Abstract
Objective
To describe the development of a minimally invasive technique aimed at surgical debridement in addition to simple drainage of the abscess cavity.
Summary Background Data
Surgical intervention for secondary infection of pancreatic necrosis is associated with a death rate of 25% to 40%. Although percutaneous approaches may drain the abscess, they have often failed in the long term as a result of inability to remove the necrotic material adequately.
Methods
Fourteen consecutive patients with infected necrosis secondary to acute pancreatitis were studied. The initial four patients underwent sinus tract endoscopy along a drainage tract for secondary sepsis after prior open necrosectomy. This technique was then modified to allow primary debridement for proven sepsis to be carried out percutaneously in a further 10 patients. The techniques and initial results are described.
Results
Additional surgery for sepsis was successfully avoided in the initial four patients managed by sinus tract endoscopy, and none died. Of the following 10 patients managed by percutaneous necrosectomy, 2 died. The median inpatient stay was 42 days. There was one conversion for intraoperative bleeding. Eight patients recovered and were discharged from the hospital after a median of three percutaneous explorations. Only 40% of patients required intensive care management after surgery.
Conclusions
These initial results in an unselected group of patients are encouraging and show that unlike with percutaneous or endoscopic techniques, both resolution of sepsis and adequate necrosectomy can be achieved. The authors’ initial impression of a reduction in postoperative organ dysfunction is particularly interesting; however, the technique requires further evaluation in a larger prospective series.
Infected pancreatic necrosis is the most feared surgical complication of acute pancreatitis and occurs in 8% to 12% of patients. 1–3 Until the 1970s, standard management of this condition involved open surgical debridement of devitalized tissue and simple postoperative drainage, 4 but the death rate remained high, mainly as a result of recurrent sepsis, requiring repeated surgery. With the development of techniques of open packing 5 and the use of prolonged postoperative lavage, 6 death rates for surgical intervention in acute pancreatitis have fallen, 7 although even in these specialist units the death rate for infected necrosis may exceed 60%. 8
Minimally invasive surgery has been consistently shown to be associated with less activation of the inflammatory response than equivalent open surgery, 9,10 and experimental evidence suggests that local sepsis and the inflammatory response may be lessened by a minimally invasive rather than an open technique. 11 We hypothesized that by minimizing the massive inflammatory “hit” of open pancreatic necrosectomy, a minimally invasive approach to the management of infected pancreatic necrosis may lessen the risk of multiple organ failure and lessen the risk of respiratory and wound complications in these patients.
We describe the development of a minimally invasive approach to pancreatic necrosectomy designed to meet the surgical ends of the open procedure—that is, debridement of devitalized tissue and establishment of a system for continuous postoperative lavage.
SURGICAL TECHNIQUE
Percutaneous Necrosectomy
Under computed tomography guidance, an 8F pigtail nephrostomy catheter is inserted into the infected cavity, the surgeon having carefully selected a path that will allow subsequent dilatation. Our path of choice is to enter the area of infected necrosis between the lower pole of the spleen and the splenic flexure. In predominately right-sided pancreatic head necrosis, we have used a path through the gastrocolic omentum, anterior to the duodenum. However, this results in a more technically difficult necrosectomy and prevents dependent postoperative drainage. The catheter is secured and the patient transferred to the operating room. With the patient under general anesthesia, access to the abscess cavity is maintained using a guidewire, over which the catheter tract is then dilated to 30F using graduated dilators and radiologic guidance. 12 This allows a 30F Amplatz sheath to be inserted. An operating nephroscope that allows intermittent irrigation and suction, with a 4-mm working channel, is then passed along the Amplatz sheath into the abscess cavity. Piecemeal removal of solid material is then performed using soft grasping forceps through the working channel by repeatedly passing the instrument into the cavity until all loose necrotic tissue is removed (Fig. 1). Finally, an 8F umbilical catheter sutured to a 28F tube drain is then passed over a 12F stiffener to the distal end of the cavity to allow continuous postoperative lavage (500 mL/hr) through the the umbilical catheter. Because of the high-volume lavage, we use a fluid normally used for peritoneal lavage (Dianil 7, Baxter Healthcare Ltd, Thetford, UK) to minimize the potential of electrolyte imbalance. The lavage is continued at this rate until the lavage fluid clears or until a further procedure.

Figure 1. Necrotic debris after piecemeal removal.
Sinus Tract Endoscopy
In patients with a previous primary debridement, either at open laparotomy or after the above technique, in whom residual sepsis is suspected, a second computed tomogram is obtained and, provided there are no satellite collections, secondary sinus tract endoscopy is performed. In the operating room and under general anesthesia, the previously sited drain or drains are removed. Either a flexible or a rigid endoscopic system is used, depending on the suspected amount of residual necrosis.
Sinus tract endoscopy using a flexible endoscope is tedious, and only small fragments of necrotic tissue can be removed with each pass of the endoscope. As a result, we have moved to using the operating nephroscope as described above for most primary explorations. The major alteration in the technique is that the Amplatz sheath is not required. Access to pockets of necrosis is occasionally limited by the rigidity of the system, and flexible endoscopy remains useful to check the tract before drain removal if residual necrosis is not suspected.
For flexible endoscopy, each tract is dilated to 45F using a balloon dilator (Maxforce TTS, Boston Scientific Ltd, St. Albans, UK). A twin-channel endoscope (Olympus GIF 2T200, Keymed Ltd, Southend-on-Sea, UK) is then passed through the skin opening. Further endoscopic antegrade dilatation of the tract is then performed until the entire length of the drain tract can be visualized. Jet irrigation using a heater probe (Olympus HPU, Keymed Ltd) and suction allows fluid collections to be cleared, and residual solid necrotic tissue or adherent slough can be teased away using a variety of endoscopic instruments (e.g., snares, stent retrieval forceps). A guidewire is then passed through the endoscope and an 8F umbilical catheter sutured to a 28F tube drain is placed in the cavity, after which lavage begins again.
PATIENTS AND RESULTS
Patient details are summarized in Table 1 (sinus tract endoscopy) and Table 2 (percutaneous necrosectomy). All 14 patients had proven infection of necrotic pancreatic or peripancreatic tissue (7 coliforms, 3 streptococci, 3 Candida, 2 Citrobacter, and 1 Staphylococcus aureus).
Table 1. PATIENT DETAILS: SINUS TRACT ENDOSCOPY
* Already in ITU before transfer.
BMI, body mass index; ERCP/ES, endoscopic retrograde cholangiopancreatography plus endoscopic sphincterotomy; IPS, inpatient stay; ITU, intensive care unit; STE, sinus tract endoscopy.
Table 2. PATIENT DETAILS: PRIMARY PERCUTANEOUS NECROSECTOMY (PPPN)
* Already in ITU before transfer.
BMI, body mass index; ERCP/ES, endoscopic retrograde cholangiopancreatography plus endoscopic sphincterotomy; IPS, inpatient stay; ITU, intensive care unit; STE, sinus tract endoscopy.
In the initial four patients, flexible sinus tract endoscopy was used after formal surgical exploration, debridement, and drainage. All had required intensive care admission after initial surgical debridement and had developed clinical signs of residual sepsis. Two were men and two were women; the median age was 43 (range 33–57). The cause was was gallstones in three and alcohol in one. In all four, the residual sepsis resolved without further surgery, and the patients were discharged home. The median total hospital stay was 49.5 (range 44–72) days. Two were subsequently readmitted for further surgery. One had reversal of an ileostomy and another underwent a right hemicolectomy for a persistent right-sided colonic fistula.
In the 10 subsequent patients, the role of the technique was extended to the primary management of retroperitoneal peripancreatic sepsis. These patients would otherwise have undergone open surgical exploration, debridement, and lavage; this group included two patients who were considered unfit for an open procedure. All had documented pancreatic and peripancreatic necrosis on contrast-enhanced computed tomography, but necrosectomy was performed only after confirmation of infection by guided fine-needle aspiration. Five of these patients had undergone prior intervention with endoscopic retrograde cholangiopancreatography (Table 1). Percutaneous necrosectomy was performed in four male and six female patients with a median age of 45 (range 32–74). The cause was gallstones in five, alcohol in three, and both alcohol and gallstones in one; one patient had pancreatitis secondary to familial hyperlipidemia. Three patients were admitted directly to the intensive care unit from referring hospitals, one 5 months after the onset of symptoms. The remainder were managed in the high-dependency surgical unit.
Two patients died. Our initial patient undergoing percutaneous necrosectomy was a 66-year-old man with severe pancreatitis and an infected, necrotic collection around the pancreatic tail. He had significant comorbidity, including end-stage liver failure, steroid-dependent chronic obstructive pulmonary disease, and morbid obesity (body mass index = 46 kg/m2). He was therefore considered unfit for a laparotomy, and a percutaneous necrosectomy was performed. After this, there was complete resolution of the pancreatic sepsis, but he died 7 weeks later as a result of combined respiratory and liver failure without leaving the hospital.
The second patient, a 74-year-old woman, was transferred to our care with multiple organ failure. Percutaneous necrosectomy was carried out, during which a laparotomy for control of intraoperative hemorrhage became necessary. This followed injury to the splenic vessels as a result of being overly fastidious in removing all slough from the cavity. The hemorrhage was initially controlled using a balloon catheter until surgical control was achieved at laparotomy. This patient underwent further surgical exploration after a deterioration in organ failure on day 8 and later died of multiple organ failure.
All of the remaining eight patients treated by percutaneous necrosectomy recovered without open surgical exploration. Three were primary admissions to our own unit, and the other five were referred to our care after developing organ dysfunction at their base hospital. All but one were referred within 10 days of onset of symptoms. The exception was a 57-year-old man with respiratory and renal failure, 6 months after multiple surgical procedures and a period of open packing resulting in an enteric fistula. There was computed tomography evidence of residual necrosis in the pancreatic bed. The necrotic material was removed using the percutaneous necrosectomy technique, with subsequent resolution of the organ failure.
These 10 patients required a median of two (range 1–4) sinus tract explorations. After surgery, only three of these patients, all of whom had established organ failure before surgery, were managed in the intensive care unit. Three further complications were observed. One patient had a prolonged gastric ileus, and despite 6 weeks of nasojejunal feeding after resolution of intraabdominal sepsis could not tolerate solid food. This was successfully managed by gastrojejunostomy. The median inpatient stay was 42 (range 23–213) days. Two further patients who had been discharged after resolution of pancreatic sepsis returned with symptomatic pancreatic pseudocysts. Both were found to communicate with the pancreatic duct on endoscopic retrograde cholangiopancreatography and were successfully managed by endoscopic transpapillary drainage. One of these patients also required percutaneous aspiration of a residual fluid collection, but both problems subsequently resolved. Resolution of the necrotic abscess cavity was confirmed by contrast-enhanced computed tomography before removal of the drain (Fig. 2).
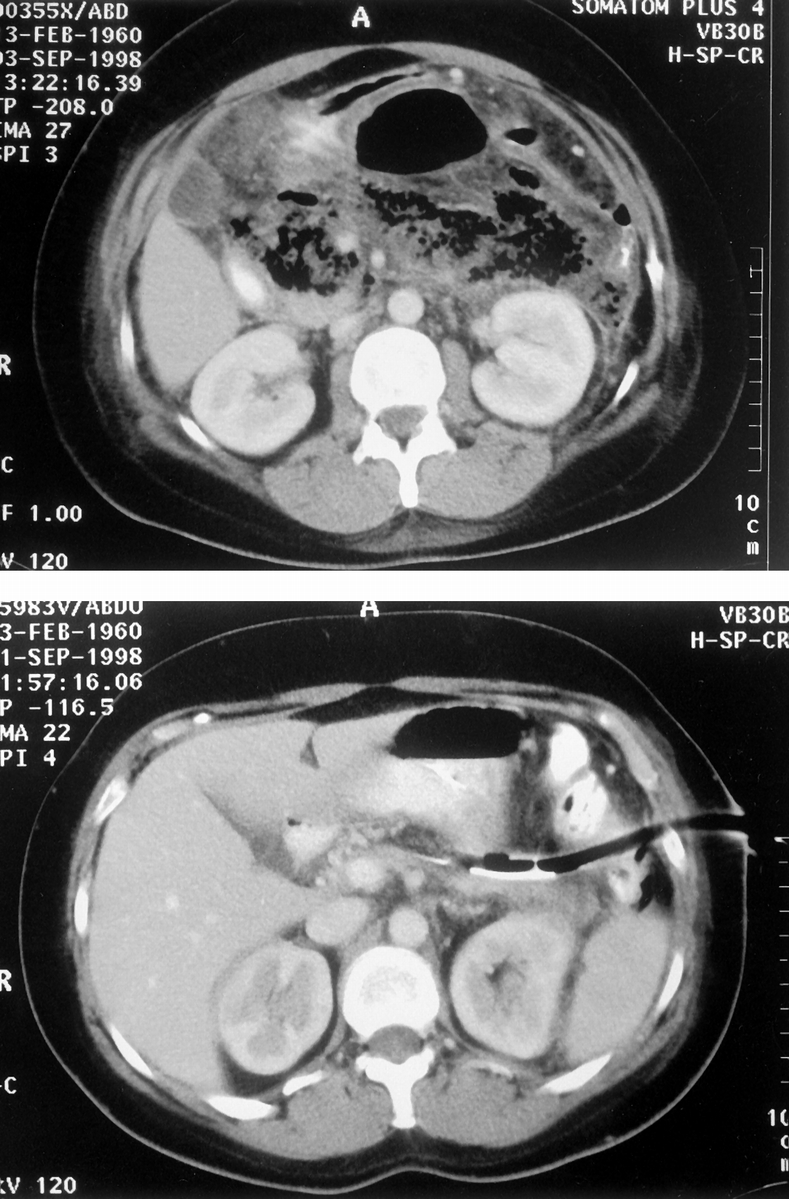
Figure 2. Preoperative and postoperative computed tomography images (patient 6) after percutaneous necrosectomy.
DISCUSSION
Infection occurs in 8% to 12% of patients with acute pancreatitis 1–3 and in up to 70% with necrotizing pancreatitis. 3,13,14 We have previously reported 14 a death rate of 28% after surgical debridement in our institution; this ranges from 15% to 80% in published series. 2,3 Delay in surgical intervention results in a higher death rate once infection occurs. 2,15,16 In the past 20 years, the technique of open surgical debridement has included necrosectomy with simple drainage, “second-look” procedures, open laparostomies, and necrosectomy with packing or with postoperative lavage. Our preference has been to perform a radical retroperitoneal debridement with cholecystectomy and cholangiography and with vigorous postoperative lavage, reserving packing for patients with significant intraoperative bleeding. In these patients, a lavage system would be created at the time of pack removal.
The surgical complication rate associated with these procedures has encouraged some authors to explore minimally invasive approaches to pancreatic collection. Simple percutaneous drainage catheters only temporize, ensuring the long-term infection of the necrotic cavity. Until recently they have been dismissed as having a limited role in the management of pancreatic sepsis. In 1996, Baron et al 17 described the technique of combining endoscopic cyst-gastrostomy with nasocyst lavage and reported good results in selected patients. These patients had pseudocysts or abscesses at a median of 7 weeks after the onset of illness, and only 28% were infected at the time of drainage. Postprocedure infection of the necrotic tissue occurred in 38% of patients, requiring further intervention and highlighting the need for removal of necrotic tissue from the abscess cavity. In the light of these results, they have since determined 18 that collections containing solid debris of more than 1 cm on preoperative imaging are unsuitable for this form of drainage. Up to 60% of patients with successful drainage developed further collections in the subsequent 2 years. 19
Freeny et al 20 reported complete resolution of sepsis in 47% of patients with established infected necrosis after aggressive percutaneous drainage with multiple catheters and lavage. This required a mean of four catheter insertions and 8-hourly lavage for a mean of 85 days. The remaining 53% required either elective (26%) or emergency surgery.
The principal advantage of our technique (percutaneous necrosectomy) over those described by Baron and Freeny is the ability to remove the necrotic material; this necessitated a second surgical intervention in more than 50% of their patients. We have used the Baron approach in some patients with a pancreatic abscess in the absence of necrosis. However, we are convinced that infected necrosis, where the solid component predominates, will not respond to this technique.
Another notable feature of these patients’ recovery has been the lack of organ dysfunction in the immediate postoperative period. After open necrosectomy, our experience was that patients were always admitted to our intensive care unit, often requiring significant respiratory and inotropic support before recovery. In our initial experience, it would appear that patients without organ dysfunction before surgery do not develop it as a result of the procedure, and we have managed 60% of these patients outside of the intensive care unit. We previously reported 21 that more than 50% of the overall cost of management is due to the period of intensive care stay; consequently, this technique may have significant resource implications in addition to benefits in clinical care. We suspect, however, that the need to return to the operating room on several occasions will make it unlikely that the overall hospital stay will be shortened in most patients.
A major initial concern involving the application of our technique was whether it was possible to perform an adequate necrosectomy. Two lessons were learned from the hemorrhage that we encountered at the end of our second procedure, necessitating conversion. First, the surgical clearance achieved by this method is similar to that achieved at open surgery. Second, fastidious clearance during the primary procedure is probably unwise: residual material will separate after lavage and may be removed with ease during a later sinus tract endoscopy.
At open surgery for infected pancreatic necrosis, there is a small but significant incidence of intestinal ischemia, usually affecting the transverse colon, requiring resection at the time of the primary laparotomy. We are aware that using the described technique, surgeons can miss these patients, because from within the organized abscess cavity, it is impossible to assess the viability of the bowel. We have tried to minimize this risk by looking for clinical or biochemical evidence of potential ischemia, in addition to assessing intestinal perfusion at the time of the contrast-enhanced computed tomogram. However, this minority is a subgroup in which this technique is not appropriate.
At our hospital, we follow a policy of performing a routine cholecystectomy and surgical cholangiography in all patients undergoing open pancreatic necrosectomy. Percutaneous necrosectomy obviously prevents this, although our protocol includes an early endoscopic retrograde cholangiopancreatogram with endoscopic sphincterotomy in all patients suspected of having severe gallstone pancreatitis. Patients requiring cholecystectomy can therefore have this performed laparoscopically as an interval procedure before discharge.
Approximately 25% to 35%2,5,7,14,16 of patients with severe acute pancreatitis who develop secondary infection require multiple surgical explorations. Each surgical procedure is associated with immediate clinical deterioration before any improvement. The significance of minimizing the magnitude of this “second hit” is of clinical interest because it is not uncommon for a patient to die during this deterioration. An adequate primary surgical procedure is of paramount importance, with debridement and lavage creating a fluid-filled cavity in the retroperitoneum. Secondary surgical exploration may release collections but often produces only small amounts of necrotic material. We have found these goals can be achieved using the percutaneous techniques described above, obviating the need for formal exploration with, in our initial experience, much less insult to the patient.
During the development of any new technique, it is always possible to achieve superficially impressive results through careful case selection. However, more universal application highlights the limitations of the procedure. Consequently, we employed percutaneous necrosectomy in a consecutive series of patients with infected pancreatic necrosis who came to our unit, including two patients thought unlikely to survive an open operation. In this context, we consider the initial results to support our clinical impression that percutaneous necrosectomy may represent an advance over open necrosectomy and postoperative lavage. Formal evaluation of this technique is ongoing.
Footnotes
Correspondence: C. Ross Carter, MD, FRCS, Dept. of Upper GI and Pancreatico-Biliary Surgery, Glasgow Royal Infirmary, Alexandra Parade, Glasgow G31 2ER, Scotland, UK.
E-mail: rcarter@clinmed.gla.ac.uk
Accepted for publication February 25, 2000.
References
- 1.Frey CF, Bradley EL III, Beger HG. Progress in acute pancreatitis. Surg Gynecol Obstet 1988; 167: 282–286. [PubMed] [Google Scholar]
- 2.Beger HG, Buchler M, Bittner R, Nevalainen T, Roscher R. Necrosectomy and post-operative local lavage in necrotising pancreatitis. Br J Surg 1988; 75: 207–212. [DOI] [PubMed] [Google Scholar]
- 3.Allardyce DB. Incidence of necrotising pancreatitis and factors related to mortality. Am J Surg 1987; 154: 295–299. [DOI] [PubMed] [Google Scholar]
- 4.Altemeier WA, Alexander JW. Pancreatic abscess. Arch Surg 1963; 87: 80–85. [DOI] [PubMed] [Google Scholar]
- 5.Bradley EL III. Management of infected pancreatic necrosis by open drainage. Ann Surg 1987; 206: 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beger HG, Krautzberger W, Bittner R, Block S, Buchler M. Results of surgical treatment of necrotising pancreatitis. World J Surg 1984; 9: 972–979. [DOI] [PubMed] [Google Scholar]
- 7.Rau B, Uhl W, Buchler MW, Beger HG. Surgical treatment of infected necrosis. World J Surg 1997; 21: 155–161. [DOI] [PubMed] [Google Scholar]
- 8.Rau B, Steinbach G, Gansuage F, et al. The potential of procalcitonin and interleukin 8 in the prediction of acute pancreatitis. Gut 1997; 41: 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labib M, Palfrey S, Paniagua E, Callender R. The postoperative inflammatory response to injury following laparoscopic assisted vaginal hysterectomy versus abdominal hysterectomy. Ann Clin Biochem 1997; 34: 543–545. [DOI] [PubMed] [Google Scholar]
- 10.Joris J, Cigarini I, Legrand M, et al. Metabolic and respiratory changes after cholecystectomy performed via laparotomy or laparoscopy. Br J Anaesth 1992; 69: 341–345. [DOI] [PubMed] [Google Scholar]
- 11.Jacobi CA, Zieren HU, Sabat R, et al. Local and systemic inflammation after laparotomy versus laparoscopy in a sepsis model in rats. Langenbecks Archiv Chirurgie 1997; 382 (4 Suppl 1):9–13. [DOI] [PubMed] [Google Scholar]
- 12.Young AT, Hulbert JC, Cardella JF, et al. Percutaneous nephrostolithotomy: Application to staghorn calculi. Am J Roentgenol 1985; 145: 1265–1269. [DOI] [PubMed] [Google Scholar]
- 13.Bittner R, Block S, Buchler M, Beger HG. Pancreatic abscess and infected pancreatic necrosis. Dig Dis Sci 1987; 32: 1082–1087. [DOI] [PubMed] [Google Scholar]
- 14.Wilson C, McArdle CS, Carter DC, Imrie CW. Surgical treatment of acute necrotising pancreatitis. Br J Surg 1988; 75: 1119–1123. [DOI] [PubMed] [Google Scholar]
- 15.Bradley EL III. A clinically based classification system for acute pancreatitis. Arch Surg 1993; 128: 586–590. [DOI] [PubMed] [Google Scholar]
- 16.Larvin M, Chalmers AG, Robinson PJ, McMahon MJ. Débridement and closed cavity irrigation for the treatment of pancreatic necrosis. Br J Surg 1988; 76: 465–471. [DOI] [PubMed] [Google Scholar]
- 17.Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organised pancreatic necrosis. Gastroenterology 1996; 111: 755–764. [DOI] [PubMed] [Google Scholar]
- 18.Morgan DE, Baron TH, Smith JK, Robbin ML, Kenney PJ. Pancreatic fluid collections prior to drainage: evaluation of MR imaging compared with CT and US. Radiology 1997; 203: 773–778. [DOI] [PubMed] [Google Scholar]
- 19.Adkisson KW, Morgan DE, Baron TH. Long-term outcome following successful endoscopic drainage of organised pancreatic necrosis (OPN): “skunk-poking” revisited [abstract]. Gastrointest Endosc 1997; 45: 514. [Google Scholar]
- 20.Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotising pancreatitis. Techniques and results. Am J Roentgenol 1998; 170: 969–975. [DOI] [PubMed] [Google Scholar]
- 21.Fenton-Lee D, Imrie CW. Pancreatic necrosis: assessment of outcome related to quality of life and cost of management. Br J Surg 1993; 80: 1579–1582. [DOI] [PubMed] [Google Scholar]