Abstract
Objective
To determine whether survival and recurrence after laparoscopic-assisted surgery for colorectal cancer is compromised by an initial laparoscopic approach.
Summary Background Data
Laparoscopic colorectal resection for malignancy remains controversial 8 years after its first description. Fears regarding compromised oncologic principles and early recurrence (particularly the phenomenon of port-site metastases) have tempered enthusiasm for this approach. Long-term follow-up data are at present scarce.
Methods
A prospective comparative trial was undertaken between December 1993 and May 1996, during which 114 patients had laparoscopic-assisted resection by a single laparoscopic colorectal surgeon or conventional open surgery by a second specialist colorectal surgeon. Intensive follow-up for at least 2 years is available on 109 patients. Analysis was performed on an intention-to-treat basis.
Results
Recurrent disease has developed in 27 patients (25%), 16 of 57 in the laparoscopic group (28%) and 11 of 52 in the conventional group (21%). Crude death rates are 26/57 (46%) in the laparoscopic group and 24/52 (46%) in the conventional group. No port-site metastases have occurred; however, wound metastases associated with disseminated disease have developed in three patients in the open group and one in the laparoscopic group. Stage-for-stage survival and recurrence figures are comparable.
Conclusion
Oncologic outcome at a minimum of 2 years is not compromised by the laparoscopic approach. Wound recurrences are a feature of laparoscopic and conventional surgery for advanced disease.
Colorectal cancer is the only commonly curable visceral malignancy. At present in the United Kingdom, approximately 50% of patients with the disease are treated with the reasonable expectation of cure, of whom 50% again can be expected to be alive 5 years later. 1,2 The use of any new treatment modality such as minimal-access surgery cannot be supported in such patients unless it is shown at least to match such a survival profile.
Although laparoscopic techniques were first applied to colorectal surgery in 1991, 3–6 the approach has not been widely adopted. This is largely because of concerns over the safety of these techniques in cancer surgery. There is evidence, albeit from nonrandomized studies, suggesting that the margins of excision of colorectal cancer achieved laparoscopically are comparable to those resulting from conventional resection. 7–11 However, the appearance of several reports of early wound recurrence after laparoscopic resection for malignancy, 12,13 including that of Dukes’ A lesions, 14,15 have led to the suggestion that the pattern of disease recurrence may be altered by the laparoscopic approach. Unfortunately, a series of elegant investigations in a variety of animal models have failed to reach a consensus: for every study in which tumor growth was facilitated by laparoscopy, 16,17 there are at least an equivalent number suggesting that growth is attenuated 18–24 or comparable. 19
Although such studies are of interest, the true assessment of the safety of laparoscopic techniques in neoplasia must come from long-term follow-up of patients operated on using such techniques. Such data are at present scarce. The Norfolk surgical group has presented the first 24-month follow-up data from 39 patients in whom the cancer-specific death rate at 24 months was 6%, with an overall recurrence rate of 9%. Importantly, no wound or port-site metastases were detected. The actuarial 3-year survival rate in their series was 92% for node-negative patients and 79% for node-positive patients. 25 The authors commented that such survival and recurrence profiles were similar to those that would be anticipated after open surgery.
Similarly, in a small number of other uncontrolled series, Gray et al 26 reported a 22.70% recurrence rate among 22 patients followed up for 24 months and Lumley et al 27 reported a recurrence rate of 6.3% at a median follow-up of 33 months. In the series of Franklin et al, 28 191 patients who had a laparoscopic approach to colorectal cancer were compared stage for stage with a control group of 224 patients undergoing conventional surgery by another group of surgeons; there was no difference in terms of survival or recurrence. Although prospective, this study allowed patients to chose which type of surgery was performed, raising questions regarding selection bias. Lacy et al,29 in a prospective and randomized trial, reported a recurrence rate of 16.1% among 31 laparoscopic-assisted resections after a mean follow-up of 21 months.
More recently, Fleshman et al 30 compared survival after laparoscopic abdominoperineal resection with a retrospective control group of patients who underwent an open procedure from several institutions. This study contained data on the radial margin of excision and demonstrated no difference in survival or recurrence between the groups after a median follow-up of 19 months.
The randomized trial from the Cleveland Clinic 31 demonstrated no difference in survival or recurrence in 42 patients undergoing laparoscopic resection for cancer after a median follow-up of 1.5 years. The authors reported four cancer-related deaths and no local or port-site recurrences during the follow-up period.
These data are reassuring, but the duration of follow-up is variable. Indeed, these studies might be considered premature, because a significant minority of patients were followed up for less than 1 year.
In the current study, we aimed to assess the safety of laparoscopic colorectal surgery for carcinoma by a prospective study of stage-for-stage recurrence and death compared with conventional surgery at a minimum follow-up of 2 years.
METHODS
From December 1993 to May 1996, patients undergoing elective surgery for colorectal carcinoma at our colorectal teaching unit were recruited into the study. Preoperative assessment included physical examination, liver function tests, carcinoembryonic antigen assay, liver ultrasound, abdominal computed tomography or magnetic resonance imaging, and usually colonoscopy and biopsy. All patients who would be considered for laparoscopy underwent a barium enema examination to determine the exact location of the lesion.
Recruitment into laparoscopic and conventional treatment groups was not randomized. Instead, in the absence of specific contraindications (multiple previous abdominal incisions, contraindications to pneumoperitoneum, gastrointestinal obstruction, or synchronous tumors), all patients to be operated on by a single surgeon (J.R.T.M.) were offered a laparoscopic approach, whereas those operated on by a second surgeon (P.W.R.L., who undertook no laparoscopy) underwent conventional resections.
Open procedures were undertaken according to the surgeon’s established technique, which conformed to standard British colorectal teaching 32 and will not be detailed further. The philosophy in undertaking a laparoscopic approach to colorectal resection was to undertake laparoscopically only what would be considered safe at open surgery. Our techniques for laparoscopic-assisted colorectal resection have been presented in detail elsewhere. 33,34 In brief, a five-port technique was used; the appropriate segment of bowel was mobilized laparoscopically, the vascular supply was wherever possible divided intracorporeally, and for all except abdominoperineal excisions, a small incision was then made to deliver the mobilized segment, divide the bowel (if not completed laparoscopically), and fashion an anastomosis. During abdominoperineal excision, the proximal colon was brought out through the left iliac fossa port site and the specimen was delivered through the perineum, thus obviating the need for an additional abdominal incision. Intraoperative staging was meticulous, and the surgeon made a definite decision, recorded in the operative notes, as to whether the procedure was to be considered curative or palliative. For this study, we have defined rectal cancer as only tumors arising below the sacral promontory; we consider procedures for tumors above this to be left colectomies.
All patients subsequently entered a structured follow-up program of 3-monthly outpatient visits for the first year and 6-monthly visits thereafter. All patients were followed up for at least 24 months. Investigations included regular physical examination, sigmoidoscopy, liver function tests, and carcinoembryonic antigen assay at each visit. Magnetic resonance imaging of the abdomen and pelvis was undertaken at 6-month intervals for rectal cancer, and computed tomography of the abdomen was performed at the same interval after resection of colonic carcinoma. Colonoscopy was performed at 1 year and thereafter at 3-year intervals unless otherwise indicated. Recurrence was biopsy-proven whenever feasible.
Statistical analyses were undertaken using the SPSS statistical package (SPSS 7.5.1 for Windows, 1996, SPSS Inc., Chicago, IL). Survival and disease-free intervals after surgery were calculated by the product-limit method of Kaplan and Meier, 35 with recurrence and survival rates compared using the log-rank test. Survival and recurrence rates at 24 months were compared by chi-square tests for categorical data using 2 × 2 tables.
RESULTS
A laparoscopic approach was attempted in 61 patients; conversion to formal laparotomy was required in 20 patients (33%). Conventional resections were undertaken in 53 patients. The two groups were comparable in terms of age, sex, pathologic stage, and type of resection undertaken (Table 1). Use of adjuvant chemotherapy and postoperative pelvic radiation therapy for rectal cancer was also comparable in the two groups (Table 2). Recurrence and survival rates were analyzed on an intention-to-treat basis, so palliative procedures were not excluded and converted cases were considered with successfully completed laparoscopic cases.
Table 1. PATIENT CHARACTERISTICS
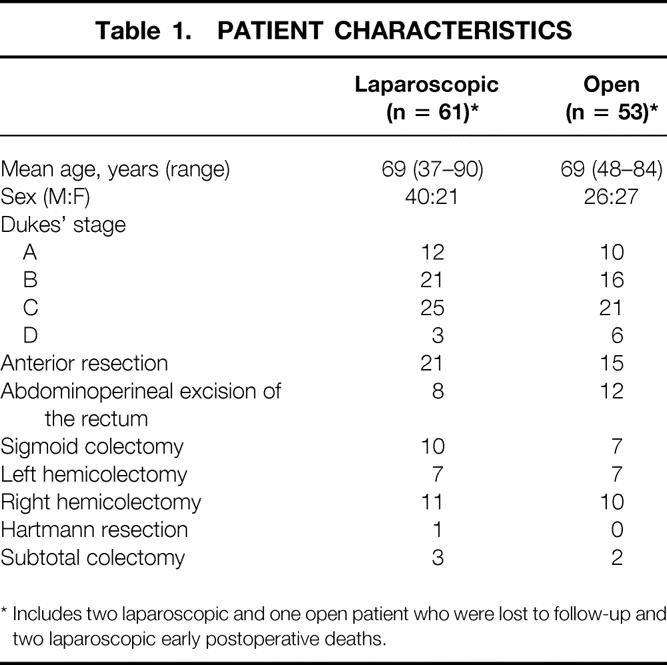
* Includes two laparoscopic and one open patient who were lost to follow-up and two laparoscopic early postoperative deaths.
Table 2. USE OF ADJUVANT CHEMOTHERAPY AND RADIATION THERAPY

There were two postoperative deaths in the laparoscopic group; the cause was myocardial infarction at days 7 and 16 after surgery. These were excluded from the survival analysis. Two patients in the laparoscopic group and one patient in the open group were lost to follow-up. A median of 42 months of follow-up (interquartile range = 14.25 months) was available on the remaining 109 patients.
To date, recurrent disease has developed in 27 of the patients (24.7%) available for follow-up (including systemic or local treatment failures). This total included 16 of 57 patients in the laparoscopic (28%) and 11 of 52 in the conventional group (21%). Crude death rates at a median of 42 months of follow-up were 26/57 in the laparoscopic group (46%) and 24/52 in the open group (46%). These differences were not significant. Stage-for-stage comparisons of disease recurrence rates (systemic and local treatment failures) and crude death rates to the limit of clinical follow-up are presented in Table 3. There was no significant difference between the groups for any stage of disease.
Table 3. CRUDE DEATH AND DISEASE RECURRENCE RATES AT MEDIAN FOLLOW-UP OF 42 MONTHS
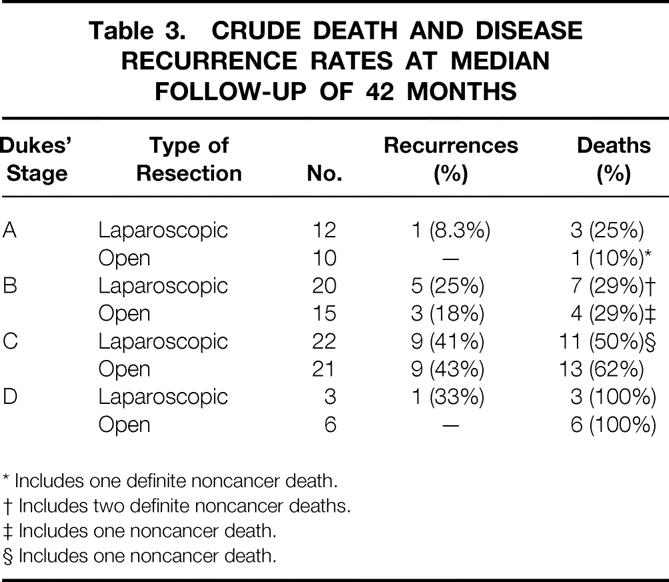
* Includes one definite noncancer death.
† Includes two definite noncancer deaths.
‡ Includes one noncancer death.
§ Includes one noncancer death.
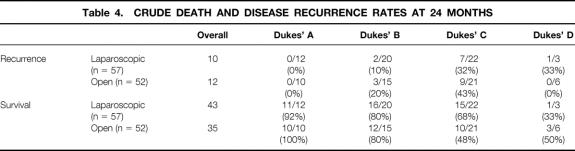
The results at 24 months of follow-up in terms of disease recurrence and crude death rates are presented in Table 4. There was no significant difference in any of these parameters between laparoscopic and open groups. The Kaplan-Meier curve for crude survival for the groups, with all patients being available or “at risk” at 24 months, is shown in Figure 1. There was no difference in calculated actuarial survival using the log-rank and Wilcoxon tests.
Table 4. CRUDE DEATH AND DISEASE RECURRENCE RATES AT 24 MONTHS

Figure 1. Kaplan-Meier curves for survival after laparoscopic (solid line) and open (dashed line) surgery for colorectal cancer (+, censored data). P = .6264, log-rank test.
The pattern of recurrent disease or treatment failure after laparoscopic and open surgery is presented in Table 5 and Table 6, respectively. Pelvic recurrence was identified in seven patients in the laparoscopic group (three of these procedures were palliative) and five patients in the conventional group (one of these procedures was palliative). The crude local recurrence rate for all cases of rectal cancer was therefore 24% (7 of 29 cases) for the laparoscopic group and 19% (5 of 27 cases) for the open group. Isolated local recurrence—that is, without evidence of systemic disease—was correspondingly less frequent and was identified in one in the laparoscopic group (3%) and one in the open group (4%). These differences between laparoscopic and open groups were not significant.
Table 5. PATIENTS WITH DISSEMINATED OR LOCAL RECURRENCE AFTER LAPAROSCOPIC SURGERY FOR COLORECTAL CANCER

APER, abdominoperineal excision of the rectum.
* Lung metastases resected.
† Hepatic resection.
‡ At 27 months presented with thickening of the abdominal scar that was biopsy-proven as recurrent disease, and then underwent laparotomy and palliative bypass for widespread intraperitoneal recurrence.
Table 6. PATIENTS WITH DISSEMINATED OR LOCAL RECURRENCE AFTER OPEN SURGERY FOR COLORECTAL CANCER
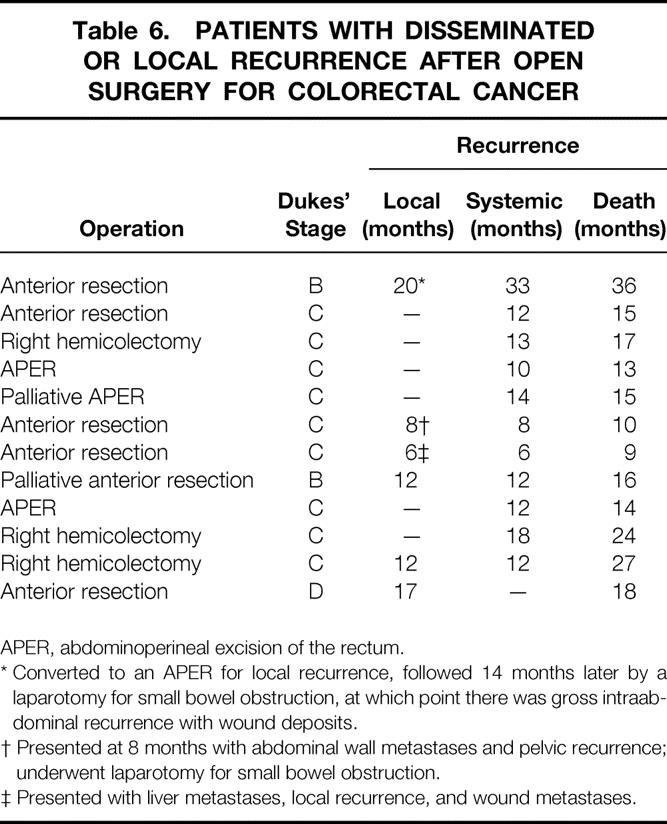
APER, abdominoperineal excision of the rectum.
* Converted to an APER for local recurrence, followed 14 months later by a laparotomy for small bowel obstruction, at which point there was gross intraabdominal recurrence with wound deposits.
† Presented at 8 months with abdominal wall metastases and pelvic recurrence; underwent laparotomy for small bowel obstruction.
‡ Presented with liver metastases, local recurrence, and wound metastases.
No port-site metastases were detected in the laparoscopic group. However, histologically confirmed wound metastases were identified in three patients in the open group at a mean of 11 months after resection (range 6–19 months) and a single patient in the laparoscopic group 27 months after resection. These were not isolated recurrences but instead occurred in the presence of widespread intraperitoneal disease and were detected at the time of repeat laparotomy (Tables 5 and 6).
DISCUSSION
The real test of the safety of any new techniques for cancer treatment comes from comparisons of patients who have the same pathologic stage and who are equivalent in all respects other than their mode of treatment. Careful prospective follow-up is then required to determine the efficacy of one form of treatment versus another. Thus far, few such studies are available for laparoscopic colorectal surgery.
The design of our study is obviously limited in that patients were not randomized into the two treatment arms. Although such an approach is clearly ideal, it is a depressing reality that no major surgical intervention has ever been introduced on the basis of a large-scale randomized controlled trial. Although not randomized, patients were recruited on the basis of their referral to the surgeon. Clearly there is the potential for bias inherent in this design, given that the referral sources may have been far from random in their allocation and instead sent a certain type of patient to the first surgeon and another type to the second, with this decision being based on factors unknown. However, this bias, although possible, seems unlikely in view of the equivalence of our two groups in terms of age and sex distributions and Dukes’ stage.
We are therefore left with the results of a prospective controlled trial of laparoscopic versus open surgery in an unselected series of patients. This study found recurrent disease, systemic or local, in 21% of patients undergoing conventional surgery and 28% of patients undergoing laparoscopic surgery at a median follow-up of 42 months. These differences were not significant, nor were the same parameters when stage-for-stage comparisons were made of the two groups at 24 months of follow-up. These data are clearly important and are among the first such comparisons to be reported with at least 2 years of follow-up.
Of comparable series, Lumley et al 27 reported a recurrence rate of 6.3% to a median follow-up of 33 months, which is superior to most data from open series. A small randomized trial from Lacy et al’s 29 group in Barcelona reported recurrence in 16.1% of 31 laparoscopic procedures compared with 15% of open colectomies during a mean follow-up of 21 months. Given that this study did not include rectal cancer cases, and that the follow-up period is shorter, the recurrence figures reported are comparable to those of the present series. The Barcelona series is also distinctive for documenting the selection criteria used and for giving detailed staging information.
The Kaplan-Meier survival curves show actuarial survival rates in the region of 50% at 5 years, which again is within the anticipated range for patients undergoing surgery for colon and rectal cancer. 36–38 Once again, there was no significant difference between laparoscopic and open groups in actuarial survival. We have chosen to treat our patients on an intention-to-treat basis rather than excluding patients who underwent palliative surgery or those in the laparoscopic arm who underwent conversion to formal laparotomy. This is because selection of subsets from within these groups carries the potential for introducing bias, and it may be difficult to determine which procedures ought to be considered palliative. In addition, it has been suggested that pneumoperitoneum may have a differential effect on tumor biology, and we believed it was appropriate for patients who may have undergone only a preliminary laparoscopy to be included in that arm of the trial. Nevertheless, most other series take pains to exclude palliative procedures, although the grounds on which this decision is made are often obscure, and this inevitably produces more favorable results.
The introductory review detailed the concern about the possibility of a change in the pattern of disease recurrence as a consequence of laparoscopy. This resulted from the alarming reports of the early development of wound metastases either at a trocar site or in the wound used to remove the specimen. 12–15,39 However, outside these reports, the evidence that wound implantation is a serious concern is scarce. Few of the major series have reported a significant incidence of such problems since Fleshman et al 40 reported four port-site recurrences in 372 accumulated resections for cancer; the multicenter registry from the American Society of Colon and Rectal Surgeons reported a similarly low incidence. 41 Our results are in keeping with the view that port-site implantation probably represents a technical error resulting from inappropriate tumor instrumentation, which is more likely to occur early in the learning curve. There were no isolated wound implantations in the 63 patients who underwent laparoscopy to the duration of follow-up. However, there was a small but significant incidence of wound metastases in both groups, although this was always part of disseminated treatment failure. It also appears that wound recurrence after open surgery may be much more common than previously thought. 42
In conclusion, this study has shown no evidence for any alteration in the pattern of disease recurrence and survival after laparoscopic and conventional surgery for colorectal cancer during at least 2 years of follow-up. This provides further evidence that the laparoscopic approach to colorectal cancer does not breach that essential tenet of medicine, primum non nocere (first, do no harm). However, the results of the ongoing randomized trials on either side of the Atlantic are awaited before we will know the true place of laparoscopic resection for malignancy in the surgeons’ armamentarium.
Footnotes
Correspondence: John R.T. Monson, MD, University of Hull, Academic Surgical Unit, Castle Hill Hospital, Cottingham, East Yorkshire HU16 5JQ, UK.
Presented in part to the American Society of Colon and Rectal Surgeons, Washington DC, May 1999.
E-mail: J.R.Monson@medschool.hull.ac.uk
Accepted for publication February 28, 2000.
References
- 1.Phillips R, Hittinger R, Blesovsky L, Fry J, Fielding L. Local recurrence following “curative” surgery for large bowel cancer: I. The overall picture. Br J Surg 1984; 71: 12–16. [DOI] [PubMed] [Google Scholar]
- 2.McArdle CS, Hole D, Hansell D, Blumgart LH, Wood CB. Prospective study of colorectal cancer in the West of Scotland: 10-year follow-up. Br J Surg 1990; 77: 280–282. [DOI] [PubMed] [Google Scholar]
- 3.Cooperman AM, Katz V, Zimmon D, Botero G. Laparoscopic colon resection: a case report. J Laparoendosc Surg 1991; 1: 221–224. [DOI] [PubMed] [Google Scholar]
- 4.Saclarides TJ, Ko ST, Airan M, Dillon C, Franklin J. Laparoscopic removal of a large colonic lipoma. Report of a case. Dis Colon Rectum 1991; 34: 1027–1029. [DOI] [PubMed] [Google Scholar]
- 5.Fowler DL, White SA. Laparoscopy-assisted sigmoid resection. Surg Laparosc Endosc 1991; 1: 183–188. [PubMed] [Google Scholar]
- 6.Schlinkert RT. Laparoscopic-assisted right hemicolectomy. Dis Colon Rectum 1991; 34: 1030–1031. [DOI] [PubMed] [Google Scholar]
- 7.Buchamen, P, Christen, D. Prolaparoscopic surgery for colorectal cancer. Dig Dis 1995; 12: 296–301. [Google Scholar]
- 8.Lord SA, Larach SW, Ferrara A, et al. Laparoscopic resections for colorectal carcinoma. A three-year experience. Dis Colon Rectum 1996; 39: 148–154. [DOI] [PubMed] [Google Scholar]
- 9.Ballantyne GH. Laparoscopic-assisted colorectal surgery: review of results in 752 patients. Gastroenterologist 1995; 3: 75–89. [PubMed] [Google Scholar]
- 10.Bokey EL, Moore JW, Chapuis PH, Newland RC. Morbidity and mortality following laparoscopic-assisted right hemicolectomy for cancer. Dis Colon Rectum 1996; 39: S24–S28. [DOI] [PubMed] [Google Scholar]
- 11.Gellman L, Salky B, Edye M. Laparoscopic assisted colectomy. Surg Endosc 1996; 10: 1041–1044. [DOI] [PubMed] [Google Scholar]
- 12.Nduka CC, Monson JRT, Menzies-Gow N, Darzi A. Abdominal wall metastases following laparoscopy. Br J Surg 1994; 81: 648–652. [DOI] [PubMed] [Google Scholar]
- 13.Wexner SD, Cohen SM. Port site metastases after laparoscopic colorectal surgery for cure of malignancy. Br J Surg 1995; 82: 295–298. [DOI] [PubMed] [Google Scholar]
- 14.Lauroy J, Champault G, Risk N, Boutelier P. Metastatic recurrence at the cannula site: should digestive carcinomas still be managed by laparoscopy? [abstract] Br J Surg 1994; 81 (suppl 1): 31. [Google Scholar]
- 15.Prasad A, Avery C, Foley RJE. Abdominal wall metastases following laparoscopy. Br J Surg 1994; 81: 1697. [DOI] [PubMed] [Google Scholar]
- 16.Jones DB, Guo DL, Reinhard MK, et al. Impact of pneumoperitoneum on trocar site implantation of colon cancer in a hamster model. Dis Colon Rectum 1995; 38: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 17.Wu JS, Jones DB, Guo LW, et al. Effects of pneumoperitoneum on tumor implantation with decreasing tumor inoculum. Dis Colon Rectum 1998; 41: 141–146. [DOI] [PubMed] [Google Scholar]
- 18.Bouvy ND, Marquet RL, Lamberts SWJ, et al. Laparoscopic bowel resection in the rat: earlier restoration of IGF-1 and less tumor growth. [abstract] Surg Endosc 1996; 10: 567. [DOI] [PubMed] [Google Scholar]
- 19.Mutter D, Hajri A, Tassetti V, et al. Experimental pancreatic tumor growth and spread after laparoscopy versus laparotomy in the rat. Surg Endosc 1996; 10: 566. [DOI] [PubMed] [Google Scholar]
- 20.Allendorf JDF, Bessler M, Kayton ML, et al. Increased tumor establishment and growth after laparotomy vs. laparoscopy in a murine model. Arch Surg 1995; 130: 649–653. [DOI] [PubMed] [Google Scholar]
- 21.Bessler M, Allendorf JDF, Chao JD. Permissive tumor growth after laparotomy versus laparoscopy is associated with altered TNF levels. Surg Forum 1994; 45: 486–487. [Google Scholar]
- 22.Jacobi CA, Ordemann J, Bohm B, et al. Increased tumor growth after laparotomy and laparoscopy with air versus CO2. Surg Endosc 1996; 10: 551. [Google Scholar]
- 23.Bouvy ND, Marquet RL, Jeekel H, Bonjer HJ. Impact of gas (less) laparoscopy and laparotomy on peritoneal tumor growth and abdominal wall metastases. Ann Surg 1996; 224: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik PS, Misawa T, Chiang M, et al. Abdominal incision tumor implantation following pneumoperitoneum laparoscopic procedure vs. standard open incision in syngeneic rat model. Dis Colon Rectum 1998; 41: 419–422. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman GC, Baker JW, Doxey JB, et al. Minimally invasive surgery for colorectal cancer. Initial follow-up. Ann Surg 1996; 6: 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray D, Lee H, Schlinkert R, Beart RW Jr. Adequacy of lymphadenectomy in laparoscopic-assisted colectomy for colorectal cancer: a preliminary report. J Surg Oncol 1994; 57: 8–10. [DOI] [PubMed] [Google Scholar]
- 27.Lumley JW, Fielding GA, Rhodes M, et al. Laparoscopic-assisted colorectal surgery. Lessons learned from 240 consecutive patients. Dis Colon Rectum 1996; 39: 155–159. [DOI] [PubMed] [Google Scholar]
- 28.Franklin ME, Rosenthal D, Abrego-Medina D, et al. Prospective comparison of open vs. laparoscopic colon surgery for carcinoma: five-year results. Dis Colon Rectum 1996; 39: S35–S46. [DOI] [PubMed] [Google Scholar]
- 29.Lacy AM, Delgado S, Garcia-Valdecasas JC, et al. Port site metastases and recurrence after laparoscopic colectomy. Surg Endosc 1998; 12: 1039–1042. [DOI] [PubMed] [Google Scholar]
- 30.Fleshman JW, Wexner SD, Anvari M, et al. Laparoscopic vs. open abdominoperineal resection for cancer. Dis Colon Rectum 1999; 42: 930–939. [DOI] [PubMed] [Google Scholar]
- 31.Milsom JW, Bohm B, Hammerhofer KA, et al. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg 1998; 187: 46–57. [DOI] [PubMed] [Google Scholar]
- 32.Heald RJ, Goligher JC. Anterior resection of the rectum. In: Fielding LP, Goldberg SM, eds. Rob and Smith’s Operative Surgery, Rectum and Anus. Oxford: Butterworth-Heinemann; 1993: 456–471.
- 33.Darzi A, Hill ADK, Henry MM, et al. Laparoscopic-assisted surgery of the colon. Operative technique. Endosc Surg 1993; 1: 13–15. [PubMed] [Google Scholar]
- 34.Monson JRT, Darzi A. Laparoscopic Colorectal Surgery. Oxford: Isis Medical Media; 1995.
- 35.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1978; 53: 457. [Google Scholar]
- 36.Welch JP, Donaldson GA. Recent experience in the management of cancer of the colon and rectum. Am J Surg 1974; 127: 258–265. [DOI] [PubMed] [Google Scholar]
- 37.Miller DR, Allbritten FF. Carcinoma of the colon and rectum. Arch Surg 1976; 111: 692–696. [DOI] [PubMed] [Google Scholar]
- 38.Wield U, Nilsson T, Knudsen JB, et al. Post-operative survival of patients with potentially curable cancer of the colon. Dis Colon Rectum 1985; 28: 332–335. [DOI] [PubMed] [Google Scholar]
- 39.Fingerhut A, Millat B, Lointier P, Hay JM. Is laparoscopic colonic resection for carcinoma feasible and safe? A collective French experience. Dig Surg 1995; 12: 280–283. [Google Scholar]
- 40.Fleshman JW, Nelson H, Peters WR, et al. Early results of laparoscopic surgery for colorectal cancer. Retrospective analysis of 372 patients treated by Clinical Outcomes of Surgical Therapy (COST) Study Group. Dis Colon Rectum 1996; 39: S53–S58. [DOI] [PubMed] [Google Scholar]
- 41.Ramos JM, Gupta S, Anthone GJ, et al. Laparoscopy and colon cancer: is the port site at risk? Arch Surg 1994; 129: 897–899. [DOI] [PubMed] [Google Scholar]
- 42.Hughes ES, McDermott FT, Polglase AI, Johnson WR. Tumor recurrence in the abdominal scar after large bowel cancer surgery. Dis Colon Rectum 1983; 26: 571–572. [DOI] [PubMed] [Google Scholar]