Abstract
Objective
To investigate the value of colonoscopic miniprobe ultrasonography for preoperative staging of colorectal neoplasms.
Summary Background Data
Endoscopic ultrasonography is the most accurate technique for staging colorectal cancer. However, limitations of this technique include the inability to examine stenotic tumors and the difficulty of reaching tumors proximal to the rectum.
Methods
Miniprobe ultrasonography (12.5 MHz) was performed in 63 patients with tumors of the colon or rectum. The results of imaging were compared with endoscopic assessment of the lesions and histopathologic findings of the resected specimens.
Results
Miniprobe ultrasonography allowed high-resolution imaging of colorectal tumors during routine colonoscopy. The infiltration depth was correctly classified in 22 adenoma, 3 T1, 10 T2, and 22 T3 or T4 tumors. The accuracy for tumors of the rectum and colon was 86% and 92%, respectively (overall accuracy 90%). The small diameter of the probe allowed examination of 21 stenotic tumors with an accuracy of 86%. Miniprobe ultrasonography revealed carcinoma in 5 of 30 broad-based polyps, although adenomas were diagnosed by endoscopy. Correct assessment of lymph node involvement was obtained in 47 of 55 patients. Based on the findings of miniprobe ultrasonography, management was modified in 7 of the 63 patients.
Conclusions
These preliminary results show that miniprobe ultrasonography improves preoperative staging of stenotic rectal cancer and colonic tumors. This technique can be easily performed during routine colonoscopy and may have considerable impact on surgical therapy.
The preoperative staging and treatment planning of rectal cancer have been significantly improved by endoscopic ultrasonography (EUS). This technique enables us to evaluate locoregional tumor spread accurately and provides the criteria to select the appropriate surgical approach. Depending on the tumor stage, different treatment concepts, including local excision, radical resection, and multimodality therapy, are available for rectal cancer. 1–3
In the past few years, minimally invasive procedures such as endoscopic and laparoscopic resection have been increasingly used in the management of colorectal tumors. Nonetheless, there is considerable controversy among surgeons regarding the appropriateness of such procedures for the management of colon cancer. 4 A sensitive technique for preoperative staging of colon cancer could improve the selection of patients who can be treated effectively by minimally invasive techniques.
However, EUS of the colon has not yet found broad clinical acceptance because of technical drawbacks. Ultrasound colonoscopes are difficult to maneuver and cannot be passed over tight strictures because of the rigidity and the large diameter of the tip. 5 Therefore, EUS of the colon is performed as a separate procedure after diagnostic endoscopy, which makes the examination expensive, time-consuming, and uncomfortable for the patient.
Recently, miniaturized ultrasound probes (miniprobes) have been developed that can be introduced through the instrument channel of the endoscope. This technique could resolve most of the problems encountered with conventional EUS.
The aim of the present study was to examine the accuracy of miniprobe ultrasonography in the preoperative staging of colorectal cancer and to determine the relevance of this technique for making therapeutic decisions.
METHODS
Miniprobe EUS was performed in 63 consecutive patients with broad-based polyps or adenocarcinoma of the colon or rectum. The tumors were located in the right hemicolon (n = 5), transverse colon (n = 4), left hemicolon (n = 40), and rectum (n = 14). Most rectal tumors were inaccessible to conventional EUS because of stenosis or proximal location. The patient group comprised 35 men and 28 women with a mean age of 62 years (range 35–87 years). All patients underwent surgical resection of the tumor (n = 47) or endoscopic snare resection (n = 16). The results of miniprobe EUS were compared with endoscopic assessment (malignant vs. benign) and histopathology of the resection specimens.
Miniprobe EUS of colorectal tumors was carried out during routine colonoscopy using ultrathin probes with a diameter of 6F (Endosound; Boston Scientific, Watertown, MA) and a B&K ultrasound unit (Bruel & Kjaer 3553, Gentofte, Denmark). The 12.5-MHz transducer of the mechanical probe provided 360° images of the intestinal wall. All examinations were performed by two experienced surgeons using the following technique. The patient underwent colonoscopy in a conventional fashion. Premedication with 5 mg midazolam was administered intravenously if required. When a broad-based polyp or a carcinoma was seen at colonoscopy, EUS was performed to determine the infiltration depth and lymph node status of the tumor. The tip of the endoscope was placed at the distal end of the tumor. The lumen was filled with 200 to 300 mL water to achieve acoustic coupling between the transducer and the intestinal wall. Subsequently, the miniprobe was introduced through the instrument channel of the colonoscope and advanced beyond the tumor. The lesion was assessed by real-time ultrasonography while the catheter was moved over the tumor region. Based on the results of miniprobe EUS, the patient underwent endoscopic or standard surgical resection of the tumor.
Staging Criteria
The tumor infiltration depth was determined according to the TNM classification of the UICC. Lesions confined to the inner hypoechoic layer (mucosa) were classified as adenoma (uT0). Tumors with infiltration of the middle hyperechoic layer were assessed as T1 carcinoma. T2 or T3 tumors were diagnosed when penetration in the outer hypoechoic layer (muscularis propria) or in the peripheral echogenic layer (serosa or adventitia) was observed. Penetration of the tumor through the serosa into the free peritoneal cavity or infiltration of adjacent structures indicated a T4 situation.
The criteria for the diagnosis of metastatic lymph node involvement were low echogenicity, clearly defined boundaries, round shape, and size of more than 1 cm. The endosonographic diagnosis was compared with the histopathologic analysis of the resection specimens. The median number of dissected lymph nodes with radical surgery was 18. Information on the lymph node status was not available in eight patients who underwent transanal excision of early rectal cancer.
RESULTS
Miniprobe EUS was successfully performed in all 63 patients with colorectal adenoma or adenocarcinoma. The 12.5-MHz transducer of the miniprobe provided high-resolution images of the colorectal wall and adjacent tissues. The normal wall of the colon and rectum was displayed in five layers (Fig. 1). The first hyperechoic layer represents the interface between water and mucosa. The second hypoechoic layer corresponds to the mucosa. The submucosa is depicted as the middle hyperechoic layer, whereas the muscularis propria appears as the outer layer with low echogenicity. Adventitia or serosa was sometimes displayed as a fifth bright layer.
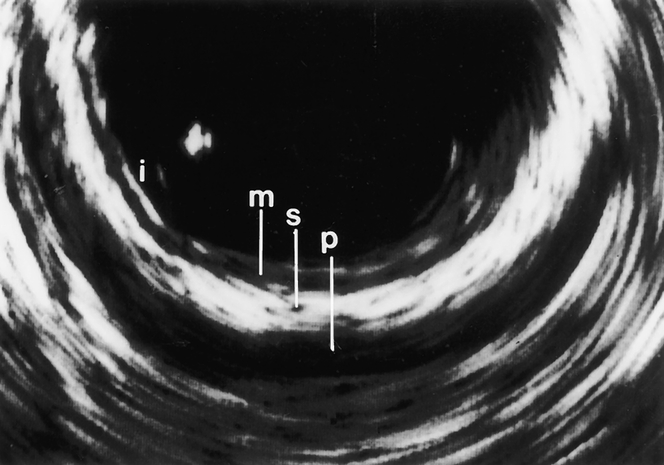
Figure 1. Miniprobe ultrasonography of the colon. All layers of the wall—mucosa (M), submucosa (S), and muscularis propria (P)—can be differentiated (i, interface between water and mucosa).
The depth of invasion was correctly determined in 57 of 63 patients with colorectal tumors (overall accuracy 90%). The detailed results are shown in Table 1. Overestimation of tumor invasion was observed in four patients. Understaging occurred in two patients with T3 tumors. Two of the misdiagnosed tumors were located in the rectum and four in the left hemicolon.
Table 1. CORRELATION BETWEEN PREOPERATIVE ENDOSCOPIC ULTRASOUND (uT) AND HISTOPATHOLOGY (pT)
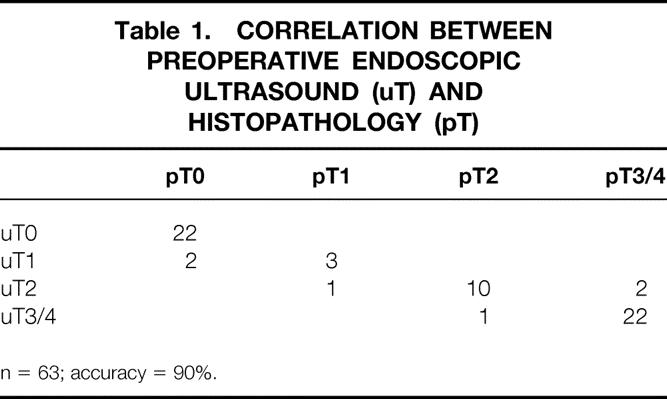
n = 63; accuracy = 90%.
Obstructing tumors that could not be traversed by the endoscope were found in 21 of the 63 patients (33%). Despite the stenosis, it was in all cases possible to advance the miniprobe over the tumor (Fig. 2). The tumor infiltration depth was correctly staged in 18 of 21 stenotic tumors (accuracy 86%). Histopathologic analysis of the resection specimens showed overstaging of a pT2 tumor. Understaging was observed in an advanced case with infiltration of the abdominal wall and a pT3 carcinoma. The accuracy of miniprobe EUS in assessing the infiltration depth of rectal and colonic tumors was 86% and 92%, respectively.
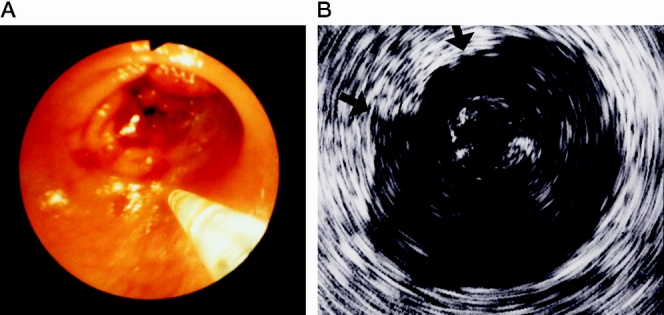
Figure 2. (A) Endoscopic view of the miniprobe and a stenotic carcinoma of the rectum. (B) Endoscopic ultrasound demonstrates a T3 tumor with infiltration of all layers of the rectum wall and penetration into the perirectal fat (arrows).
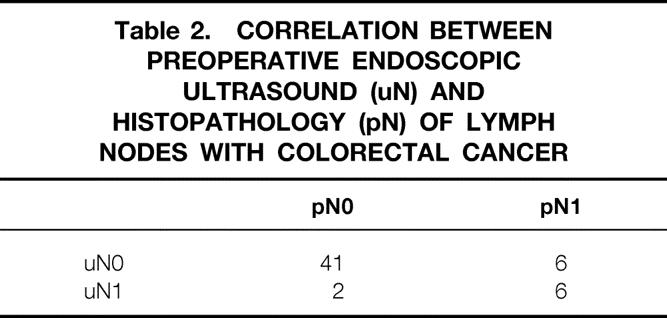
The accuracy of EUS in predicting lymph node involvement was 85%. Lymph node metastases were diagnosed in 6 of 12 patients. The absence of lymph node metastases was demonstrated in 41 of 43 patients (Table 2).
Table 2. CORRELATION BETWEEN PREOPERATIVE ENDOSCOPIC ULTRASOUND (uN) AND HISTOPATHOLOGY (pN) OF LYMPH NODES WITH COLORECTAL CANCER
Miniprobe scanning was very accurate in the diagnosis of broad-based polyps of the rectum and colon (Table 3). Benign and malignant lesions were correctly classified in 29 of 30 patients (97%). Carcinoma with infiltration of the submucosa was identified in five patients, although endoscopy or biopsy had suggested a benign adenoma (Fig. 3). The infiltration depth was overestimated in an adenoma with severe dysplasia that was classified as T1 carcinoma.
Table 3. ASSESSMENT OF POLYPOID COLORECTAL TUMORS BY ENDOSCOPY, BIOPSY, AND MINIPROBE
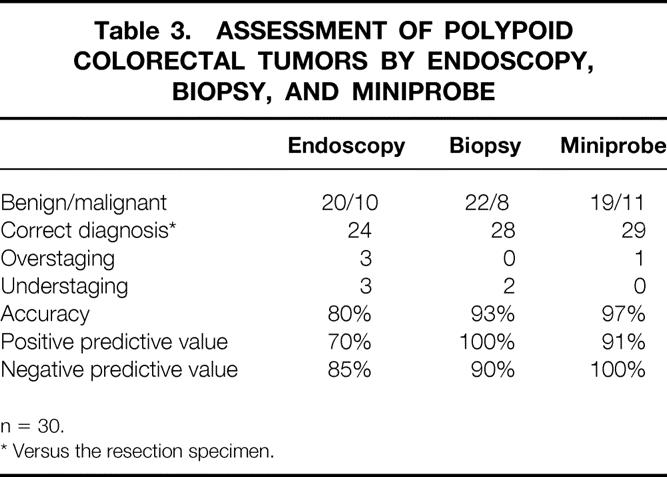
n = 30.
* Versus the resection specimen.
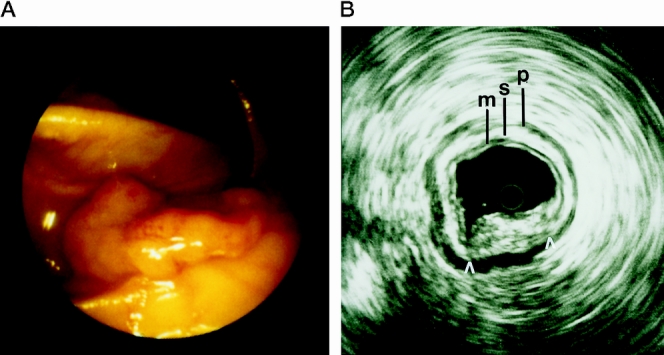
Figure 3. (A) Endoscopic view of a broad-based polyp of the colon. (B) Miniprobe ultrasonography shows infiltration of the submucosa (arrow) (M, mucosa; S, submucosa; P, muscularis propria).
DISCUSSION
Endoscopic ultrasonography is the most sensitive technique for the preoperative staging of colorectal cancer. 6–8 However, one major limitation of conventional transrectal EUS with rigid probes is the inability to examine stenotic tumors. 9 Moreover, EUS of the colon is far from being a routine procedure for the evaluation of colon cancer because of the technical limitations of ultrasound colonoscopes. The impact of this technique on therapy planning in colon cancer remains unclear.
We prospectively examined the value of colonoscopic miniprobe ultrasonography in 63 patients with colorectal tumors. Miniprobe EUS improved the preoperative evaluation of lesions in the proximal colon and stenotic colorectal cancer. The 12.5-MHz transducer of the miniprobe enabled high-resolution imaging of colorectal tumors during routine colonoscopy. The overall accuracy of miniprobe EUS in assessing the infiltration depth of colorectal tumors was 90%. There was no significant difference in the accuracy of EUS whether the tumor was located in the rectum or colon. The lymph node status was correctly predicted in 85% of cases. These results are comparable to the figures reported for ultrasound colonoscopes. 10
There is limited experience with miniprobe EUS in colorectal cancer.11–14 Hamada et al 14 performed miniprobe EUS in 33 patients with colorectal cancer using a 15-MHz miniprobe. The accuracy of the miniprobe for the depth of invasion and the nodal status was 82% and 87%, respectively. The number of patients with positive lymph nodes in this and our study was limited and may not yet allow valid assessment of lymph node staging with miniprobes. However, it has already been shown that miniprobe ultrasonography permits accurate evaluation of lymph node involvement in esophageal and gastric cancer. 15,16 Nonetheless, the penetration depth of the miniprobes and the inability to perform fine-needle aspiration of suspicious nodes may be critical for the diagnosis of metastatic lymph node involvement.
In our experience, miniprobe EUS proved to be particularly valuable for staging stenotic rectal tumors that were not accessible to conventional probes. Correct assessment of the infiltration depth was obtained in 86% of cases. In most patients, EUS demonstrated advanced carcinomas. In some centers, preoperative radiochemotherapy is performed to improve resectability and local tumor control in patients with advanced rectal cancer (Dukes’ C), although no overall survival benefit has been proven. 17,18 It has been demonstrated that obstruction is not necessarily associated with advanced tumor stage and poor prognosis. 19 Accurate staging is therefore crucial to select patients for trials evaluating neoadjuvant treatment. Miniprobe EUS could help to validate the therapeutic approach in obstructing rectal tumors.
One problem in the staging of advanced colon cancer was the differentiation of T3 and T4 tumors. Most advanced tumors were accurately visualized with miniprobe EUS. However, it proved difficult to distinguish penetration into the free peritoneal cavity (T4) from penetration into the mesocolon (T3), because both are associated with a complete disruption of the wall layers and irregular outer borders. We have therefore combined both categories into one group. Retrospectively, T4 tumors could have been correctly diagnosed in eight of nine patients in whom EUS showed circular infiltration of the colon.
Miniprobe EUS could be valuable for minimally invasive surgery of colon tumors. Laparoscopic surgery is safe, effective, and possibly even beneficial for many benign conditions, such as colorectal polyps. However, there is ongoing controversy over laparoscopic resection of colorectal cancer, because some cases of port site metastases have been reported. 20–22 Several mechanisms, including direct contamination, intraabdominal tumor cell spilling, and effects of the CO2 pneumoperitoneum, have been discussed in the literature. 23 The true incidence and the exact pathogenesis of port site metastases are still unknown. However, there is evidence of an increased risk of port site metastases in carcinomas with penetration through the serosa. 24 Wexner and Cohen 25 have calculated a risk of 4% port site recurrences for all stages of colorectal cancer and 21% for Dukes’ B and C tumors.
Our results and those of other published studies show that EUS can differentiate between early colorectal carcinoma (T1/2) and advanced cancer (T3/4). Consequently, preoperative miniprobe EUS may influence the decision to perform laparoscopic colectomy. Moreover, it has recently been suggested that the extent of laparoscopic lymph node dissection can be modified according to the tumor stage. Hida et al 26 analyzed the distribution of lymph node metastases in 164 patients with colorectal cancer and concluded that central lymph node dissection may not be required in patients with T1 carcinoma. However, dissection of the intermediate and main lymph nodes should be performed in T2 tumors and more advanced tumors.
Many surgeons believe that endoscopic treatment of broad-based adenoma is inappropriate because occult cancer may be found in approximately 30% of the specimens. 27,28 Despite apparently complete snare resection, local recurrence, lymph node metastases, or both can be observed in 10% to 20% of the patients. 29,30 A reliable method for detecting invasive cancer is therefore essential to avoid inadequate endoscopic treatment of broad-based polyps with carcinoma.
Hizawa et al 31 performed EUS in 60 patients with colorectal tumors confined to the mucosa or submucosa using a flexible colonoscope (7.5 MHz). The accuracy for the detection of early cancer was only 77%. In our experience with a 12.5-MHz miniprobe, a correct diagnosis was obtained in 97% of the broad-based polyps. Notably, EUS revealed carcinoma in two patients, although endoscopic biopsy had suggested adenoma with dysplasia. The combined accuracy of EUS and biopsy in the detection of invasive carcinoma was 100%.
Miniprobe ultrasonography had a considerable impact on therapy. Endoscopic resection of broad-based polyps was abandoned in 5 of the 63 patients because infiltration of the submucosa or muscularis propria was detected. As a consequence, the patients underwent conventional surgery, and the diagnosis was confirmed in the resection specimens.
CONCLUSION
Miniprobe ultrasonography can be easily performed during routine colonoscopy without additional discomfort for the patient. This technique permits high-resolution imaging of colorectal lesions. Preoperative evaluation of stenotic rectal cancer and broad-based polyps of the colon with miniprobes could improve planning of minimally invasive surgery. This technique may become a routine examination in the preoperative evaluation of colorectal neoplasms. It is well known that EUS is operator-dependent, and the procedure involves a significant learning curve. Our preliminary experience with miniprobes shows that this technique holds promise for the preoperative staging of colorectal cancer, but further studies will be required to define the exact role of this technique.
Footnotes
Correspondence: Peter M. Schlag, MD, PhD, Dept. of Surgery and Surgical Oncology, Robert Rössle Klinik, Humboldt University, 13122 Berlin, Germany.
Accepted for publication December 22, 1999.
References
- 1.Beart RW, Steele GD Jr, Menck HR, et al. Management and survival of patients with adenocarcinoma of the colon and rectum: a national survey of the Commission on Cancer. J Am Coll Surg 1995; 181:225–236. [PubMed] [Google Scholar]
- 2.Zaheer S, Pemberton JH, Farouk R, et al. Surgical treatment of adenocarcinoma of the rectum. Ann Surg 1998; 227:800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heimann TM, Steinhagen RM, Greenstein AJ, et al. Surgical treatment of tumors of the distal rectum with sphincter preservation. Ann Surg 1992; 216:432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wexner SD, Cohen SM, Ulrich A, Reissmann P. Laparoscopic colorectal surgery: are we being honest with our patients? Dis Colon Rectum 1995; 36:723–727. [DOI] [PubMed] [Google Scholar]
- 5.Kuntz C, Kienle P, Buhl K, et al. Flexible endoscopic ultrasonography of colonic tumors: indications and results. Endoscopy 1997; 29:865–870. [DOI] [PubMed] [Google Scholar]
- 6.Beynon J, Mortensen NJ, Foy DMA, et al. Preoperative assessment of mesorectal lymph node involvement in rectal cancer. Br J Surg 1989; 76:276–279. [DOI] [PubMed] [Google Scholar]
- 7.Rifkin MD, Ehrlich SM, Marks G. Staging of rectal carcinoma: prospective comparison of endorectal US and CT. Radiology 1989; 170:319–322. [DOI] [PubMed] [Google Scholar]
- 8.Milsom JW, Lavery IC, Stolfi VM, et al. The expanding utility of endoluminal ultrasonography in the management of rectal cancer. Surgery 1992; 112:832–840. [PubMed] [Google Scholar]
- 9.Hünerbein M, Schlag PM. 3D-endosonography for staging of rectal cancer. Ann Surg 1997; 225:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tio TL, Coene PP, van Delden OM, Tytgat GN. Colorectal carcinoma: preoperative TNM classification with endosonography. Radiology 1991; 179:165–170. [DOI] [PubMed] [Google Scholar]
- 11.Reference not provided
- 12.Frank N, Grieshammer B, Zimmermann W. A new miniature ultrasonic probe for gastrointestinal scanning: feasibility and preliminary results. Endoscopy 1994; 26:603–608. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida M, Tsukamoto Y, Niwa Y, et al. Endoscopic assessment of invasion of colorectal tumors with a new high-frequency ultrasound probe. Gastrointest Endosc 1994; 41:587–592. [DOI] [PubMed] [Google Scholar]
- 14.Hamada S, Akahoshi K, Chijiiwa Y, et al. Preoperative staging of colorectal cancer by a 15-MHz ultrasound miniprobe. Surgery 1998; 123:264–269. [PubMed] [Google Scholar]
- 15.Hünerbein M, Ghadimi B, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheters. Gastrointest Endosc 1998; 48:371–375. [DOI] [PubMed] [Google Scholar]
- 16.Chak A, Canto M, Stevens PD, et al. Clinical application of a new through-the-scope probe: prospective comparison with an ultrasound endoscope. Gastrointest Endosc 1997; 45:291–295. [DOI] [PubMed] [Google Scholar]
- 17.Minsky B, Cohen A, Enker W, et al. Preoperative 5-fluorouracil, low-dose leucovorin, and concurrent radiation therapy for rectal cancer. Cancer 1994; 73:273–280. [DOI] [PubMed] [Google Scholar]
- 18.Rau B, Wust P, Hohenberger P, et al. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: a phase II clinical trial. Ann Surg 1998; 227:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Valdecasas JC, Lovera JM, deLacy AM. Obstructing colorectal carcinomas: a prospective study. Dis Colon Rectum 1991; 34:759–762. [DOI] [PubMed] [Google Scholar]
- 20.Vukasin P, Ortega AE, Greene FL, et al. Wound recurrence following laparoscopic colon cancer resection. Dis Colon Rectum 1996; 83:S20–23. [DOI] [PubMed] [Google Scholar]
- 21.Martinez J, Targarona EM, Balague C, et al. Port site metastasis: an unresolved problem in laparoscopic surgery. A review. Int Surg 1995; 80:315–321. [PubMed] [Google Scholar]
- 22.Cirocco WC, Schwartzman A, Golub RW. Abdominal wall recurrence after laparoscopic colectomy for colon cancer. Surgery 1994; 116:842–846. [PubMed] [Google Scholar]
- 23.Paik PS, Beart RWJ. Laparoscopic colectomy. Surg Clin North Am 1997; 77:1–13. [DOI] [PubMed] [Google Scholar]
- 24.Ramos JM, Gupta S, Anthone GJ, et al. Laparoscopy and colon cancer. Is the port site at risk? Arch Surg 1994; 129:897–899. [DOI] [PubMed] [Google Scholar]
- 25.Wexner SD, Cohen SM. Port site metastases after laparoscopic colorectal surgery for cure of malignancy. Br J Surg 1995; 82:295–298. [DOI] [PubMed] [Google Scholar]
- 26.Hida J, Yasutomi M, Maruyama T, et al. The extent of lymph node dissection for colon carcinoma: the potential impact on laparoscopic surgery. Surgery 1997; 80:188–192. [PubMed] [Google Scholar]
- 27.Muto T, Sawada T, Sugihara T. Treatment of carcinoma in adenomas. World J Surg 1991; 15:35–40. [DOI] [PubMed] [Google Scholar]
- 28.Fried GM, Hreno A, Duguid WP. Rational management of malignant colon polyps based on long-term follow-up. Surgery 1984; 96:815–821. [PubMed] [Google Scholar]
- 29.Richards WO, Webb WA, Morris SJ, et al. Patient management after endoscopic removal of cancerous colon adenoma. Ann Surg 1987; 205:665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stulc JP, Ptrelli NJ, Herrera L, Mittelman A. Colorectal villous and tubulovillous adenomas equal to or greater than four centimeters. Ann Surg 1988; 207:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hizawa K, Suekane H, Aogiy K. Use of endosonographic evaluation of colorectal tumor depth in determining the appropriateness of endoscopic mucosal resection. Am J Gastroenterol 1996; 91:768–771. [PubMed] [Google Scholar]


