google Scholar]
- 8.Mori K, Nagata I, Yamagata S, et al. The introduction of microvascular surgery to hepatic artery reconstruction in living-donor liver transplantation: its surgical advantages compared with conventional procedures. Transplantation 1992; 54:263–268. [DOI] [PubMed] [Google Scholar]
- 9.Egawa H, Inomata Y, Uemoto S, et al. Hepatic vein reconstruction in 152 living-related donor liver transplantation patients. Surgery 1997; 121:250–257. [DOI] [PubMed] [Google Scholar]
- 10.Jurim O, Shackleton CR, McDiarmid SV, et al. Living-donor liver transplantation at UCLA. Am J Surg 1995; 169:529–532. [DOI] [PubMed] [Google Scholar]
- 11.Fuchinoue W, Tanaka K, Takasaki K, et al. Living-related liver transplantation for fulminant hepatic failure. Transplant Proc 1997; 29:424–427. [DOI] [PubMed] [Google Scholar]
- 12.Mori K, Nishizawa F, Sasaki H, et al. Hepatic artery reconstruction under microvascular surgery in partial liver transplantation from living donor. Transplant Proc 1993; 25 (1 Pt 2):1093–1095. [PubMed] [Google Scholar]
- 13.Sheil AG. Living-donor transplantation: a view from Australia. Clin Transplant 1994:360–361. [PubMed]
- 14.Haberal M, Bilgin N, Buyukpamukcu N, et al. Living donor hepatectomy in partial liver transplantation: surgical technique and results. Transplant Proc 1993; 25:1899–1901. [PubMed] [Google Scholar]
- 15.Millis JM, Seaman DS, Piper JB, et al. Portal vein thrombosis and stenosis in pediatric liver transplantation. Transplantation 1996; 62:748–754. [DOI] [PubMed] [Google Scholar]
- 16.Grewal HP, Thistlethwaite JR Jr, Loss GE, et al. Complications in 100 living-liver donors. Ann Surg 1998; 228:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emond JC, Heffron TG, Whitington PF, Broelsch CE. Reconstruction of the hepatic vein in reduced size hepatic transplantation. Surg Gynecol Obstet 1993; 176:11–17. [PubMed] [Google Scholar]
- 18.Alonso EM, Piper JB, Echols G, et al. Allograft rejection in pediatric recipients of living related liver transplants. Hepatology 1996; 23:40–43. [DOI] [PubMed] [Google Scholar]
- 19.Randall HB, Wachs ME, Somberg KA, et al. The use of the T tube after orthotopic liver transplantation. Transplantation 1996; 61:258–261. [DOI] [PubMed] [Google Scholar]
- 20.Feith MP, Klompmaker IJ, Maring JK, et al. Biliary reconstruction during liver transplantation in patients with primary sclerosing cholangitis. Transplant Proc 1997; 29:560–561. [DOI] [PubMed] [Google Scholar]
- 21.Chaib E, Friend PJ, Jamieson NV, Calne RY. Biliary tract reconstruction: comparison of different techniques after 187 paediatric liver transplantations. Transpl Int 1994; 7:39–42. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SR, Marterre WF, Alonso MH, Hanto DW. A percutaneous technique for venovenous bypass in orthotopic cadaver liver transplantation and comparison with the open technique. Liver Transpl Surg 1996; 2:354–361. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen A, Braidley P, Jamieson NV, et al. Biliary complications in orthotopic liver transplantation. Transplant Proc 1994; 26:1792. [PubMed] [Google Scholar]
- 24.Rasmussen LS, Ejlersen E, Hjortrup A, et al. Veno-venous bypass during human liver transplantation. Transplant Proc 1994; 26:1791. [PubMed] [Google Scholar]
- 25.Rasmussen A, Palfelt I, Larsen PN, et al. Importance of early arterialization of the liver graft. Transplant Proc 1994; 26:1790. [PubMed] [Google Scholar]
- 26.Scherer R, Giebler R, Schmutzler M, et al. Effect of high shuntflows during portofemoro-subclavian venovenous bypass in human orthotopic liver transplantation. Transplant Proc 1993; 25:2591. [PubMed] [Google Scholar]
- 27.Scherer R, Giebler R, Schmutzler M, et al. Shuntflow vs. renal perfusion pressure during venovenous bypass in human orthotopic liver transplantation. Transplant Proc 1993; 25:2590. [PubMed] [Google Scholar]
- 28.Scherer RU, Giebler RM, Schmutzler MJ, et al. Shunt flow and caval pressure gradient during veno-venous bypass in human orthotopic liver transplantation. Br J Anaesth 1993; 70:689–690. [DOI] [PubMed] [Google Scholar]
- 29.Stieber AC. One surgeon’s experience with the piggyback versus the standard technique in orthotopic liver transplantation: is one better than the other? Hepato-Gastroenterology 1995; 42:403–405. [PubMed] [Google Scholar]
- 30.Nery J, Jacque J, Weppler D, et al. Routine use of the piggyback technique in pediatric orthotopic liver transplantation. J Pediatr Surg 1996; 31:1644–1647. [DOI] [PubMed] [Google Scholar]
- 31.Neuhaus P, Blumhardt G, Bechstein WO, et al. Technique and results of biliary reconstruction using side-to-side choledochocholedochostomy in 300 orthotopic liver transplants. Ann Surg 1994; 219:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broelsch CE, Whitington PF, Emond JC. Evolution and future perspectives for reduced-size hepatic transplantation. Surg Gynecol Obstet 1990; 171:353–360. [PubMed] [Google Scholar]
- 33.Inomoto T, Nishizawa F, Sasaki H, et al. Experiences of 120 microsurgical reconstructions of hepatic artery in living related liver transplantation. Surgery 1996; 119:20–26. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto M, Moriyasu F, Nada T, et al. Influence of spontaneous portosystemic collateral pathways on portal hemodynamics in living-related liver transplantation in children. Doppler ultrasonographic study. Transplantation 1995; 60:41–45. [DOI] [PubMed] [Google Scholar]
- 35.Rogiers X, Burdelski M, Broelsch CE. Liver transplantation from living donors. Br J Surg 1994; 81:1251–1253. [DOI] [PubMed] [Google Scholar]
- 36.Malago M, Rogiers X, Burdelski M, Broelsch CE. Living related liver transplantation: 36 cases at the University of Hamburg. Transplant Proc 1994; 26:3620–3621. [PubMed] [Google Scholar]
- 37.Otte JB, de Ville de Goyet J, Reding R, et al. Living related donor liver transplantation in children: the Brussels experience. Transplant Proc 1996; 28:2378–2379. [PubMed] [Google Scholar]
- 38.Ozawa K, Uemoto S, Tanaka K, et al. An appraisal of pediatric liver transplantation from living relatives. Initial clinical experiences in 20 pediatric liver transplantations from living relatives as donors. Ann Surg 1992; 216:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saad S, Tanaka K, Inomata Y, et al. Portal vein reconstruction in pediatric liver transplantation from living donors. Ann Surg 1998; 227:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van der Werf WJ, D’Alessandro AM, Knechtle SJ, et al. Infant pediatric liver transplantation results equal those for older pediatric patients. J Pediatr Surg 1998; 33:20–23. [DOI] [PubMed] [Google Scholar]
- 41.Chardot C, Herrera JM, Debray D, et al. Portal vein complications after liver transplantation for biliary atresia. Liver Transpl Surg 1997; 3:351–358. [DOI] [PubMed] [Google Scholar]
- 42.Stevens LH, Emond JC, Piper JB, et al. Hepatic artery thrombosis in infants. A comparison of whole livers, reduced-size grafts, and grafts from living-related donors. Transplantation 1992; 53:396–399. [DOI] [PubMed] [Google Scholar]
- 43.Lallier M, St-Vil D, Dubois J, et al. Vascular complications after pediatric liver transplantation. J Pediatr Surg 1995; 30:1122–1126. [DOI] [PubMed] [Google Scholar]
- 44.Cacciarelli TV, Esquivel CO, Moore DH, et al. Factors affecting survival after orthotopic liver transplantation in infants. Transplantation 1997; 64:242–248. [DOI] [PubMed] [Google Scholar]
- 45.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg 1998; 228:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka K, Uemoto S, Tokunaga Y, et al. Living related liver transplantation in children. Am J Surg 1994; 168:41–48. [DOI] [PubMed] [Google Scholar]