Abstract
Objective
To assess whether recent practice has improved, the authors created detailed, evidence-based guidelines and assessed the quality of early-stage breast cancer care at four hospitals in the metropolitan New York area.
Summary Background Data
Adjuvant treatments for early-stage breast cancer have been shown to improve health and longevity. However, reports from the 1980s showed marked underuse of these therapies.
Methods
All 723 women with early-stage breast cancer who had a definitive surgical procedure at four participating hospitals in the Mount Sinai-NYU Health System between April 1994 and August 1996 were included. Inpatient and outpatient records were abstracted.
Results
Fifty-nine percent of women underwent breast-conserving surgery, of whom 81% received radiation therapy. Hospital-specific radiation therapy rates varied from 69% to 87%. Seventy-eight percent of women with stage 1B or greater cancer received systemic treatment, with hospital-specific rates varying from 71% to 86%. Between 18% and 33% of women who could have benefited from local or systemic adjuvant treatments did not receive them. The risk of not getting a beneficial adjuvant treatment varied more than twofold by the hospital where the breast cancer surgery was performed.
Conclusions
The hospital where breast cancer surgery is performed is associated with the likelihood that women receive effective local and systemic adjuvant treatments. Surgeons and members of hospital quality improvement programs should encourage multidisciplinary approaches to breast cancer care.
In the 1990s, meta-analyses summarized the results of randomized clinic trials that enrolled 30,000 women to assess the efficacy of tamoxifen, 26,000 to evaluate chemotherapy, and 28,000 to study radiation therapy. These analyses showed that for patients with early-stage disease, adjuvant hormone therapy reduces the annual death rate by 17%, chemotherapy reduces the annual death rate by 16%, 1 and radiation therapy after breast-conserving surgery reduces local recurrence rates by two thirds. 2 Research in the 1980s documented marked underuse of these adjuvant therapies. 3–8 In one study, 48% of patients undergoing breast-conserving surgery did not receive radiation, and 44% of women with stage II breast cancer did not receive systemic treatment. 3 It is unknown how the quality of early-stage breast cancer care has changed since publication of the large meta-analyses of the efficacy of adjuvant systemic and radiation therapies. A recent Institute of Medicine report assessing the quality of cancer care notes that serious underuse of treatments may be due to older practice patterns from the 1980s, as well as to questionable reliability of data in cancer registries. 9 They call for reports of current, reliable data on key processes and outcomes to assess breast cancer care. We undertook a study to determine the current quality of breast cancer care at a sample of hospitals in our health system.
METHODS
We recruited four teaching hospitals in the New York metropolitan area to participate in an early-stage breast cancer quality improvement project. All hospitals performed at least 100 breast cancer surgeries per year. The hospitals were a 493-bed community teaching hospital, a 420-bed community teaching hospital, a 705-bed tertiary care teaching hospital, and a 1,171-bed tertiary care teaching hospital. We assembled a steering committee of breast cancer experts from the four participating hospitals and created evidence-based guidelines (Table 1) . Guidelines were created based on studies from the mid-1980s through the mid-1990s and represented standard practice in breast cancer care. Guidelines were mailed to all physicians with an accompanying letter from the chairman of the surgery department encouraging support of and participation in the project. 10
Table 1. GUIDELINES FOR CARE OF PATIENTS WITH BREAST CANCER

We created data collection instruments permitting abstractors to collect the clinical data necessary to determine whether care for individual patients was consistent with quality measures derived from the guidelines. We developed training materials and procedures for ensuring data quality to achieve a high degree of interrater reliability. To test interrater reliability, 10% of records were abstracted by two abstractors. The resulting kappa of ≥0.85 on adjuvant treatment questions indicated a high level of interrater reliability.
Using tumor registries, hospital discharge and ambulatory surgery databases, and pathology databases and files, we identified a study population consisting of all women who received their initial definitive surgical procedure for primary stage 1 or 2 breast cancer at each participating hospital during the study period (March 1994 through August 1996). We assessed care provided during a period before the development and dissemination of our guidelines to describe baseline performance before planned interventions for improvement.
A total of 1,258 potentially eligible women were identified. Of these, 400 were excluded: 183 received their definitive surgery at other hospitals, 93 had recurrent cancers, 53 had late-stage cancer, 49 were not treated during the study period, 21 did not have breast cancer, and 1 was a man. An additional 135 women were stage 0 (ductal carcinoma in situ) and were not included in these analyses.
Because most adjuvant treatments are provided in the outpatient setting, records were abstracted from both inpatient and outpatient sources. All physicians agreed to participate. Two hundred eighty physician offices provided access to data about their patients for this study. The median number of different information sources required to complete data abstraction on each patient was two (range 1–6).
Insurance was categorized by primary outpatient payor. All patients with Medicaid were categorized as Medicaid; patients with Medicare only or Medicare and commercial insurance were categorized as Medicare. Patients with commercial insurance only were categorized as commercial. Analyses included chi-square tests for categorical and t tests for continuous bivariate comparisons and logistic regression for multivariable modeling. Interaction terms for age, hospital, and insurance were not statistically significant and are not included in the final models. The final model and model fit were assessed using the log likelihood and the Hosmer Lemeshow goodness-of-fit test, respectively. Logistic models calculate odds ratios that may overestimate the relative risk of outcomes of interest if the incidence of that outcome is 10% or greater. We corrected our logistic models’ odds ratios using a method to approximate a risk ratio from the adjusted odds ratio for common outcomes. 11
RESULTS
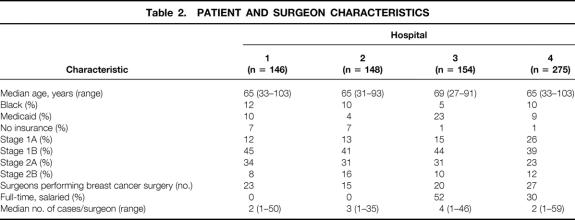
The final study population consisted of 723 women receiving definitive surgical treatment of primary stage 1 or 2 breast cancer: 146 patients at hospital 1, 148 at hospital 2, 154 at hospital 3, and 275 at hospital 4. Patient and surgeon characteristics are listed in Table 2. The mean age at each of the hospitals was 65 years. One of the hospitals had a slightly older patient population with a median age of 69 years, compared with age 65 at the other three facilities. Between 5% and 12% of patients were black. Between 4% and 23% had Medicaid, and 1% to 7% did not have insurance. The number of surgeons performing breast cancer surgery at each institution varied from 15 to 27. The proportion of surgeons at each hospital whose status was full-time salaried varied from 0% to 43%. Individual surgeon volume at the participating hospital ranged from 1 to 59 cases, with median values between 2 and 4.
Table 2. PATIENT AND SURGEON CHARACTERISTICS
Rates of performance on the quality measures for each hospital are listed in Table 3. No woman underwent a radical mastectomy. The rate of axillary dissection for the whole population was 87%, with hospital rates varying from 79% to 92%. Sentinel node biopsy was performed at one of the participating institutions in the context of a study trial; all 10 women who underwent the procedure also underwent axillary dissection. Eighty-five percent of all women had their tumors assayed for hormone receptors. Three of the four hospitals performed the assays frequently; in one hospital, only 56% of tumors were tested for hormone receptors.
Table 3. QUALITY OF EARLY-STAGE BREAST CANCER CARE
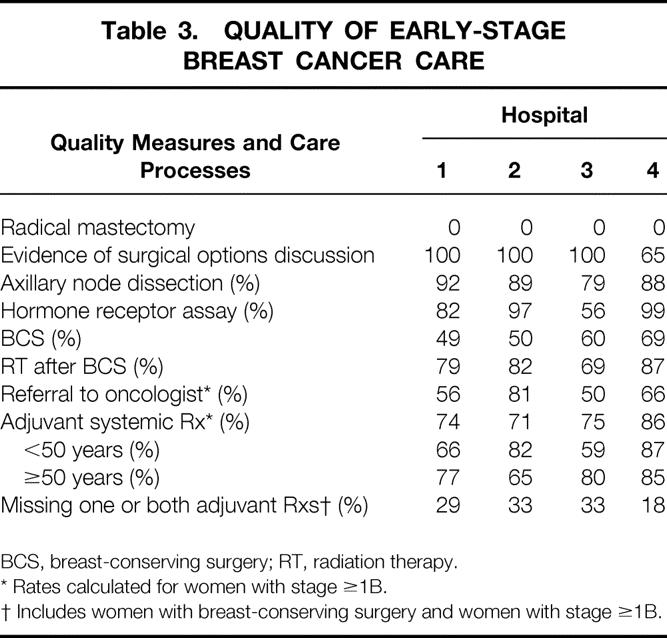
BCS, breast-conserving surgery; RT, radiation therapy.
* Rates calculated for women with stage ≥1B.
† Includes women with breast-conserving surgery and women with stage ≥1B.
Overall, 59% of women underwent breast-conserving surgery. At the two community hospitals, the breast-conserving surgery rate was approximately 50%; the rates were higher at the tertiary care hospitals. Eighty-one percent of women who underwent breast-conserving surgery received radiation therapy afterward, with rates varying from 69% to 87%. The radiation therapy rate among women 65 years and older was 75%. Seventy-eight percent of women with stage 1B or stage 2 tumors received adjuvant systemic treatment, with rates varying from 71% to 86%. We found that 64% of women with stage 1B or stage 2 breast cancer were referred to a medical oncologist. Referral to an oncologist was associated with receipt of systemic therapy. Of women with stage 1B or stage 2 cancer, 91% of women referred to an oncologist received systemic therapy compared with 53% of women who were not referred (P < .0001). Overall, 18% to 33% of women treated at these hospitals who could have benefited from an adjuvant treatment did not receive either radiation after breast-conserving surgery or adjuvant systemic treatment, or both.
Risk factors for omission of adjuvant radiation and systemic therapies are listed in Table 4. Adjusting for important clinical and demographic factors, we found that age is a significant independent predictor of receipt of radiation therapy. Women age 75 and older were 2.4 times more likely than younger women to have radiation therapy omitted after breast-conserving surgery. Insurance is also associated with receipt of radiation therapy. Compared with women who had commercial insurance, uninsured women had a fourfold increased risk of not receiving radiation therapy. The hospital where a woman had her breast cancer surgery performed was associated with receipt of radiation therapy. Women who underwent breast-conserving surgery at hospital 3 had a 2.2 greater risk of not receiving radiation therapy as women whose surgery was at hospital 4.
Table 4. ADJUSTED RISKS OF MISSING ADJUVANT TREATMENTS FOR BREAST CANCER
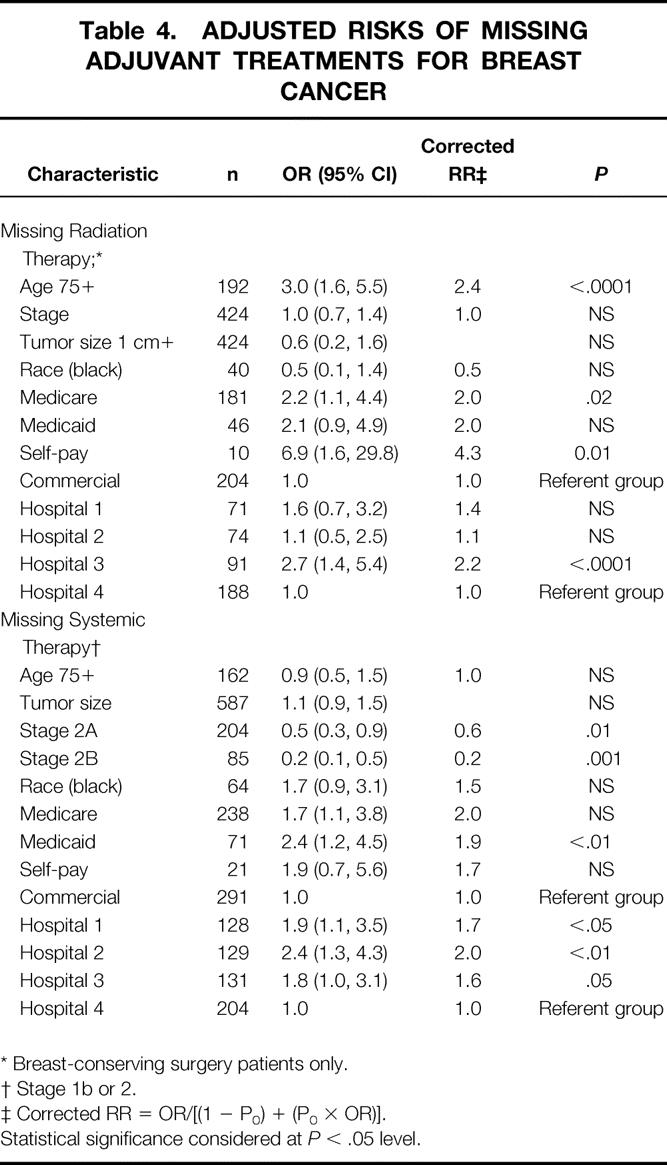
* Breast-conserving surgery patients only.
† Stage 1b or 2.
‡ Corrected RR = OR/[(1 − P0) + (P0 × OR)].
Statistical significance considered at P < .05 level.
Receipt of adjuvant systemic treatment was found to be related to stage, insurance, and the hospital where the breast cancer surgery was performed. Age did not affect the likelihood of receiving systemic treatment. The more advanced the stage, the more likely women were to receive systemic treatment. Women with stage 2B were 80% more likely than those with stage 1B to receive systemic treatment. Women with stage 1B or higher breast cancer who had Medicaid insurance appeared to be at double the risk of not receiving systemic treatment. Women whose surgery was performed at hospitals 1 or 2 had a significantly increased risk of not receiving systemic therapy compared with women who had their surgery at hospital 4.
DISCUSSION
We found that the quality of early-stage breast cancer care has improved since reports published in the 1980s. The rates of performance we found were substantially higher than those previously published; however, there continues to be a substantial percentage of women who could benefit from adjuvant treatments who do not receive them. Between 18% and 33% of women did not receive guideline-recommended adjuvant therapy known to improve health outcomes. We found that receipt of adjuvant therapies was affected by clinical factors including age and stage, and the hospital where the woman had her definitive breast cancer surgery performed.
We found a high rate of receipt of radiation therapy in the total population, comparable to the highest rates previously documented. Studies from the late 1980s and early 1990s documented rates of radiation therapy after breast-conserving surgery of 46% to 87%. 12–15 In particular, our 75% rate of radiation therapy among older women with breast-conserving surgery was markedly higher than found in previous work. However, age remains a significant factor in omission of radiation therapy: the older old are less likely to receive radiation therapy. A study of Medicare beneficiaries from 1985 to 1989 reported a 46% rate of radiation therapy among women undergoing breast-conserving surgery. 13 Our rate of systemic treatment was high compared with the 44% to 83% rates reported in the late 1980s and early 1990s. 12–15 Past studies of older women reported that 59% to 63% of node-positive, estrogen receptor-positive postmenopausal women received adjuvant systemic treatment. 14 Our higher rates of systemic treatment among older women may indicate that higher proportions of older women are now receiving systemic therapy, but may also result from the intense efforts we used to find treatment data from outpatient sources.
Women with Medicaid or no insurance have lower rates of cancer screening, are diagnosed with breast cancer at later stages, and have a lower 5-year survival rate from early-stage breast cancer than women with other forms of insurance. 16–18 Our finding of an increased risk of not receiving efficacious radiation and systemic treatments among women with Medicaid or no insurance provides a possible explanation for their lower survival rates. Although suggestive, this result must be interpreted with caution because there were only 82 women with Medicaid and 24 with no insurance. Our finding of a significant relation between Medicare and omission of radiation therapy is probably due to the high correlation of older age and Medicare status.
The three hospitals in our study in which almost one third of newly diagnosed breast cancer patients did not receive a beneficial adjuvant treatment were hospitals with a moderate volume of breast cancer cases (50–150 cases per year). The hospital in which 18% of newly diagnosed breast cancer patients did not receive a beneficial adjuvant treatment was a high-volume facility (>150 cases per year). The variation we found in receipt of adjuvant treatments by hospital suggests a possible explanation for the relation between hospital volume of breast cancer surgery and 5-year survival rates described by Roohan et al. 19 Demonstrating a direct relation between the volume of breast cancer operations and receipt of adjuvant treatments across the full range of hospital volume, including very low (<10 cases per year) and low (10–49 cases), would provide confirming evidence.
Our study has several limitations. We assessed care at only four hospitals. All the hospitals in this study were teaching hospitals in the metropolitan New York area and had relatively high volumes of breast cancer cases. Previous studies have shown higher rates of performance of breast cancer adjuvant treatment and better quality of care in general at teaching hospitals and larger hospitals. 7,20 It may be impossible to generalize our findings to other types of settings. The guidelines we created were based on literature from the mid-1980s and early 1990s and should have had adequate time to disseminate and affect usual practice in the absence of a specific intervention to improve practice. The present study was designed to assess current practice and to inform the design of subsequent specific interventions to improve the quality of breast cancer care; it was not designed to evaluate an effort to increase compliance with a guideline. Some patients refused treatments; we documented 13 such refusals, 0 at hospital 1, 4 at hospital 2, 3 at hospital 3, and 6 at hospital 4. This may be an underestimate of patient refusal of treatment. If a patient chose not to follow through on treatment recommendations and never returned, we considered this to be a failure to receive treatment but did not attribute it to patient refusal.
Treatment of breast cancer frequently requires multispecialty care. We found that after controlling for age, stage, tumor size, insurance status, and race, the hospital where a woman undergoes breast cancer surgery affects the likelihood of her receiving beneficial adjuvant local and systemic treatments. The variability in rates of performance by hospital emphasizes the critical role hospitals may be able to play in improving the care of patients whose treatments frequently extend beyond the inpatient stay and continue in the outpatient setting. For example, our finding of substantially higher rates of adjuvant systemic therapy among patients referred to medical oncologists suggests that a system intervention, such as automatic referral of stage 1B or 2 patients from surgeon to oncologist, may increase the rate of appropriate systemic treatment. Surgeons and members of hospital-based quality improvement activities should encourage multidisciplinary approaches to breast cancer care to ensure that women with early-stage breast cancer receive beneficial adjuvant therapies.
Acknowledgments
The authors thank the members of the Mount Sinai-NYU Health System and Mount Sinai Medical Center Early-Stage Breast Cancer Treatment Quality Improvement Steering Committees and all the physicians who voluntarily opened their offices to us.
Footnotes
Correspondence: Nina Bickell, MD, MPH, Dept. of Health Policy, Box 1077, Mount Sinai School of Medicine, 1 Gustave L. Levy Pl., New York, NY 10029.
Funded in part by a grant from the United Hospital Fund.
E-mail: Nina_Bickell@mountsinai.org
Accepted for publication February 8, 2000.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75000 women. Lancet 1992; 339: 1–15,71–85. [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. N Engl J Med 1995; 333: 1444–1455. [DOI] [PubMed] [Google Scholar]
- 3.Hand R, Sener S, Imperato J, et al. Hospital variables associated with quality of care for breast cancer patients. JAMA 1991; 266: 3429–3432. [PubMed] [Google Scholar]
- 4.Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med 1992; 326: 1097–1101. [DOI] [PubMed] [Google Scholar]
- 5.Lazovich DA, White E, Thomas DB, Moe RE. Underutilization of breast-conserving surgery and radiation therapy among women with stage I or II breast cancer. JAMA 1991; 266: 3433–3438. [PubMed] [Google Scholar]
- 6.Lee-Feldstein A, Anton-Culver H, Feldstein PJ. Treatment differences and other prognostic factors related to breast cancer survival. Delivery systems and medical outcomes. JAMA 1994; 271: 1163–1168. [PubMed] [Google Scholar]
- 7.Nattinger AB, Gottlieb MS, Veum J, Yahnke D, Goodwin JS. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med 1992; 326: 1102–1107. [DOI] [PubMed] [Google Scholar]
- 8.Sainsbury R, Haward H, Johnston C, Round C. Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet 1995; 345: 1265–1270. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine, Commission on Life Sciences National Research Council. Hewitt M, Simone J, eds. Ensuring Quality Cancer Care. Washington DC: National Academy Press; 1999.
- 10.Bickell NA, Aufses AH Jr, Chassin MR. Engaging clinicians in a QI strategy for early-stage breast cancer treatment. Qual Manag Health Care 1998; 6: 63–68. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Kai FY. What’s the relative risk? A method of correcting the odds ration in cohort studies of common outcomes. JAMA 1998; 280: 1690–1691. [DOI] [PubMed] [Google Scholar]
- 12.Hillner BE, McDonald K, Penberthy L, et al. Measuring standards of care for early breast cancer in an insured population. J Clin Oncol 1997; 15: 1401–1408. [DOI] [PubMed] [Google Scholar]
- 13.Hillner BE, Penberthy L, Desch CE, et al. Variation in staging and treatment of local and regional breast cancer in the elderly. Breast Cancer Res Treat 1996; 40: 75–86. [DOI] [PubMed] [Google Scholar]
- 14.Guadagnoli E, Shapiro CL, Weeks JC, et al. The quality of care for treatment of early-stage breast carcinoma. Is it consistent with national guidelines? Cancer 1998; 83: 302–309. [PubMed] [Google Scholar]
- 15.Silliman RA, Guadagnoli E, Weitberg AB, Mor V. Age as a predictor of diagnostic and initial treatment intensity in newly diagnosed breast cancer patients. J Gerontol 1989; 33: M46–50. [DOI] [PubMed] [Google Scholar]
- 16.Hayward RA, Shapiro M, Freeman HE, Corey CR. Who gets screened for cervical and breast cancer? Results from a new national study. Arch Intern Med 1988; 148: 1177–1181. [PubMed] [Google Scholar]
- 17.Blustein J. Medicare coverage, supplemental insurance, and the use of mammography by older women. N Engl J Med 1995; 332: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 18.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med 1993; 329: 326–331. [DOI] [PubMed] [Google Scholar]
- 19.Roohan PJ, Bickell NA, Baptiste MS, et al. Hospital volume differences and five-year survival from breast cancer. Am J Public Health 1998; 88: 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keeler EB, Rubenstein LV, Kahn KL, et al. Hospital characteristics and quality of care. JAMA 1992; 268: 1709–1714. [PubMed] [Google Scholar]