Abstract
Objective
To determine whether ischemic preconditioning protects the human liver against a subsequent period of ischemia in patients undergoing hemihepatectomy, and to identify possible underlying protective mechanisms of ischemic preconditioning, such as inhibition of hepatocellular apoptosis.
Summary Background Data
Ischemic preconditioning is a short period of ischemia followed by a brief period of reperfusion before a sustained ischemic insult. Recent studies in rodents suggest that ischemic preconditioning is a simple and powerful protective modality against ischemic injury of the liver. The underlying mechanisms are thought to be related to downregulation of the apoptotic pathway.
Methods
Twenty-four patients undergoing hemihepatectomy for various reasons alternatively received ischemic preconditioning (10 minutes of ischemia and 10 minutes of reperfusion) before transection of the liver performed under inflow occlusion for exactly 30 minutes. Liver wedge and Tru-cut biopsy samples were obtained at the opening of the abdomen and 30 minutes after the end of the hepatectomy. Serum levels of aspartate transferase, alanine transferase, bilirubin and prothrombin time were determined daily until discharge. Hepatocellular apoptosis was evaluated by in situ terminal deoxynucleotidyl transferase mediated d-UTP nick end-labeling (TUNEL) assay and electron microscopy. Caspase 3 and 8 activities were measured in tissue using specific fluorometric assays.
Results
Serum levels of aspartate transferase and alanine transferase were reduced by more than twofold in patients subjected to ischemic preconditioning versus controls. The analysis of a subgroup of patients with mild to moderate steatosis indicated possible increased protective effects of ischemic preconditioning. In situ TUNEL staining demonstrated a dramatic reduction in the number of apoptotic sinusoidal lining cells in the ischemic preconditioning group. Electron microscopy confirmed features of apoptosis present in control but not in ischemic preconditioning patients. There was no significant difference in caspase 3 and 8 activity when patients with ischemic preconditioning were compared with controls.
Conclusions
Ischemic preconditioning is a simple and effective modality protecting the liver against subsequent prolonged periods of ischemia. This strategy may be a more attractive technique than intermittent inflow occlusion, which is associated with increased blood loss during each period of reperfusion.
Clamping of the portal triad (inflow occlusion), also called the Pringle maneuver, is often used during liver surgery to minimize blood loss. Excessive blood loss during surgery and the need for transfusion have been shown to hinder the postoperative course. 1–3 Several recent clinical 4–6 and experimental 7,8 studies have demonstrated that intermittent clamping of the portal triad is better tolerated than prolonged continuous periods of ischemia. 5,6 However, the beneficial effect of intermittent clamping on ischemic injury, as assessed by postoperative serum transaminase and bilirubin levels, is counterbalanced by increased blood loss during the various reperfusion periods. If the protective mechanisms of intermittent ischemia could be identified, then innovative strategies could be developed to protect the liver against ischemic and reperfusion injury without increased blood loss.
One strategy that could be applicable to hepatic surgery is ischemic preconditioning. Ischemic preconditioning is a short period of ischemia followed by a brief period of reperfusion before a sustained ischemic insult. Most of the data on ischemic preconditioning have been gathered in the heart, with a focus on the ability of preconditioning to reduce the size of a myocardial infarction. A few of the involved extracellular mediators, such as adenosine 9 and nitric oxide, 10 have been identified. Protection by ischemic preconditioning has been subsequently documented in a variety of tissues, including skeletal muscle, 11 brain, 12 spinal cord, 13 retina, 14 and intestine, 15 and is thought to be part of a ubiquitous protective mechanism against repetitive stress on cells and cell systems. Only a few investigators have studied the effects of ischemic preconditioning in the liver, 16–21 and only data in rodents are available. These studies have suggested that the liver could also be protected against ischemic insults by preconditioning.
We recently identified an ischemic preconditioning protocol (10 minutes of ischemia followed by 15 minutes of reperfusion) that completely prevented animal death in mice subjected to 75 minutes of total hepatic ischemia, a lethal condition without preconditioning. 16 In view of our previous findings that apoptosis of sinusoidal endothelial cells and hepatocytes is a prominent feature of reperfusion injury in the warm ischemic liver, 22 we also studied the effects of ischemic preconditioning on postreperfusion apoptosis in a mouse model of partial hepatic ischemia. 16 Ischemic preconditioning was associated with complete abrogation of the massive hepatocellular apoptosis seen after prolonged periods of ischemia and downregulation of cytoplasmic caspase activities. Caspases are a family of cysteine proteases initiating complex proteolytic reactions leading to cell disassembly and death. 23
The beneficial effects of intermittent clamping of the portal triad, as reported by others, 4–8 might be related to a preconditioning effect. If so, a short period of ischemia may protect the liver during subsequent liver resection performed under inflow occlusion. This effect should occur without increased blood loss as seen during intermittent clamping. To test this hypothesis, we designed a study involving 24 patients undergoing major hepatectomy. To minimize variability, we adopted a rigid protocol including fixed periods of prolonged clamping after ischemic preconditioning, and a standardized technique of hepatectomy performed by a single surgeon (P.A.C.). We also attempted to identify underlying mechanisms of injury and protection from ischemic preconditioning in these patients.
METHODS
Experimental Design
We based the ischemic preconditioning protocol on the data obtained in rodents, where a period of 10 minutes of clamping followed by 10 to 15 minutes of reperfusion maximally protected the liver against a subsequent prolonged period of ischemia. 16 Previous studies in the human liver have shown that ischemic injury becomes detectable after 30 minutes of ischemia. 6 Because most anatomical hemihepatectomies can be performed during this time period in our center, we designed a pilot study in 24 patients undergoing anatomical right or left hemihepatectomy under continuous inflow occlusion (clamping of the portal triad) for exactly 30 minutes. This time was also chosen considering the possibility of negative effects of ischemic preconditioning in humans. Twelve patients were subjected to ischemic preconditioning consisting of 10 minutes of clamping of the portal triad followed by 10 minutes of reperfusion before hepatectomy. To minimize bias in patient selection, ischemic preconditioning was performed in an alternate fashion. Patients with hepatic steatosis, but not cirrhosis, were included. Variables studied included intraoperative blood loss, need for blood products, duration of surgery, postoperative serum transaminases and bilirubin levels, hospital and intensive care unit stay, and clinical outcome. The effect of ischemic preconditioning was also evaluated in a subgroup of patients with mild to moderate steatosis (20–50% fat). Wedge and Tru-cut biopsies were performed on opening of the abdomen and exactly 30 minutes after reperfusion after the 30-minute clamping period for histologic examination of apoptotic cell death (terminal deoxynucleotidyl transferase mediated d-UTP nick end-labeling [TUNEL] assay and electron microscopy), and to measure cytoplasmic caspase 3 and 8 activities. Caspase 3 and 8 are at opposite ends of the apoptotic cascade, one initiating the cascade (caspase 8) and the other effecting cell death (caspase 3).
Patients and Surgical Procedure
The protocol was approved by the investigation and review board at our institution, and consent was obtained from each patient before surgery. Those included in this study were patients scheduled to undergo anatomical right or left hemihepatectomy for various benign and malignant conditions. Patients with wedge or segmental resections were not included. Characteristics of the patients are shown in Table 1. At the time of surgery, each patient was assigned, after intraoperative ultrasound, to either ischemic preconditioning (10 minutes of portal clamping followed by 10 minutes of reperfusion) or no ischemic preconditioning (control group), followed by liver resection. Each hemihepatectomy was performed with clamping of the porta hepatis for exactly 30 minutes.
Table 1. PATIENTS CHARACTERISTICS

Right and left hemihepatectomies were performed by one surgeon using a standard technique. 24 The decision to proceed with anatomical hemihepatectomy was made after an exploratory laparotomy and intraoperative ultrasound. Patients were then alternatively allocated to the ischemic preconditioning or control group. Baseline Tru-cut and wedge biopsy samples were obtained from the part of the liver to be resected. The porta hepatis was dissected and the right or left portal vein and arteries were ligated and divided, depending on the part of the liver to be removed, before parenchymal dissection. In patients undergoing right hemihepatectomies, all short caudate veins were ligated and divided before ligation of the right hepatic vein. In patients undergoing left hemihepatectomies, the left hepatic vein was ligated. Clamping of the portal triad was performed with the tourniquet technique using an umbilical tape. Separate clamping of aberrant left hepatic arteries was carefully performed, when present. Transection of the liver was initiated immediately after complete hepatic inflow occlusion. Transection was performed using the Kelly clamp “crushing” technique; small vessels and bile ducts were clipped with an automatic clip applicator, and larger structures were ligated with 2–0 silk suture. After exactly 30 minutes, the portal clamping was released, and careful hemostasis was obtained. Tru-cut and wedge biopsy samples were obtained after 30 minutes of reperfusion, usually just before closure of the abdomen. Each surgery was performed with a low central venous pressure to minimize blood loss, 25 and no postoperative drain was used.
Tissue Biopsy
Samples of liver tissue (wedge and Tru-cut) obtained during surgery were immediately put in liquid nitrogen (−70°C) or stored in 4% paraformaldehyde. Some specimens were also stored in glutaraldehyde for electron microscope processing.
TUNEL Staining
Cleavage of genomic DNA during apoptosis yields DNA strand breaks that can be identified by labeling free 3′-OH ends with fluorescent nucleotides in an enzymatic reaction involving terminal deoxynucleotidyl transferase. A TUNEL assay was used to localize and assess cells undergoing DNA fragmentation, as previously described. 26 Briefly, samples were immediately stored in 4% paraformaldehyde for fixation. Frozen sections (6 μm) of the fixed tissue were prepared and stained by the TUNEL method using a commercial kit (Boehringer Mannheim Co., Indianapolis, IN).
Morphometric analysis of the fluorescent cells in tissue stained with the TUNEL method was performed under high-power magnification (×400) in a masked fashion. Ten random fields were analyzed for each TUNEL-stained tissue sample. To identify cells showing features of apoptosis, some tissue samples were processed for electron microscopy.
Assay for Transaminases and Bilirubin and Prothrombin Time
Blood samples were obtained 24 hours after surgery and daily until discharge. All blood samples were analyzed using an Ektachem 950 (Johnson and Johnson, Rochester, NY) for aspartate transferase (AST), alanine transferase (ALT), and bilirubin levels. Prothrombin times were evaluated using the Multi-Diagnostic Analyzer 180 (Organon Teknika, Boxtel, The Netherlands).
Electron Microscopy
Tru-cut biopsy samples were immediately fixed in 4% glutaraldehyde and phosphate-buffered saline. The specimens were postfixed with osmium tetroxide, dehydrated in graded alcohol, and embedded in an Epon 812 mixture. Sections were cut on an ultramicrotome, stained with uranyl acetate and lead citrate, and examined using a Philips 201 electron microscope (Eindhoven, The Netherlands).
Caspase Assays
Caspase 3 is distinguished by its ability to cleave poly(ADP-ribose) polymerase during apoptosis. The cleavage site in poly(ADP-ribose) polymerase is C-terminal to Asp-216. The upstream sequence, DEVD, is the basis for the substrate and inhibitors used in this assay. Caspase 8 is an upstream apoptotic signaling protease that is released from procaspase 8 on binding to intracellular domains of receptors, such as Fas and tumor necrosis factor α, after extracellular stimulation. This leads to processing and initialization of the apoptotic caspase cascade. Caspase 3 and caspase 8-like activity was determined by measuring proteolytic cleavage of the respective specific substrates N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin (Ac-DEVD-AFC; Biomol, Plymouth Meeting, PA) or N-acetyl-Ile-Glu-Thr-Asp-7-amino-4-trifluoromethyl coumarin (Ac-IETD-AFC) in the presence or absence of the specific caspase 3 or 8 aldehyde inhibitor based on the same amino acid sequence (Ac-DEVD-CHO and Ac-IETD-CHO, respectively; Biomol). Liver tissue was quickly excised and sonicated in assay buffer (1 mmol/L EDTA, 145 mmol/L NaCl, 100 mmol/L Tris, 0.1 mmol/L DTT, 0.1% CHAPS, 10% glycerol). Protein content was determined using the Bradford protein assay. The samples were diluted and incubated at room temperature with Ac-DEVD-AFC substrate in the presence or absence of the inhibitor Ac-DEVD-CHO. AFC release was assayed during a 2-hour period in a fluorometer, using 400 nm excitation and measurement of 505 nm emission. AFC release is expressed as arbitrary fluorescence units per milligram of liver tissue after subtracting the reading in the inhibited sample from the noninhibited sample.
Statistical Analysis
The results are expressed as mean ± standard deviation. SAS statistical software (SAS Institute, Inc., Cary, NC) was used for all statistical analyses, and a P < .05 was considered significant.
RESULTS
Effects of Ischemic Preconditioning on Intraoperative Parameters, Blood Loss, and Need for Blood Transfusion
No significant differences were detected between the ischemic preconditioning group versus the control group in terms of the duration of surgery, presence of low blood pressure (mean <60 mmHg) during surgery, or blood loss (range 30–800 mL, median 350). Only 3 of the 24 patients received blood during or after surgery; 2 patients received one unit of packed red cells, and 1 patient received two units of packed red cells. These patients were in the control group. No patient subjected to ischemic preconditioning received any blood products.
Effects of Ischemic Preconditioning on Intensive Care Unit and Hospital Stay and Postoperative Complications
There were no deaths in this series. No significant difference was noted in the length of hospital (median 5.5 days, range 4–17) or intensive care unit (median 1 day, range 0–4) stay, although the two patients with a hospital stay of more than 10 days were in the control group. One patient in the control group was readmitted to the intensive care unit 2 days after surgery for a postoperative stroke related to bilateral stenosis of the carotid arteries. Other complications within 30 days of surgery in the control group included one myocardial infarction requiring a coronary bypass 3 weeks after surgery, and two minor wound infections. Thus, the complication rate in the control group was 33% (4/12). In comparison, no serious complications occurred in the preconditioning group; two patients had minor wound infections. The complication rate in this group was 17% (2/12). No patient was readmitted after discharge in both groups.
Effects of Ischemic Preconditioning on Postoperative Serum Transaminase and Bilirubin Levels and Prothrombin Time
The severity of reperfusion injury is best assessed by serial serum AST levels, an established marker of injury of the liver. 27 Peak AST levels in the ischemic human liver occurred within 12 and 36 hours of reperfusion and returned to normal levels within 2 to 5 days. Patients subjected to ischemic preconditioning had significantly lower serum AST and ALT levels than the control group at 24 hours after reperfusion (Fig. 1). Mean AST levels were 469 ± 85 IU/L (median 418, range 302–650) versus 206 ± 37 IU/L (median 203, range 100–498). Mean ALT levels were 407 ± 93 IU/L (median 418, range 398–650) versus 202 ± 37 IU/L (median 187, range 117–468). The Student t test and rank sum test were both statistically significant (P < .01) when comparing AST and ALT levels between the preconditioning and control groups, respectively.
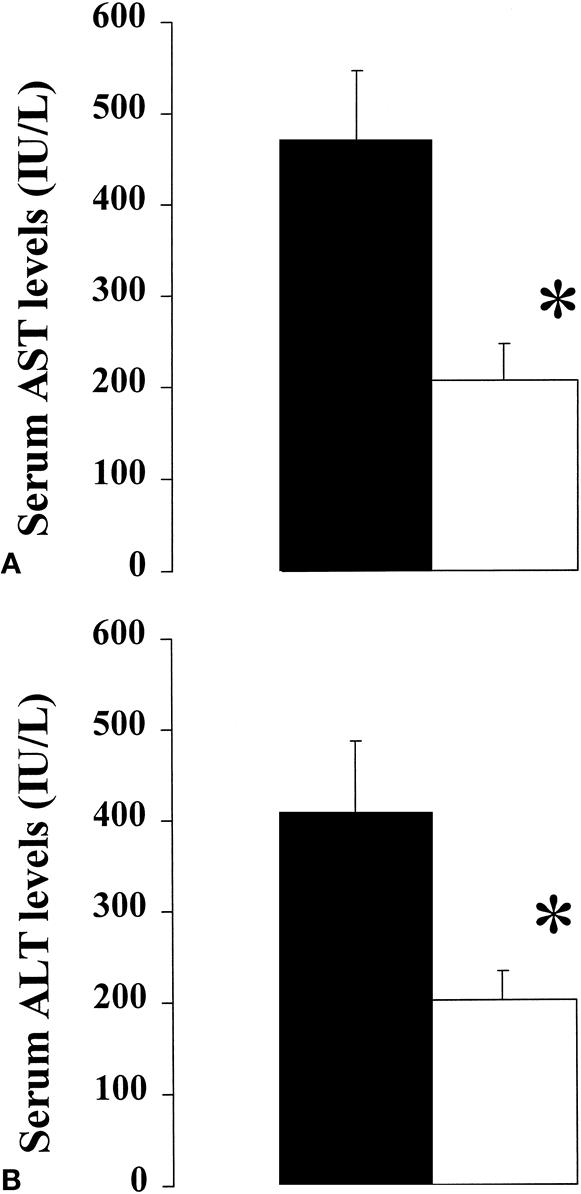
Figure 1. Serum aspartate transferase (A) and alanine transferase (B) levels (mean ± standard deviation) 24 hours after surgery were significantly lower in patients subjected to ischemic preconditioning (□) versus controls (▪) (n = 12 in each group; *P < .01 in each respective comparison, Student t test).
Of the 24 patients, 7 were found to have mild to moderate steatosis; 4 of these were in the ischemic preconditioning group (25%, 25%, 50%, 50% steatosis) and 3 were in the control group (25%, 30%, 50%). The amount of fat in each liver was assessed on hematoxylin-and-eosin–stained biopsy samples by one pathologist (R.C.B.) without knowledge of the group allocation of the patient. In this small subgroup, the differences in serum transaminase levels between the preconditioned and control patients were even more pronounced. AST levels were 159 ± 54 IU/L in the preconditioned group versus 599 ± 48 IU/L in the control group, and ALT levels were 221 ± 27 IU/L versus 517 ± 78 IU/L (P < .01; Student t test in each respective comparison). To exclude that the presence of patients with steatosis was solely responsible for the significant difference between the preconditioning and control groups, the Student t test was performed excluding the seven patients with steatosis. The difference in AST and ALT levels between the two groups remained statistically significant (P < .04).
No significant differences were noted in bilirubin levels or prothrombin time at any time point. For transaminase levels on postoperative day 2 and thereafter, no significant differences were observed between the two groups, although a trend for lower values in the preconditioning group was present until postoperative day 4 (data not shown).
Effect of Ischemic Preconditioning on Apoptosis
The effect of ischemic preconditioning on liver cell apoptosis after reperfusion was evaluated on Tru-cut biopsy samples using in situ TUNEL staining and electron microscopy in the last 10 patients of the study. Liver samples were evaluated before inflow occlusion (baseline) and 30 minutes after reperfusion. Only exceptional cells stained positive for TUNEL assay in baseline biopsy samples. In contrast, in the control group after 30 minutes of reperfusion, many sinusoidal lining cells stained positive for TUNEL, but no hepatocyte staining was observed (Fig. 2). A significant decrease in sinusoidal lining cell TUNEL staining was observed in the preconditioned livers after 30 minutes of reperfusion, and again no hepatocyte was found to be positive for TUNEL assay (Fig. 2). Morphometric assessment of TUNEL-positive sinusoidal endothelial cells between control and preconditioned livers is shown in Figure 3.

Figure 2. In situ TUNEL staining in Tru-cut liver biopsy samples obtained 30 minutes after the prolonged period of ischemia (30 minutes). Controls (A) showed more positive sinusoidal lining cells than patients subjected to ischemic preconditioning (B). No hepatocyte was found to be TUNEL-positive in either group.
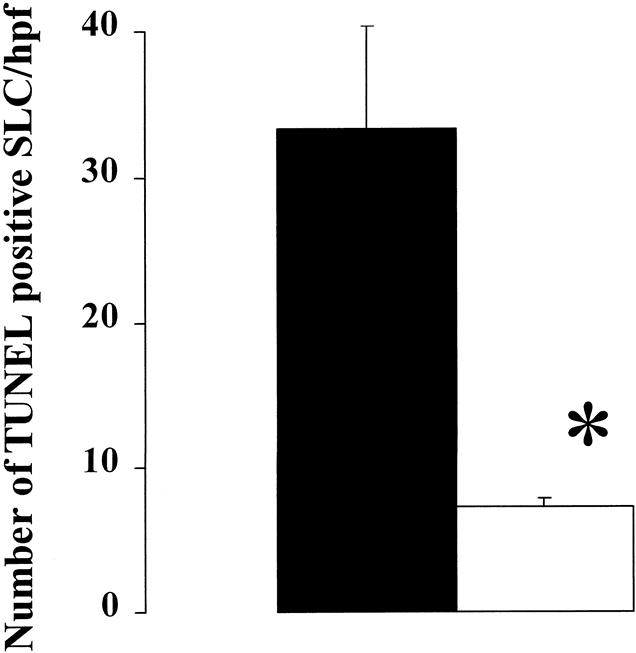
Figure 3. Morphometric analysis of sinusoidal lining cell apoptosis as assessed by the in situ TUNEL assay showed significantly more positive cells in the control group (▪) than in patients subjected to ischemic preconditioning (□). (Data expressed as mean count ± standard deviation per high-power field; n = 5 in each group; *P < .01, Student t test).
To characterize apoptosis further, biopsy samples were taken 30 minutes after reperfusion in three patients in each group and processed for electron microscopy. A pathologist (R.C.B.) examined the slides without knowledge of the specimens studied. Numerous features consistent with apoptosis of the sinusoidal endothelial cells were found in the control group, but no evidence of apoptosis was observed in the preconditioning group. The most frequent finding was a dramatic increase in the nucleus/cytoplasmic ratio and detachment from the sinusoidal plate (Fig. 4). Only a few cells showed evidence of chromatin condensation. In contrast, these features were absent in biopsy samples from patients subjected to ischemic preconditioning.

Figure 4. Electron microscopic examination of a Tru-cut tissue biopsy showing the most common finding seen after 30 minutes of reperfusion in control livers subjected to 30 minutes of inflow occlusion. A rounded sinusoidal endothelial cell with a dramatic increase in the cytoplasmic/nucleus ratio, which appears detached from the sinusoidal plate, is shown (arrow). These features are suggestive of early apoptosis (H, hepatocyte, RBC, red blood cell).
Effects of Ischemic Preconditioning on Caspase 3 and 8 Activities
The effect of ischemic preconditioning on caspase activity was evaluated using specific fluorometric assays for caspase 3 and caspase 8. Caspase 8 is a membrane-bound mediator initiating the cellular cascade of apoptosis; caspase 3 is an effector mediator leading to DNA fragmentation. Tissue samples (n = 5 in each group) were analyzed at baseline and 30 minutes after hepatic resection. Both wedge and Tru-cut (core) biopsy samples were examined. We did not observe any significant differences in the samples from the wedge biopsies, but there was a trend toward increased caspase 8 activity in the postresection Tru-cut biopsy samples obtained in the control group (2.3 u/mg) when compared with preconditioned samples (0.4 u/mg) (Student t test, P < .15). Caspase 8 activity was minimal in all baseline biopsy samples, and caspase 3 activity was low in all the examined tissue samples (<0.5 u/mg).
DISCUSSION
This is the first study demonstrating a protective effect of ischemic preconditioning in the human liver. The results suggest that mechanisms of injury in the ischemic human liver are similar to those in rodents, in which sinusoidal endothelial cells appear to be the initial target of injury. Furthermore, the reduced number of apoptotic sinusoidal endothelial cells by TUNEL staining and electron microscopy in livers subjected to ischemic preconditioning points to inhibition of apoptotic cell death as a key mechanism of protection from ischemic preconditioning in the human liver. The results may have important clinical applications, given that ischemic preconditioning was associated with minimal intraoperative blood loss. This may represent a significant advantage over techniques using only intermittent clamping of the portal triad during liver resection.
Ischemic preconditioning was found to confer protection to the liver, as evidenced by a decrease in serum AST and ALT levels measured 24 hours after surgery. These enzymes are currently the most sensitive markers of ischemic injury to the liver. Studying a variety of markers of injury in an isolated perfused rat liver model, Iu et al 27 showed that release of AST best correlates with the degree of ischemic injury. Most clinical studies have used transaminase levels to assess hepatic injury resulting from ischemia. 6,28,29
In this study, we alternated the use of ischemic preconditioning to minimize bias in patient selection, and we adopted a rigid protocol regarding ischemic time, type of hepatectomy, and surgical technique. Only anatomical hemihepatectomies were included. Resections in patients with cirrhosis or those requiring a technique of total vascular exclusion (e.g., in the presence of tumor involving the vena cava) were not included. The clamping period of the porta hepatis was set at exactly 30 minutes, a time during which most hemihepatectomies can safely be performed. This period is also the shortest ischemic time associated with elevated transaminase levels after surgery. 6 The lack of significant effects of ischemic preconditioning on other markers of hepatic injury and patient outcome may be related to this relatively short period of ischemia.
Belghiti et al 6 recently showed in a series of patients undergoing liver resection that intermittent clamping of the portal triad was better tolerated than continuous clamping. The difference in transaminase levels between the two groups was comparable to that observed in our study. Others 4 have shown that the optimal timing for intermittent clamping is 15 minutes of ischemia followed by a short period of reperfusion; similar periods were found to be effective in animal models of ischemic preconditioning of the liver. 16,18 These findings may suggest that the beneficial effects, and perhaps the underlying mechanisms, are similar in techniques of intermittent clamping and ischemic preconditioning. Therefore, the results of this study provide a possible mechanistic explanation for the beneficial effects of intermittent clamping during hepatic surgery.
An important shortcoming of intermittent clamping is the risk of blood loss during each period of reperfusion (release of the clamp) during transection of the liver. The French series 6 documented twice as much blood loss during liver transection in the intermittent clamping group versus the continuous clamping group. Our ischemic preconditioning protocol consisted of a short period of ischemia and reperfusion before transection of the liver performed under continuous clamping of the portal trial. Blood loss was minimal, with only three patients in the control group requiring blood transfusion. Thus, ischemic preconditioning not only protects against ischemic injury but also minimizes blood loss. The routine use of low central venous pressure (0–3 mmHg), as is standard at our institution, may also have contributed to the minimal bleeding during hepatic surgery in this series.
The diseased liver is particularly susceptible to ischemia, and a poor outcome is not uncommon in patients with fatty liver undergoing major hepatectomy under a long period of inflow occlusion. 2,4–6 Our data are again consistent with those of Belghiti et al, 6 in which the most pronounced effects of intermittent clamping occurred in patients with hepatic steatosis. In a subgroup of seven patients with mild to moderate steatosis (>20%), we found a higher level of injury at day 1, as determined by serum AST and ALT levels, in the fatty livers versus normal livers. Ischemic preconditioning of these fatty livers appears to be particularly protective, with serum transaminase levels on postoperative day 1 less than 260 IU/L in each patient.
A secondary goal of the current study was the search for underlying mechanisms of protection from ischemic preconditioning. Our group 22,26 and others 30,31 have demonstrated that apoptosis is a prominent feature of ischemia-reperfusion of the liver. Gao et al 26 and Kohli et al 22 have shown in rodents that the first targets of injury are sinusoidal endothelial cells, in which morphologic features of apoptosis develop within 30 minutes of reperfusion. Studies at later time points in the same models have shown that injury to the hepatocyte occurred between 3 and 8 hours of reperfusion. 16,22 In the current clinical study, we did not study mechanisms of injury after closure of the abdomen (i.e., 30 minutes after reperfusion); therefore, later mechanisms related to hepatocyte injury could not be evaluated in this study. Our data indicated similar early mechanisms of injury as seen in the rodents, with clear features of apoptosis in sinusoidal endothelial cells (TUNEL and electron microscopy) without detectable injury in other hepatic cells, including Kupffer cells and hepatocytes.
In a murine model of ischemic preconditioning, our group 16 recently showed that ischemic preconditioning dramatically reduced cell death by apoptosis of the endothelial cell followed by the hepatocyte at later stages of reperfusion. This experimental study also showed that ischemic preconditioning is associated with a significant decrease in caspase 3 activity, the executioner caspase in the complex cascade leading to apoptosis. In this clinical study, we found similar protective mechanisms, with a decreased number of apoptotic cells (TUNEL assay) in livers subjected to ischemic preconditioning, and an absence of morphologic features consistent with apoptosis on electron microscopy. Although caspase 3 activity was not different, a trend toward decreased caspase 8 activity (P = .15) was documented. Caspase 8 is a membrane-bound caspase usually activated by extracellular stimuli, and it triggers cytoplasmic and mitochondrial changes, eventually leading to caspase 3 activity and DNA fragmentation. We speculate that the lack of difference in caspase 3 activity in this study is due to the early stage of reperfusion, when only a few sinusoidal endothelial cells are apoptotic. The trend in lower caspase 8 activity may suggest that extracellular mediators such as oxygen-free radicals, nitric oxide, Fas, or tumor necrosis factor α trigger the apoptotic cascade. Another finding relevant to the design of future studies is the lack of any difference among wedge biopsy samples. Unlike Tru-cut biopsies, wedge biopsies are obtained on the surface of the liver and are subject to various manipulations during surgery.
In conclusion, we provide the first clinical evidence suggesting a beneficial effect of ischemic preconditioning during major hepatic surgery. These data might be particularly important in complex cases where long periods of ischemia are necessary and in livers with underlying disease or steatosis, which tolerate injury poorly. Another application might be in living-related adult liver transplantation, in which ischemia is currently carefully avoided. An effective protective technique allowing continuous clamping of the portal triad during hepatectomy would be very attractive to prevent bleeding and thereby to decrease the risk of surgery in the healthy donor. Future studies are necessary to evaluate the impact of ischemia in the general practice of liver surgery, as well as its role compared with intermittent clamping.
Acknowledgments
The authors thank Peggy Cotton, PA, and Jaye Danko for their help in data collection and analysis.
Footnotes
Correspondence: Pierre-Alain Clavien, MD, PhD, FACS, Dept. of Visceral and Transplantation Surgery, Zurich University Medical Center, Raemistrasse 100, Zurich 8091, Switzerland.
Supported by NIH Grant DK54048–01A1 (to PAC).
Accepted for publication November 29, 1999.
References
- 1.Wobbes T, Bemelmans BLH, Kuypers JHC, et al. Risk of postoperative septic complications after abdominal surgery treatment in relation to perioperative blood transfusion. Surg Gynecol Obstet 1990; 171: 5962–5965. [PubMed] [Google Scholar]
- 2.Makuuchi M, Mori T, Gunven P, et al. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet 1989; 130: 824–831. [PubMed] [Google Scholar]
- 3.Nagorney DMHJ, Illstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery 1989; 106: 740–749. [PubMed] [Google Scholar]
- 4.Horiuchi T, Muraoka R, Tabo T, et al. Optimal cycles of hepatic ischemia and reperfusion pedicle clamping during liver surgery. Arch Surg 1995; 130: 754–758. [DOI] [PubMed] [Google Scholar]
- 5.Hardy KJ, Tancheroen S, Shulkes A. Comparison of continuous versus intermittent ischemia-reperfusion during liver resection in an experimental model. Br J Surg 1995; 82: 833–-836. [DOI] [PubMed] [Google Scholar]
- 6.Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection. A controlled study. Ann Surg 1999; 229: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isozaki H, Adam R, Gigou M, et al. Experimental study of the protective of intermittent hepatic pedicle clamping in the rat. Br J Surg 1992; 79: 310–313. [DOI] [PubMed] [Google Scholar]
- 8.Hewitt G, Halliday I, MacCaigue M, et al. Mortality, endotoxaemia and cytokine expression after intermittent and continuous hepatic ischemia. Br J Surg 1995; 82: 1424–1426. [DOI] [PubMed] [Google Scholar]
- 9.Leesar MA, Stoddard M, Ahmed M, et al. Preconditioning of human myocardium with adenosine during coronary angioplasty. Circulation 1997; 95: 2500–2507. [DOI] [PubMed] [Google Scholar]
- 10.Vegh A, Szekeres L, Parratt J. Preconditioning of the ischemic myocardium: involvement of the l -arginine nitric oxide pathway. Br J Pharmacol 1992; 107: 648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang CY, Yang RZ, Zhong A, et al. Acute ischemic preconditioning protects against skeletal muscle infarction in the pig. Cardiovasc Res 1995; 29: 1546–1577. [PubMed] [Google Scholar]
- 12.Glazier SS, O’Rourke DM, Graham DI, Welsh FA. Induction of ischemic tolerance following brief focal ischemia in rat brain. J Cereb Blood Flow Metab 1994; 14: 545–553. [DOI] [PubMed] [Google Scholar]
- 13.Sakurai M, Hayashi T, Abe K, et al. Enhancement of heat shock protein expression after transient ischemia in the preconditioned spinal cord of rabbits. J Vasc Surg 1998; 27: 720–725. [DOI] [PubMed] [Google Scholar]
- 14.Roth S, Li B, Rosenbaum PS, et al. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci 1998; 39: 777–785. [PubMed] [Google Scholar]
- 15.Hotter G, Closa D, Prados M, et al. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Comm 1996; 222: 27–32. [DOI] [PubMed] [Google Scholar]
- 16.Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the liver by inhibition of apoptosis through a caspase dependent pathway. Hepatology 1999; 30: 1223–1231. [DOI] [PubMed] [Google Scholar]
- 17.Peralta C, Closa D, Hotter G, et al. Liver ischemic preconditioning is mediated by the inhibitory action of nitric oxide on endothelin. Biochem Biophys Res Comm 1996; 229: 264–270. [DOI] [PubMed] [Google Scholar]
- 18.Peralta C, Hotter G, Closa D, et al. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology 1997; 25: 934–937. [DOI] [PubMed] [Google Scholar]
- 19.Peralta C, Closa D, Xaus C, et al. Hepatic preconditioning in rats is defined by a balance of adenosine and xanthine. Hepatology 1998; 28: 768–773. [DOI] [PubMed] [Google Scholar]
- 20.Kume M, Yamamoto Y, Saad S, et al. Ischemic preconditioning of the liver in rats: implications of heat shock protein induction to increase tolerance of ischemia-reperfusion injury. J Lab Clin Med 1996; 128: 251–258. [DOI] [PubMed] [Google Scholar]
- 21.Hardy KJ, McClure DN, Subwongcharoen S. Ischemic preconditioning of the liver: a preliminary study. Austr NZ J Surg 1996; 66: 707–710. [DOI] [PubMed] [Google Scholar]
- 22.Kohli V, Selzner M, Madden JF, et al. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation 1999; 67: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 23.Kothakota S, Azuma T, Reinhard C, et al. Caspase-3 generated fragment of gelsolin: effector of morphological change in apoptosis. Science 1997; 278: 5336. [DOI] [PubMed] [Google Scholar]
- 24.Selzner M, Clavien PA. Resection of liver tumors: special emphasis on neoadjuvant and adjuvant therapy. In: Clavien PA, ed. Malignant Liver Tumors: Current and Emerging Therapies. Malden: Blackwell Science; 1999: 137–149.
- 25.Hill RP, Gan TJ. Anesthetic management of liver surgery. In: Clavien PA, ed. Malignant Liver Tumors: Current and Emerging Therapies. Malden: Blackwell Science; 1999: 341–351.
- 26.Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology 1998; 27: 1652–1660. [DOI] [PubMed] [Google Scholar]
- 27.Iu S, Harvey PRC, Makowka L, et al. Markers of allograft viability in the rat. Relationship between transplantation viability and liver function in the isolated perfused rat liver. Transplantation 1987; 45: 562–569. [DOI] [PubMed] [Google Scholar]
- 28.Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997; 226: 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delva E, Camus Y, Nordlinger B, et al. Vascular occlusions for liver resections: operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg 1989; 209: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cursio R, Gugenheim J, Ricci JE, et al. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. FASEB J 1999; 13: 253–261. [DOI] [PubMed] [Google Scholar]
- 31.Bilbao G, Contreras JL, Eckhoff DE, et al. Reduction of ischemia-reperfusion injury of the liver by in vivo adenovirus mediated gene transfer of the antiapoptotic Bcl-2 gene. Ann Surg 1999; 230: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]


