Abstract
Objective
To characterize the longer-term therapeutic response of permanent sacral nerve stimulation for fecal incontinence and to delineate suitable indications and the mode of action.
Summary Background Data
A single report of permanent sacral nerve stimulation in three patients followed up for 6 months showed marked improvement in fecal continence. Acute evaluation has shown that the effect may be mediated by altered rectal and anal smooth muscle activity, and facilitation of external sphincter contraction.
Methods
Five women (age 41–68 years) with fecal incontinence for solid or liquid stool at least once per week were followed up for a median of 16 months after permanent implantation. All had passive incontinence, and three had urge incontinence. The cause was scleroderma in two, primary internal sphincter degeneration in one, diffuse weakness of both sphincters in one, and disruption of both sphincters in one.
Results
All patients had marked improvement. Urgency resolved in all three patients with this symptom. Passive soiling resolved completely in three and was reduced to minor episodes in two. Continence scores (scale 0–20) improved from a median of 16 before surgery to 2 after surgery. There were no early complications, and there have been no side effects. One patient required wound exploration at 6 months for local pain, and a lead replacement at 12 months for electrode displacement. The quality of life assessment improved in all patients. The resting pressure increased in four patients, but there was no consistent measured physiologic change that could account for the symptomatic improvement.
Conclusions
In patients with sphincter degeneration and weakness, and possibly in those with sphincter disruption, sacral nerve stimulation markedly improves fecal incontinence.
There is no simple surgical procedure that reliably restores continence in patients with structurally intact but weak anal sphincter muscles or in patients with disruption of the internal anal sphincter. These patients are traditionally managed with conservative therapies such as antidiarrheal agents and dietary manipulation. More recently, reports on the use of other pharmacologic therapies, such as phenylephrine and oral amitriptyline, have shown success in some patients with internal sphincter dysfunction. Phenylephrine applied topically to the anus has been shown to increase anal sphincter resting pressure. 1 Low-dose oral amitriptyline may inhibit rectal motor activity and reduce passive leakage caused by a weakened anal sphincter. One report on sphincter injection augmentation with GAX (gluteraldehyde cross-linked) collagen as a bulking agent found marked short-term improvement in 71% of patients with internal sphincter dysfunction or an internal sphincter defect. 2 Although biofeedback behavioral treatment produces improvement in most patients in the short term, benefit is more consistently observed in patients with external as opposed to internal sphincter dysfunction. 3 The medium- and long-term efficacy of all these treatments are unknown.
An alternative approach to the management of these patients may be to modulate the neurologic control of the anorectum. This approach, known as sacral nerve stimulation, has previously been used with success for urologic incontinence. 4–6 The short-term results of its use in three patients with fecal incontinence with a weak but structurally intact external sphincter have also been reported. 7 Using this technique with temporary percutaneously placed electrodes, we have shown in a previous acute study that there is an effect on rectal and internal sphincter smooth muscle activity, in addition to facilitation of the external sphincter striated muscle function. 8
This study describes the use of permanently implanted sacral nerve stimulation in patients with internal or external sphincter (or both) weakness, and one patient with weakness associated with structural sphincter damage.
METHODS
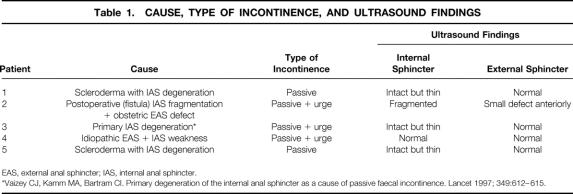
Five women, median age 59 years (range 41–68), underwent implantation of a permanent sacral electrode and stimulator. All had fecal incontinence to solid or liquid stool at least once per week, documented on a prospectively recorded diary card. The median preoperative duration of symptoms was 3 years (range 3–6). All had failed to improve adequately with conservative treatment with antidiarrheal drugs. Details of the causes of incontinence, preoperative symptoms, and ultrasound findings are shown in Table 1. Patient 2, with internal sphincter fragmentation and an external sphincter defect, had undergone a postanal repair 2 years previously. Patient 4, with external and internal sphincter weakness, had failed to improve with a full course of biofeedback treatment.
Table 1. CAUSE, TYPE OF INCONTINENCE, AND ULTRASOUND FINDINGS
EAS, external anal sphincter; IAS, internal anal sphincter.
*Vaizey CJ, Kamm MA, Bartram CI. Primary degeneration of the internal anal sphincter as a cause of passive faecal incontinence. Lancet 1997; 349:612–615.
The surgical procedure and equipment used for permanent electrode and stimulator implantation have been described previously. 4,7 Before permanent implantation, patients underwent temporary percutaneous stimulation to assess their likely response to treatment. Patients 1 and 3 had a 1-week trial of sacral nerve stimulation using a percutaneously placed wire electrode (Medtronic 041830–004; Medtronic, Minneapolis, MN) attached to an external stimulator (Medtronic Screener Model 3625). 8 The remaining three patients had a 3-week trial of stimulation with a surgically implanted fixed sacral electrode (Medtronic 3080); it was connected by a subcutaneously tunneled extension lead (Medtronic 7495) with a percutaneous connection to the same type of external stimulator. To ensure optimal placement of unilateral implanted electrodes, the sacral nerve root that produced the maximal anal response to stimulation (S3 in all cases) was identified by percutaneous needle stimulation. An incision over the sacrum allowed access to the sacral foramen, and the implanted electrodes were inserted directly into the foramen and were secured with sutures to the sacral periosteum.
Stimulation for the 1- or 3-week test periods was at a level just above the sensory threshold for each patient. It varied from 0.4 to 2.0 volts. Pulse frequency (15 pulses/sec) and pulse width (210 microseconds) were the same for all patients and were kept constant throughout the period of study.
For all patients, the decision to progress from temporary to permanent stimulation with the implantation of a complete stimulating system was made on the basis of a greater than 50% improvement in either the number of episodes of incontinence or incontinence-free days during the test period. This was documented using a symptom diary card that detailed the number of episodes of incontinence to solid or liquid stool each day. The use of a diary has been previously validated for the assessment of fecal incontinence in our department. 9 All patients clearly satisfied the criteria for permanent stimulation.
The two patients who had been evaluated with a percutaneous electrode had a permanent sacral electrode surgically placed. All five patients had a stimulator (Medtronic Interstim Implantable Pulse Generator 3023) placed in the anterior abdominal wall, superficial to the rectus muscle. Stimulators were connected to the implanted sacral electrode by a subcutaneously tunneled lead. Once implanted, stimulators were left turned on continuously at a level just above that required to produce threshold perineal sensation.
Fecal continence scores (Cleveland Clinic Continence Scoring System) 10 and quality of life assessments (Short Form Health Survey Questionnaire [SF-36]) 11 were obtained before stimulation and with permanent stimulation. Anorectal physiologic testing was performed before stimulation, during the period of trial stimulation with the external stimulator, and with the permanently implanted stimulator. This included maximum resting and squeeze anal pressures, and rectal sensory threshold, sensation of urgency, and maximum tolerated rectal volume to balloon distention with air. Manometry was undertaken using a stationary pull-through technique 12 with an eight-channel water-perfused system, and the squeeze pressure was measured as the mean incremental rise above resting pressure. Rectal sensation was tested using balloon distention with air. 13
In view of the small number of patients in this study, only limited statistical analysis has been performed, and data are presented in full.
RESULTS
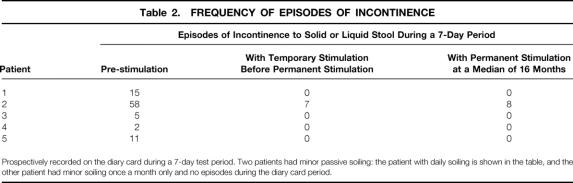
All five patients had marked symptom improvement. Table 2 shows the episodes of incontinence during a 1-week period before implantation, with temporary stimulation, and with permanent stimulation at a median of 16 months (range 3–26). Fecal urgency resolved in all three patients with this as a presenting symptom. Passive soiling resolved in three patients, was reduced to less than monthly minor episodes in one, and was reduced to daily minor episodes in one. Cleveland Clinic continence scores (0–20 scale; 0 = perfect continence, 20 = total incontinence) improved from a median of 16 (range 13–20) before implantation to 2 (range 0–13) with implantation (P < .001). The patient with a postoperative continence score of 13 and daily episodes of minor fecal soiling had near-perfect continence immediately after implantation, and again after reimplantation of her displaced sacral electrode at 12 months. She has had a recent deterioration in symptoms with the onset of diarrhea, and this is thought to relate to possible lead displacement.
Table 2. FREQUENCY OF EPISODES OF INCONTINENCE
Prospectively recorded on the diary card during a 7-day test period. Two patients had minor passive soiling: the patient with daily soiling is shown in the table, and the other patient had minor soiling once a month only and no episodes during the diary card period.
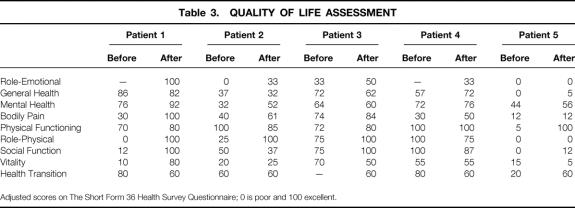
Table 3 details the SF-36 quality of life assessment measured before implantation and at a median follow-up of 16 months (range 3–16). All patients showed an overall improvement with permanent sacral stimulation in several of the parameters measured.
Table 3. QUALITY OF LIFE ASSESSMENT
Adjusted scores on The Short Form 36 Health Survey Questionnaire; 0 is poor and 100 excellent.
Anorectal Physiologic Testing
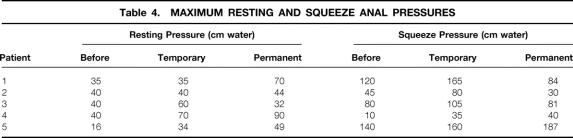
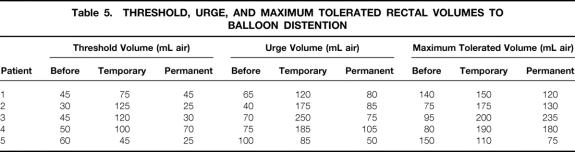
Results of anorectal physiologic testing before stimulation, with temporary stimulation, and with permanent stimulation at a median of 16 months (range 3–26) are shown in Tables 4 and 5. Although the involuntary resting pressure was improved in four of the five patients, there was no clearly definitive change in either resting pressure (40 [range 16–40] vs. 49 [32–90] cm water, before vs. after stimulation;P = .10) or squeeze pressure (80 [10–140] vs. 81 [30–187] cm water;P = .72) with permanent, continuous stimulation. In particular, voluntary contraction showed no consistent change. Threshold and urge sensory volumes to distention were altered in the short term but returned to original values in the long term. In the long term, there appeared to be no change in threshold volume to balloon distention (45 [30–60] vs. 30 [25–70] mL;P = .48) or in urge volume (70 [40–100] vs. 80 [50–105] mL;P = .61]. There was a possible increase in maximum tolerated volume (95 [75–150] vs. 130 [75–235] mL;P = .36). Pudendal nerve-terminal motor latencies were normal before surgery on both sides in four patients, and on one side in the remaining patient. After stimulation, pudendal latencies were difficult to measure because of stimulation artifact and have not been recorded.
Table 4. MAXIMUM RESTING AND SQUEEZE ANAL PRESSURES
Table 5. THRESHOLD, URGE, AND MAXIMUM TOLERATED RECTAL VOLUMES TO BALLOON DISTENTION
Complications
There were no perioperative complications, and there have been no side effects of chronic stimulation. One patient required wound exploration at the site of connection of the sacral and stimulator extension leads (left loin) at 6 months for local discomfort. There were no abnormal findings, and the discomfort subsequently resolved. The same patient required reimplantation of the sacral lead at 12 months after traumatic lead displacement from the sacral foramen, with restoration of continence.
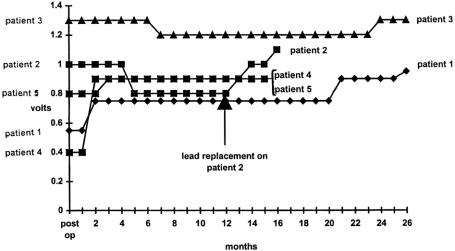
Stimulation Voltages
Permanent stimulation amplitude was adjusted with time to fine-tune continence. Stimulation values over time are shown in Figure 1. There was no set pattern to stimulation requirements. Wavelength (210 msec), frequency (15 Hz), and stimulation mode (continuous) were constant throughout the period of the study.
Figure 1. Permanent stimulation amplitude as a function of time in individual patients.
DISCUSSION
This study has shown that permanent sacral nerve stimulation is an effective treatment for fecal incontinence in patients with both internal and external sphincter insufficiency. A novel aspect of this therapy for fecal incontinence is the ability to perform a trial of treatment over a 1- to 3-week test period before the decision is made to proceed with permanent implantation. The high incidence of displacement of a percutaneously placed test electrode 14 led to a modification in our technique in three patients in this study. The introduction of a surgically fixed electrode for the test period ensured that these three patients had a 3-week test period with no loss of effect of the stimulation. With either technique, the operation is simple and carries a low complication rate. No bowel preparation is necessary, and patients are mobilized and given a full diet the same day as their surgery. In addition to the symptomatic improvement documented on the diary card, quality of life was also shown to improve in the medium term.
The only previous report of the results of treatment with this technique in fecal incontinence was confined to patients with external sphincter weakness. 7 All three patients had an intact external sphincter, and it was assumed that the main effect of stimulation was by means of the alpha motor fibers, with resultant facilitation of the external sphincter muscle and enhancement of squeeze pressures. A detailed study looking at the possible modes of action of sacral nerve stimulation, 8 using both static and ambulatory physiologic techniques, showed that in addition to facilitation of the external sphincter muscle, there is also neuromodulation of sacral reflexes that regulate rectal sensitivity and contractility, and anal motility. In this study, none of the five patients had isolated external sphincter weakness. All had internal sphincter weakness, one had external sphincter weakness in addition to internal sphincter weakness, and one had a small defect in the external sphincter. This study failed to show any definite long-term evidence of external sphincter muscle facilitation. Internal sphincter tone may have been improved.
In a previous study of two patients who used implants for a mean of 9 months, we turned the stimulator off and on again in a double-blind manner to assess whether stimulation remains effective and to distinguish the effect from placebo. That study demonstrated recurrence of incontinence when the stimulator was turned off, suggesting that the beneficial effect is related to the stimulation and is dependent on continued stimulation—that is, stimulation does not induce any permanent change in function, at least not in the duration of treatment we observed.
As for other available treatments for fecal incontinence of varying causes, this treatment needs to be considered in the context of long-term efficacy. Although short- to medium-term benefit has been shown in up to 80% of patients undergoing anterior overlapping sphincter repair 15 or postanal repair 16 for fecal incontinence, the long-term results are less encouraging. The long-term success of overlapping repair is approximately 50%, 17 and 5-year continence rates may be as low as 26% with postanal repair. 16,18 This study has shown that the clinical effects of sacral nerve stimulation for fecal incontinence are maintained in the medium term (median 16 months). Using similar techniques as described in this article, sacral nerve stimulation appears to maintain good long-term results when used for the treatment of urologic disorders. 19–21 There is some evidence to support the long-term efficacy of dynamic graciloplasty 22 and the artificial bowel sphincter 23,24 for fecal incontinence, but series are few and numbers are small. Further studies are indicated to refine the indications for sacral nerve stimulator implantation, to prove its efficacy in a multicenter trial, and to assess the long-term results.
Footnotes
Correspondence: Michael A. Kamm, MD, St. Mark’s Hospital, Watford Road, Harrow, Middlesex HA1 3UJ, United Kingdom.
Dr. Vaizey is currently with The Middlesex Hospital, London.
Accepted for publication October 7, 1999.
References
- 1.Carapeti EA, Kamm MA, Evans BK, Phillips RKS. Topical phenylephrine increases anal sphincter resting pressure. Br J Surg 1999; 86:267–270. [DOI] [PubMed] [Google Scholar]
- 2.Kumar D, Benson M, Bland J. Glutaraldehyde cross-linked collagen in the treatment of faecal incontinence. Br J Surg 1998; 85:978–979. [DOI] [PubMed] [Google Scholar]
- 3.Norton C, Kamm MA. Outcome of biofeedback for faecal incontinence. Br J Surg 1999; 86:1159–1163. [DOI] [PubMed] [Google Scholar]
- 4.Bosch JLHR, Groen J. Sacral (S3) segmental nerve stimulation as a treatment for urge incontinence in patients with detrusor instability: results of chronic electrical stimulation using an implantable neural prosthesis. J Urol 1995; 154:504–507. [DOI] [PubMed] [Google Scholar]
- 5.Hassouna MM, Elhilali MM. Role of the sacral root stimulator in voiding dysfunction. World J Urol 1991; 9:145–148. [Google Scholar]
- 6.Thon WF, Baskin LS, Jonas U, et al. Neuromodulation of voiding dysfunction and pelvic pain. World J Urol 1991; 9:138–141. [Google Scholar]
- 7.Matzel KE, Stadelmaier U, Hohenfeller M, Gall FP. Electrical stimulation of spinal nerves for treatment of faecal incontinence. Lancet 1995; 346:1124–1127. [DOI] [PubMed] [Google Scholar]
- 8.Vaizey CJ, Kamm MA, Turner I, et al. Effects of short-term sacral nerve stimulation on anal and rectal function in patients with anal incontinence. Gut 1999; 44:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaizey CJ, Carapeti E, Cahill J, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut 1999; 44:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum 1993; 36:77–97. [DOI] [PubMed] [Google Scholar]
- 11.Brazier JE, Harper R, Jones NMB, et al. Validating the SF-36 Health Survey Questionnaire: new outcome measure for primary health care. Br Med J 1992; 305:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neill ME, Parks AG, Swash M. Physiology studies of the pelvic floor in idiopathic faecal incontinence and rectal prolapse. Br J Surg 1981; 68:531–536. [DOI] [PubMed] [Google Scholar]
- 13.Kamm MA, Lennard-Jones JE. Rectal mucosal electrosensory testing—evidence for a rectal sensory neuropathy in idiopathic constipation. Dis Colon Rectum 1990; 33:419–423. [DOI] [PubMed] [Google Scholar]
- 14.Janknegt RA, Weil EH, Eerdmans P. Improving neuromodulation technique for refractory voiding dysfunctions: two-stage implant. Abstracts from the Proceedings of the International Continence Society. Neurol Urodyn 1996; 15:403–404. [Google Scholar]
- 15.Engel AF, Sultan AH, Kamm MA, et al. Anterior sphincter repair for patients with obstetric trauma. Br J Surg 1994; 81:1231–1234. [DOI] [PubMed] [Google Scholar]
- 16.Jameson JS, Speakman CT, Darzi A, et al. Audit of postanal repair in the treatment of faecal incontinence. Br J Surg 1994; 37:369–372. [DOI] [PubMed] [Google Scholar]
- 17.Malouf AJ, Norton CS, Engel AF, et al. Long-term results of overlapping anterior anal sphincter repair for obstetric trauma. Lancet 2000; 355:260–265. [DOI] [PubMed] [Google Scholar]
- 18.Setti Carraro P, Kamm MA, Nicholls RJ. Long-term results of postanal repair for neurogenic faecal incontinence. Br J Surg 1994; 81:140–144. [DOI] [PubMed] [Google Scholar]
- 19.Bosch JLHR. Sacral neuromodulation in the treatment of the unstable bladder. Curr Opin Urol 1998; 8:287–291. [DOI] [PubMed] [Google Scholar]
- 20.Ruud Bosch JLH, Groen J. Neuromodulation: urodynamic effects of sacral (S3) spinal nerve stimulation in patients with detrusor instability or detrusor hyperflexia. Behav Brain Res 1992; 92:141–150. [DOI] [PubMed] [Google Scholar]
- 21.Weil EHJ, Ruiz-Cerda JL, Eerdmans PHA, et al. Clinical results of sacral neuromodulation for chronic voiding dysfunction using unilateral sacral foramen electrodes. World J Urol 1998; 16:313–321. [DOI] [PubMed] [Google Scholar]
- 22.Cavina E. Outcome of restorative perineal graciloplasty with simultaneous excision of the anus and rectum for cancer. A ten-year experience with 81 patients. Dis Colon Rectum 1996; 39:182–190. [DOI] [PubMed] [Google Scholar]
- 23.Wong WD, Jensen LL, Bartolo DCC, Rothenberger DA. Artificial anal sphincter. Dis Colon Rectum 1996; 39:1345–1351. [DOI] [PubMed] [Google Scholar]
- 24.Lehur PA, Glemain P, Bruley des Varannes S, et al. Outcome of patients with an implanted artificial anal sphincter for severe faecal incontinence. Int J Colorect Dis 1998; 13:88–92. [DOI] [PubMed] [Google Scholar]