Abstract
Objective
To compare the clinical outcome and restenosis incidence of patients who underwent carotid endarterectomy with patch closure (CEAP) on one side and carotid eversion endarterectomy (CEE) on the other.
Summary Background Data
Although a few investigators have compared the results of CEAP versus CEE, no reports have compared the outcome of CEAP versus CEE in the same patient.
Methods
Eighty-six patients were randomly selected for sequential surgical treatment involving either CEAP/CEE or CEE/CEAP. All patients underwent postoperative duplex ultrasound study and clinical follow-up at 1, 6, and 12 months and every year thereafter. Various factors were analyzed to ascertain any association with restenosis, and Kaplan-Meier analysis was used to estimate the risk of restenosis.
Results
Demographic and clinical data were similar in the CEAP and CEE groups. The selective shunting rate was statistically higher in the CEAP group. There were no perioperative deaths. Although the incidence of perioperative ipsilateral stroke was not significant, CEAP patients had a rate of combined transient ischemic attacks and strokes that approached statistical significance. The mean follow-up was 40 months. CEAP patients had a significantly higher incidence of restenosis and combined occlusive events and restenoses. Kaplan-Meier analysis showed that CEE had a significantly better cumulative patency rate than CEAP and that freedom from restenoses at 24 and 36 months was 87% and 83% for CEAP and 98% and 98% for CEE, respectively.
Conclusions
CEE is less likely to cause perioperative neurologic complications and restenoses than CEAP. The significantly higher rate of unilateral recurrence suggests that local factors play a more important role than systemic factors in the occurrence of restenosis.
Carotid endarterectomy (CEA) is one of the few surgical procedures whose efficacy has been tested with randomized controlled clinical trials. 1–6 Controversy has remained, however, concerning the best method for arterial closure after CEA to improve perioperative stroke and internal carotid artery (ICA) occlusion rates and to reduce the incidence of early and late restenosis. A few prospective randomized studies 7–14 have demonstrated that, when data on the three principal outcomes (perioperative stroke, early ICA occlusion, and restenosis >50% at 1 year) are pooled, the statistical results strongly favor patch-plasty over primary closure. Although patch reconstruction, regardless of the type of patch material used (autologous saphenous or cervical vein, Dacron graft, and polytetrafluoroethylene [PTFE]), has become popular in the past decade, a technique first described by DeBakey et al in 1959 15 —the carotid eversion endarterectomy (CEE)–has also gained growing approval, providing excellent early and late outcomes. Recent randomized and nonrandomized studies by several institutions, 16–19 including ours, 20 have compared the results of CEA with patch closure (CEAP) versus CEE. Although the findings reported in these studies vary, primarily because the sample sizes were too small to achieve sufficient statistical power to detect small significant differences, CEE generally seems to be superior to CEAP in reducing perioperative neurologic complications, recurrence, or late occlusive events.
Whereas most perioperative strokes and ICA occlusions can be traced back to technical errors in surgical technique, 21 several factors have been implicated in the pathogenesis of restenosis, including local and systemic factors. No randomized prospective studies have examined this occurrence in a population undergoing bilateral CEA, in whom CEAP was performed on one side and CEE on the other. In this case, each patient would serve as his or her own control, because both CEAs would be subjected to the same systemic risk factors for restenosis. Any restenosis occurring on one side would consequently be related to local factors. This is the first prospective study that compares the clinical outcome and the incidence of restenosis in patients undergoing bilateral CEA who were randomized to receive CEAP on one side and CEE on the other.
METHODS
Patients
The study involved 86 patients who underwent bilateral CEA (CEAP on one side and CEE on the other); some of these patients joined a prospective randomized study on 310 patients evaluated for the early and late clinical outcome of CEAP versus CEE. 20 There were 64 men (74.5%) with a mean age of 70 years (range 41–84) and 22 women (25.5%), with a mean age of 72 years (range 54–82). Patients were randomized to sequential surgical treatment involving either CEAP/CEE or CEE/CEAP. Bilateral CEAs were planned at the time of admission in 63 patients (73%), with a mean time of 7 ± 2 weeks between the two procedures, whereas 23 (27%) developed indications for contralateral CEA a mean of 23 ± 9 months after the first procedure. Informed consent was obtained from all patients, and the study was approved by the Ethical Committee of the University of Padua, School of Medicine, Italy. Patients scheduled for repeat CEA or CEA with concomitant coronary artery bypass grafting, or patients with associated supraaortic trunk lesions requiring concurrent surgery were excluded.
Before surgery, all patients underwent either carotid duplex ultrasound scanning on an ATL Ultramark 9 HDI System (Advanced Technology Laboratory, Inc., Bothel, WA) or angiographic studies of the supraaortic trunks, with biplanar extracranial and intracranial views. Preoperative risk factors including coronary artery disease, hypertension, hyperlipidemia, diabetes mellitus, and a history of smoking, as well as associated diseases (peripheral vascular disease and abdominal aortic aneurysm), were determined for each patient.
Preoperative patient preparation was standardized. Antiplatelet therapy (aspirin and/or dipyridamole; ticlopidine) was suspended at least 2 weeks before surgery and was not resumed until the patient was discharged from the hospital. Indications for surgery were classified as hemispheric transient ischemic attack (TIA), amaurosis fugax, fixed or partial nonprogressing stroke, and no symptoms (Table 1). In asymptomatic patients, the angiographic finding was characterized at the carotid bifurcation as stenosis greater than 70% or stenosis less than 70% but with ulcerated or hemorrhagic plaque. Percentage diameter stenosis was calculated from arteriograms in accordance with the North American Symptomatic Carotid Endarterectomy Trial. 1 Preoperative computed cerebral tomography was performed in all symptomatic patients.
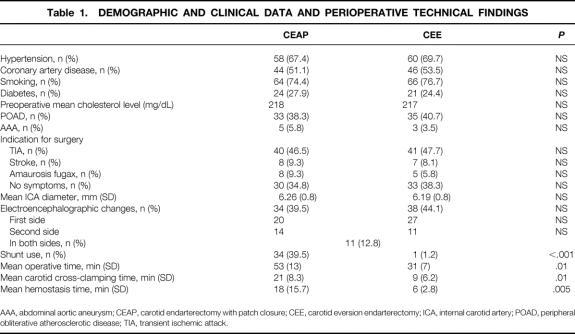
Table 1. DEMOGRAPHIC AND CLINICAL DATA AND PERIOPERATIVE TECHNICAL FINDINGS
AAA, abdominal aortic aneurysm; CEAP, carotid endarterectomy with patch closure; CEE, carotid eversion endarterectomy; ICA, internal carotid artery; POAD, peripheral obliterative atherosclerotic disease; TIA, transient ischemic attack.
Surveillance Protocol
All patients were followed up clinically, and duplex ultrasound scans were systematically performed before discharge, assessing the quality of the repair. These studies were repeated regularly at 1, 6, and 12 months and every year thereafter to assess the presence of residual ICA stenosis, angulation, ICA dilation, recurrent ICA disease, and occlusion. Residual stenosis was recorded as present when a stenotic area was noted at the proximal or distal end of the endarterectomy site at the 30-day duplex ultrasound follow-up. Residual angulation was recorded as present when a greater than 60° kink was detected just beyond the distal end of the patch or endarterectomized zone at the 30-day duplex scan. Dilation of the ICA was graded from 0% to 100% of its distal diameter; significant postoperative dilation was defined as a dilation at least twice the diameter of the adjacent normal artery. Recurrent stenosis was judged to be present only if the abnormality detected by the ultrasound scan was not visible on the first postoperative scan, and if it persisted for two or more tests performed within 6 months of the original scan. It was defined as a greater than 50% narrowing of the lumen diameter. A peak systolic velocity greater than 210 cm/sec and an end-diastolic velocity greater than 110 cm/sec were consistent with ICA stenosis greater than 70%. 22 Arteriography was obtained for noninvasive evidence of any restenosis exceeding 70%.
Study end points were perioperative stroke and death, restenosis, and occlusion and were sought in all patients. Stroke was defined as a neurologic deficit persisting more than 24 hours, regardless of the mechanism, and related to either cerebral hemisphere. Other perioperative complications and reportable events during follow-up were recorded in accordance with the guidelines set by the Ad Hoc Committee on Reporting Standards for Cerebrovascular Disease, Society for Vascular Surgery/North American Chapter of the International Society of Cardiovascular Surgery. 23
Surgical Techniques
All surgical CEAs were performed with the patient under deep general anesthesia. The sole method for cerebral protection was continuous perioperative electroencephalographic monitoring for selective use of an indwelling shunt (carotid Pruitt-Inahara T-shunt; Ideas for Medicine, St. Petersburg, FL). All operations were performed using intravenous heparin (5,000 U) before carotid cross-clamping; blood pressure was maintained at average preoperative levels or slightly higher. The heparin was not reversed with protamine. The “parachute” suture technique, using 5–0 polypropylene suture material (Prolene; Ethicon, Somerville, NJ), was used in all patients. Thrombin-soaked oxidized cellulose and digital pressure were applied to stop any bleeding before closure.
The technical details of CEAP have changed very little from those described in our 1984 report. 24 All patched arteries were closed using expanded PTFE (Gore-Tex; W. L. Gore, Elkton, MD). An attempt was made to obtain a good-quality distal end point, with complete removal of the tongue of plaque. CEA was thorough in every instance, thus avoiding the need for end point tacking sutures. In the CEE technique, unlike most surgeons who reanastomose the transected vessel in situ, 16–19,25–29 we always reimplant the endarterectomized ICA end to side, more proximally, in the lateral wall of the common carotid artery, after cutting a matching elliptic longitudinal tongue of arterial wall. 20
Statistical Analysis
Continuous variables were examined using the Student t test, and discrete variables were compared with either chi-square analysis or the Fisher exact test. Analysis included multiple logistic regression and Kaplan-Meier method of life-table analysis. Life-table data were compared with the log-rank test. Statistical significance was inferred at P < .05.
RESULTS
Table 1 shows that the demographics and clinical data and the mean ICA diameters were comparable for the CEAP and CEE groups. Although the incidence of electroencephalographic changes was similar in the two groups, the rate of indwelling shunt use was significantly higher in the CEAP group (39.5% vs. 1.2%;P < .001). The surgical (P = .01), carotid cross-clamping (P = .01), and hemostasis times (P = .005) were significantly shorter for the CEE group than for the CEAP group.
Perioperative Results
There were no perioperative deaths (Table 2). The incidence of perioperative stroke secondary to ipsilateral ICA thrombosis was similar in the two groups (3.5% 2/86 for CEAP vs. 0% for CEE). Both the patients who had stroke awoke from the anesthesia with no apparent neurologic deficit or stroke; neither were shunted. Their major event occurred 10 and 12 hours after surgery, while in the recovery room, where duplex ultrasound scanning immediately confirmed the ICA thrombosis. Both patients underwent surgical exploration consisting of a thrombectomy and new PTFE patch-plasty, with some improvement in the neurologic status in one and none in the other. At reoperation, the ICA was occluded in both patients and acutely angulated at the end of the distal tip of the patch closure; the patch had made the artery stiff, and with systolic thrust the ICA had been driven forward against the angulation and thrombosed.
Table 2. EARLY AND LATE OUTCOMES
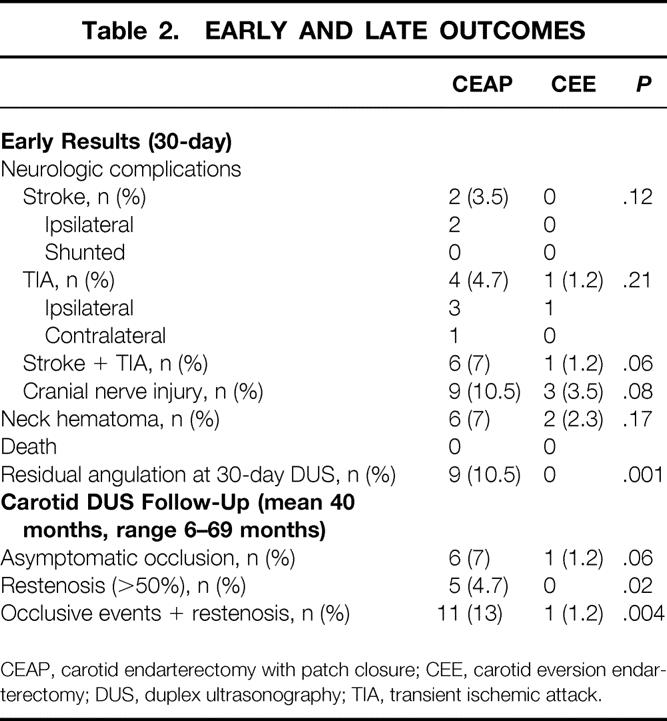
CEAP, carotid endarterectomy with patch closure; CEE, carotid eversion endarterectomy; DUS, duplex ultrasonography; TIA, transient ischemic attack.
Patients with CEAP had a higher incidence of neurologic complications (TIAs and strokes combined) than CEE, with the difference reaching significance (7% vs. 1.2%;P = .06). A further four perioperative TIAs (all but one ipsilateral), which showed spontaneous recovery within a few hours, occurred in the CEAP group and were associated with a normal duplex scan. One patient with CEE had an ipsilateral TIA 10 days after surgery and 7 days after hospital discharge: the duplex scan was normal. Although CEE required a more extensive distal dissection of the ICA, cervical and cranial nerve injury rates were comparable in the two groups. At the first duplex ultrasound follow-up, there was a significantly higher incidence of residual ICA angulations in the CEAP group (10.5% vs. 0%;P = .001).
Late Results
The mean follow-up was 40 months (range 6–69 months). There were no significant differences in the mean follow-up between the CEAP and CEE groups. Table 2 summarizes the duplex ultrasound findings according to the type of surgery. A significant difference was found in the rate of restenosis (>50%) between the CEAP and CEE groups (4.7% vs. 0%;P = .02). When combined restenosis and occlusion rates were analyzed, the CEE group was superior to the CEAP group (1.2% vs. 13%;P = .004); when only occlusive events were compared, eversion was still better than patching (1.2% vs. 7%), with a difference approaching significance (P = .06). All occlusive events and restenoses occurred in ICAs that were not shunted. All occlusive events occurred without symptoms within the first postoperative year, because all duplex ultrasound examinations at 1 and 6 months were normal. In addition, all late occlusions in the CEAP group developed in arteries evidencing a residual ICA angulation at the 30-day scan. All restenoses occurred without symptoms after the first postoperative year and were in the ICA segment (one was at the distal end of the patch). All but one of the restenoses were moderate lesions (50–70%) that remained unchanged at subsequent ultrasound follow-up, and all but one were treated medically. In one patient it progressed rapidly and became severe enough (>90%) to require reoperation 19 months after the first operation (this involved an interposition bypass of an autologous reversed saphenous vein graft between the common carotid and the ICA, 2 cm beyond the distal end of the patch).
When the influence of sex on the incidence of restenosis was considered, a higher incidence was found in men than in women (8% 5/64 vs. 0% 0/22), but the difference was not significant. However, overall women had a higher incidence of occlusive events than men (13.6% 3/22 vs. 6.2% 4/64), all in the CEAP group, but again the difference was not significant. When combined restenosis and occlusion rates were considered, the incidence was still comparable between women and men (13.6% 3/22 vs. 14% 9/64).
Kaplan-Meier analysis (Fig. 1) showed that CEE had a significantly better cumulative patency rate than CEAP (P = .001). This analysis showed that the rate of freedom from restenosis was 87% at 24 months and 83% at 36 months for CEAP and 98% and 98% for CEE, respectively.
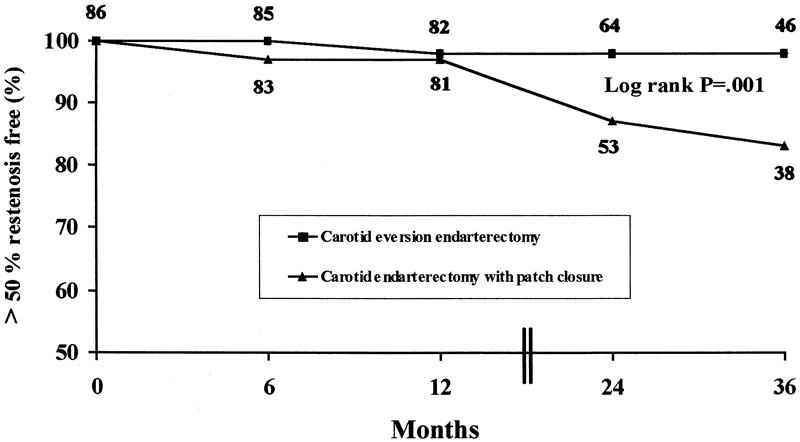
Figure 1. Kaplan-Meier analysis curve showing the cumulative patency rate and freedom from restenosis (>50%) or occlusive events for the two groups.
Six late deaths occurred in the whole series, four related to myocardial infarction and two secondary to malignancies.
DISCUSSION
The efficacy of surgical treatment for carotid bifurcation disease in preventing stroke has been well documented in several prospective randomized trials. 1–6 The best method for arterial closure after CEA remains controversial, however. A few prospective randomized studies have shown that by avoiding the technical hazards of longitudinal ICA closure, patch-plasty minimizes the role of the closure in the incidence of early and late complications. 7–14 Early and late outcomes seem to be even better, however, when the proximal ICA is transversely resected and anastomosed to the common carotid, thus obviating the need for patch closure and avoiding any interference with the endarterectomized zone, as in the CEE technique.
Five studies have compared the outcome of conventional CEA and CEE. 16–20 Two were not randomized. 17,19 In two series, not all conventional CEAs were patched. 18,19 In three studies, the perioperative stroke rate for CEAP and CEE was similar, whereas in the other two 17,19 a significant superiority of CEE over CEAP was demonstrated. However, if the perioperative stroke rates from these studies are pooled (metaanalysis), the incidence is 2.2% for 2,131 CEAP procedures and 0.9% for 3,935 CEE procedures (two-tailed P = .0001). The incidence of perioperative stroke in the present series was similar in the CEAP and CEE groups, although it was higher in patched than in everted ICAs (3.5% vs. 0%). It correlates closely with the results of a larger recently published study. 20 Although the difference was not significant, the absence of early major neurologic events in the CEE group appears impressive and must be taken into account.
In reviewing our data, there are several statistically and clinically significant findings relating to the improved early results with CEE. The higher incidence of residual angulation after patching, compared with the lack of residual distal elongation in eversion (10.5% vs. 0%;P =.001), indicates that this defect may be a major determinant of perioperative stroke secondary to primary thrombosis and a predictive factor for late occlusive events. We found a residual angulation in the two patched patients with perioperative occlusion who underwent reexploration immediately after surgery, and this was detected during long-term follow-up in all patched arteries subsequently found to be occluded. The same finding was reported by Vanmaele et al. 16 The endarterectomized vessel is known to be thrombogenic, however, and this, combined with a zone of angulation, may be enough to produce thrombosis. The atherosclerotic plaque may act as a stent for the carotid axis, with redundancy becoming more apparent once the plaque has been removed. Patch closure does not necessarily solve the problem of redundant ICA length, whereas this is always rectified by the eversion technique and reimplantation. However, many authors have reported an improvement in their outcome after standard CEA when they started resecting all kinks, or shortening or transversely folding all elongated ICAs. 21,25,30,31
CEE is a rapid surgical procedure. The carotid cross-clamping (P = .01), hemostasis (P = .005), and overall surgical (P = .01) times are significantly lower than for CEAP. The need for an indwelling shunt is significantly reduced (P < .001), although its insertion proved no more difficult than during a conventional CEA, 28,32 and all the technical problems and clinical situations leading to shunt misplacement are consequently minimized. In our hands, CEE takes an average of 9 minutes (range 5–18). Whenever electroencephalographic abnormalities appear immediately after clamping, the transection, eversion, and reimplantation of the ICA into the common carotid takes no more than 5 or 6 minutes. To reduce clamping time further, blood flow is restored in the ICA first, then the defect in the common carotid is closed. When the onset of electroencephalographic changes is delayed, the procedure is nearing completion. In both circumstances, the indwelling shunt is not used because carotid clamping is always kept within a safe time interval of less than 6 minutes. 33
Although CEE has been criticized as not suitable for every patient for technical reasons, 17,19,26,28 no patient was rejected for CEE as a result of inadequate exposure of the ICA or perceived inability to obtain an adequate reconstruction, no CEE was aborted or incomplete, and no special exposure techniques (e.g., physiologic mandibular subluxation) were used. In our experience, the hypoglossal nerve is always exposed and the elongation distal to the plaque is always sought. To facilitate circumferential mobilization of the ICA, best performed after its transection, to evert the adventitia completely over the atherosclerotic core of an extensive plaque, the vessel is sometimes transposed anteriorly to the hypoglossal nerve. After eversion, the end point may be closely and directly visualized for its entire circumference, thus enabling careful débridement of all circular muscular fibers and complete removal of loose fragments. The CEE technique preserves the original carotid configuration, especially with regard to the inclination and amplitude of the ostium of the ICA. This streamlined configuration, in which the ICA diameter is increased, 34 resembles the nonstenotic unoperated artery more closely than after patch closure, thus offering theoretical hemodynamic advantages in terms of minimizing turbulence and the potential for restenosis. 35
The rate of restenosis after CEA can be interpreted as a measure of CEA durability. In four of the above-mentioned five studies, data were available on stenoses greater than 50% in the first year after CEA. 16,18–20 The restenosis rate was 2.7% (38/1,416) for the CEAP group and 0.7% (23/3,196) for the CEE group (two-tailed P < .0001). These pooled results are consistent with the 4.7% and 0% restenosis rates reported here for the CEAP and CEE groups, respectively (P = .02). In addition, CEE had a significantly better cumulative patency rate than CEAP (P = .001), and freedom from restenosis at 24 and 36 months was 87% and 83% for CEAP and 98% and 98% for CEE, respectively. All these findings on the durability of the CEE procedure are consistent with our previously published data. 20
The present series, however, allows an evaluation of whether local or systemic factors predominate in the pathogenesis of the restenoses. Because patients received CEAP on one side and CEE on the other, each patient served as his or her own control: unilateral restenosis would suggest a predominance of local factors, and bilateral restenosis would implicate systemic factors. Multiple regression analysis revealed that none of the commonly implicated systemic etiologic factors were associated with restenosis. A trend toward the predominance of local factors was suggested by Rossi et al, 36 however, and confirmed by AbuRahma et al 37 in the only two randomized studies found in the English medical literature (Medline, 1966 to July 1999) that analyze the durability of bilateral CEA in patients who received primary closure on one side and patching on the other. These findings correlate closely with the absence of bilateral restenoses in our study, and because all restenoses were in the CEAP group, the influence of technical factors in the type of closure appears evident.
In conclusion, although the sample size is small, this prospective randomized series offers a valid comparison between traditional longitudinal patched carotid arteriotomy and transverse carotid arteriotomy in the same patient. Our findings provide further evidence that CEE is less likely than CEAP to cause perioperative neurologic complications and restenosis. In addition, the significantly higher incidence of unilateral restenosis in the CEAP group supports the hypothesis that local factors play an important role in the cause of recurrent stenosis.
Footnotes
Correspondence: Enzo Ballotta, MD, Service of Vascular Surgery, Dept. of Medical & Surgical Sciences, University of Padua, School of Medicine, Policlinico Universitario, Via N. Giustiniani, 2, 35128 Padova, Italy.
E-mail: ballotta@ux1.unipd.it
Accepted for publication September 30, 1999.
References
- 1.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325:445–453. [DOI] [PubMed] [Google Scholar]
- 2.European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991; 337:1235–1243. [PubMed] [Google Scholar]
- 3.Mayo Asymptomatic Carotid Endarterectomy Group. Results of a randomized controlled trial of carotid endarterectomy for asymptomatic carotid stenosis. Mayo Clin Proc 1992; 67:513–518. [DOI] [PubMed] [Google Scholar]
- 4.Asymptomatic Study Executive Committee. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995; 273:1421–1428. [PubMed] [Google Scholar]
- 5.Hobson RW, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. N Engl J Med 1993; 328;221–227. [DOI] [PubMed] [Google Scholar]
- 6.European Carotid Surgery Trialists’ Collaborative Group. Randomized trial of endarterectomy for recently symptomatic carotid stenosis: final result of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387. [PubMed] [Google Scholar]
- 7.Eikelboom BC, Ackerstaff RGA, Hoeneveld H, et al. Benefits of carotid patching: a randomized study. J Vasc Surg 1988; 7:240–247. [DOI] [PubMed] [Google Scholar]
- 8.Lord RSA, Raj TB, Stary DL, et al. Comparison of saphenous vein patch, polytetrafluoroethylene patch and direct arteriotomy closure after carotid endarterectomy—Part I: perioperative results. J Vasc Surg 1989; 9:521–529. [PubMed] [Google Scholar]
- 9.Clagett GP, Patterson CB, Fisher DF Jr, et al. Vein patch versus primary closure for carotid endarterectomy. A randomized prospective study in a selected group of patients. J Vasc Surg 1989; 9:213–223. [DOI] [PubMed] [Google Scholar]
- 10.Ranaboldo CJ, D’sa AABB, Bell PRF, et al, for the Joint Vascular Research Group. Randomized controlled trial of patch angioplasty for carotid endarterectomy. Br J Surg 1993; 80:1528–1530. [DOI] [PubMed] [Google Scholar]
- 11.Katz D, Snyder SO, Gandhi RH, et al. Long-term follow-up for recurrent stenosis: a prospective randomized study of expanded polytetrafluoroethylene patch angioplasty versus primary closure after carotid endarterectomy. J Vasc Surg 1994; 19:198–205. [DOI] [PubMed] [Google Scholar]
- 12.Myers SI, Valentine RJ, Chervu A, et al. Saphenous vein patch versus primary closure for carotid endarterectomy: long-term assessment of a randomized prospective study. J Vasc Surg 1994; 19:15–22. [DOI] [PubMed] [Google Scholar]
- 13.AbuRahma AF, Khan JH, Robinson PA, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein and polytetrafluoroethylene: perioperative (30-day) results. J Vasc Surg 1996; 24:998–1007. [DOI] [PubMed] [Google Scholar]
- 14.AbuRahma AF, Robinson PA, Saied S, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein and polytetrafluoroethylene: long-term follow-up. J Vasc Surg 1998; 27:222–234. [DOI] [PubMed] [Google Scholar]
- 15.DeBakey ME, Crawford ES, Cooley DA, Morris GC. Surgical considerations of occlusive disease of innominate, carotid, subclavian and vertebral arteries. Ann Surg 1959; 149:690–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanmaele RG, Van Schil PE, De Maeseneer MG, et al. Division-endarterectomy-anastomosis of the internal carotid artery: a prospective randomized comparative study. Cardiovasc Surg 1994; 2:573–581. [PubMed] [Google Scholar]
- 17.Entz L, Jaranyi ZS, Nemes A. Comparison of perioperative results obtained with eversion endarterectomy and with conventional patch plasty. Cardiovasc Surg 1997; 5:16–20. [DOI] [PubMed] [Google Scholar]
- 18.Cao P, Giordano G, De Rango P, et al, and collaborators of the EVEREST Study Group. A randomized study on eversion versus standard carotid endarterectomy: study design and preliminary results: the EVEREST trial. J Vasc Surg 1998; 27:595–605. [DOI] [PubMed] [Google Scholar]
- 19.Shah DM, Darling RC III, Chang BB, et al. Carotid endarterectomy by eversion technique. Its safety and durability. Ann Surg 1998; 228:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballotta E, Da Giau G, Saladini M, et al. Carotid endarterectomy with patch closure versus carotid eversion endarterectomy and reimplantation: a prospective randomized study. Surgery 1999; 125:271–279. [PubMed] [Google Scholar]
- 21.Riles TS, Imparato AM, Jacobowitz GR, et al. The cause of perioperative stroke after carotid endarterectomy. J Vasc Surg 1994; 19:206–216. [DOI] [PubMed] [Google Scholar]
- 22.Ballotta E, Da Giau G, Abbruzzese E, et al. Carotid endarterectomy without angiography: can clinical evaluation and duplex ultrasonography scanning alone replace traditional arteriography for carotid surgery workup? A prospective study. Surgery 1999; 126:20–27. [DOI] [PubMed] [Google Scholar]
- 23.Baker JD, Rutherford RB, Bernstein EF, et al. Suggested standards for reports dealing with cerebrovascular disease. J Vasc Surg 1988; 8:721–729. [DOI] [PubMed] [Google Scholar]
- 24.Deriu GP, Ballotta E, Bonavina L, et al. The rationale for patch graft angioplasty after carotid endarterectomy: early and long-term results. Stroke 1984; 15:972–979. [DOI] [PubMed] [Google Scholar]
- 25.Bosse A, Ansorg P, Mayer B, Mulch J. Eversion endarterectomy of the internal carotid artery. Thorac Cardiovasc Surg 1991; 39:371–375. [DOI] [PubMed] [Google Scholar]
- 26.Berguer R. Eversion endarterectomy of the carotid bifurcation. In: Veith FJ, ed. Current Critical Problems in Vascular Surgery. Vol. 5. St. Louis: Quality Medical Publishing; 1993: 441–447.
- 27.Kieny R, Hirsch D, Seiller C, et al. Does carotid eversion endarterectomy and reimplantation reduce the risk of restenosis? Ann Vasc Surg 1993; 7:407–413. [DOI] [PubMed] [Google Scholar]
- 28.Koskas F, Kieffer E, Bahnini A, et al. Carotid eversion endarterectomy: short- and long-term results. Ann Vasc Surg 1995; 9:9–15. [DOI] [PubMed] [Google Scholar]
- 29.Raithel D. Carotid eversion endarterectomy: a better technique than standard operation? Cardiovasc Surg 1997; 5:471–472. [DOI] [PubMed] [Google Scholar]
- 30.Archie JP. Carotid endarterectomy with reconstruction techniques tailored to operative findings. J Vasc Surg 1993; 17:141–151. [DOI] [PubMed] [Google Scholar]
- 31.Coyle KA, Smith RB III, Chapman RL, et al. Carotid artery shortening: a safe adjunct to carotid endarterectomy. J Vasc Surg 1995; 22:257–263. [DOI] [PubMed] [Google Scholar]
- 32.Reigner B, Reveilleau P, Gayral M, et al. Eversion endarterectomy of the internal carotid artery: mid-term results of a new technique. Ann Vasc Surg 1995; 9:141–146. [DOI] [PubMed] [Google Scholar]
- 33.Sundt TM, Sharbrough FW, Marsh WR, et al. The risk-benefit ratio of intraoperative shunting during carotid endarterectomy. Ann Surg 1986; 203:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah DM, Darling RC III, Chang BB, et al. Carotid endarterectomy by eversion technique: comparison of results with standard technique. In: Veith FJ, ed. Current Critical Problems in Vascular Surgery. Vol. 8. St. Louis: Quality Medical Publishing; 1997.
- 35.Baan J Jr, Thompson JM, Reul GJ, et al. Vessel wall and flow characteristics after carotid endarterectomy: eversion endarterectomy compared with Dacron patch plasty. Eur J Vasc Endovasc Surg 1997; 13:583–591. [DOI] [PubMed] [Google Scholar]
- 36.Rossi PJ, Valentine RJ, Myers SI, et al. The durability of bilateral carotid endarterectomy. Ann Vasc Surg 1995; 9:16–20. [DOI] [PubMed] [Google Scholar]
- 37.AbuRahma AF, Robinson PA, Saiedy S, et al. Prospective randomized trial of bilateral carotid endarterectomy: primary closure versus patching. Stroke 1999; 30:1185–1189. [DOI] [PubMed] [Google Scholar]